Abstract
Objective
To assess the prevalence and risk factors of erectile dysfunction (ED) in HIV patients from the HIV clinic of a tertiary referral center in Mexico City.
Design
Prevalence was obtained from cross-sectional studies, and the International Index of Erectile Function (IIEF), a standardized method, was used to assess ED.
Methods
A cross-sectional study was performed in the HIV clinic. Participants completed the IIEF to allow ED assessment. Information on demographics, clinical and HIV-related variables was retrieved from their medical records.
Results
One hundred and nine patients were included, with a mean age of 39.9 ± 8.8 years. ED was present in 65.1% of the individuals. Patients had been diagnosed with HIV for a mean of 92.7 ± 70.3 months and had undergone a mean 56.4 ± 45.5 months of HAART. The only variable associated with ED in the univariate analysis was dyslipidemia, and this association was also found in the multivariate analysis (P = 0.01).
Conclusions
ED is highly prevalent in HIV patients. Dyslipidemia should be considered as a risk factor for ED in HIV patients. Romero-Velez G, Lisker-Cervantes A, Villeda-Sandoval CI, Sotomayor de Zavaleta M, Olvera-Posada D, Sierra-Madero JG, Arreguin-Camacho LO, and Castillejos-Molina RA. Erectile dysfunction among HIV patients undergoing highly active antiretroviral therapy: Dyslipidemia as a main risk factor. Sex Med 2014;2:24–30.
Keywords: Erectile Dysfunction, HIV, HAART, Dyslipidemia, IIEF, AIDS
Introduction
Erectile dysfunction (ED) is defined as the inability to achieve or maintain an erection for sexual intercourse [1]. It affects approximately 10–20 million men in the USA, with a global prevalence of 16%, which increases with advancing age [2]. In Mexico, one study reported a 33.7% prevalence of ED in young men [3]. ED is a multifactorial disease and is associated with comorbidities that include vascular, metabolic, psychogenic, and neurologic diseases.
Human immunodeficiency virus (HIV) is an RNA retrovirus transmitted through sexual intercourse, through contaminated blood, or vertical route. It has become a pandemic infection, and it is estimated that over 34 million people are infected worldwide [4]. In Mexico, its prevalence has been estimated at 0.4% [5]. AIDS-related mortality has decreased since the introduction of highly active antiretroviral therapy (HAART). A longer survival rate has made chronic degenerative diseases more prevalent in HIV-infected patients.
ED among HIV patients is estimated to have a prevalence of 46% [6]. Several studies have investigated this relationship. HAART therapy, protease inhibitors, hypogonadism, an increase in life expectancy in HIV patients, type 2 diabetes (DM2), depression, dyslipidemia, and direct virus effect are possible explanations. However, there are contradictions between studies, which may indicate that the underlying cause of ED in this population is multifactorial.
Aim
The aim of our study was to assess the prevalence of ED in patients on HAART from the HIV clinic at a tertiary care center in Mexico. We also looked for demographic and clinical variables that could be related to ED in this population.
We hypothesized that ED would be more prevalent in men with HIV undergoing HAART. We expected that medical comorbidities would be significantly associated with the rate of ED in these men.
Methods
We conducted a cross-sectional study from January 2008 to December 2008, in which patients on HAART from the HIV clinic at our institution were invited to participate. The study was approved by the local ethics committee. Once informed consent was provided by participants, they completed the International Index of Erectile Function 15 (IIEF-15) questionnaire [7], in the validated Spanish version [8]. The erectile function domain was used to evaluate the patients, and those with a score of >22 were classified as individuals without ED. A score of ≤21 was considered ED, classified as mild (score of 21–17), moderate (16–8), or severe (≤7).
Participants' medical records were reviewed for demographic and clinical data. Demographic variables included age, educational level, sexual preference, tobacco use, and drug and alcohol consumption. HIV-related variables included months since HIV diagnosis, CD4 count nadir, actual CD4 count, months on HAART, history of protease inhibitor (PI) use, current PI use, and use of non-nucleoside reverse transcriptase inhibitors (NNRTIs), nucleoside reverse transcriptase inhibitors (NRTIs), and integrase inhibitors (IIs). Comorbidities recorded included DM2, dyslipidemia (triglycerides >200 mg/dL, total cholesterol >240 mg/dL or both), history of myocardial infarction, hypertension, depression, hepatitis B infection, and obesity. DM2, dyslipidemia, and hypertension were diagnosed based on the definitions of the American Diabetes Association [9], the Adult Treatment Panel III [10], and Joint National Committee 7 [11], respectively.
An independent-samples Student's t-test was performed to compare means between the groups; while nonparametric variables were compared using Kruskal–Wallis and Mann–Whitney tests. A multivariate regression analysis was performed using the most significant variables. All analyses were performed using Statistical Product and Service Solutions (SPSS) version 17.0 (IBM, Armonk, NY, USA). Statistical significance was considered to be P < 0.05.
Results
The final sample included 109 patients. The enrollment procedure is depicted in Figure 1. ED was found in 71 individuals (65.1%). Mild, moderate, and severe ED were found in 48 (67.6%), 9 (12.67%) and 14 (19.71%) patients, respectively.
Figure 1.
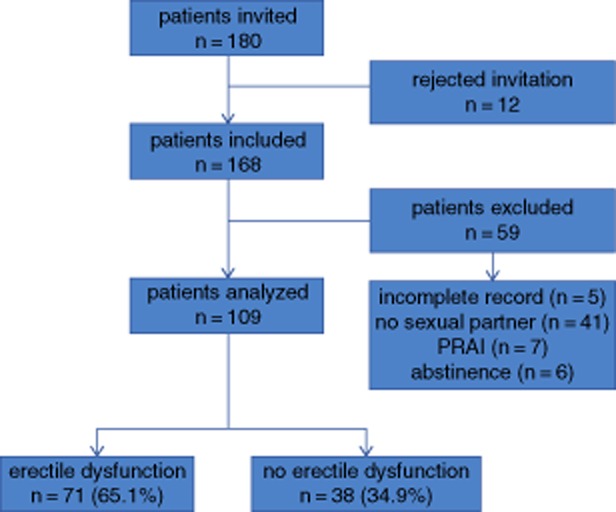
Enrollment process. PRAI = passive-role anal intercourse.
The mean age was 39.9 ± 8.8 years; 70.6% were men who have sex with men (MSM). High school was the minimum educational level in 75.2% of the individuals. In patients with ED, 60.5% had a history of alcohol use, and 38.05% had a history of smoking, while in those without ED these rates were 50% and 31.6%, respectively. Table 1 shows the prevalence of these variables in patients with and without ED.
Table 1.
Demographic and clinical characteristics
| Erectile dysfunction | No erectile dysfunction | P | |
|---|---|---|---|
| Age, mean (SD) | 40.6 (8.6) | 38.8 (9.1) | 0.31 |
| Educational level, n (%) | 0.38 | ||
| Below high school | 15 (21.1) | 5 (13.1) | |
| Above high school | 53 (74.6) | 29 (76.3) | |
| Missing | 3 (4.3) | 4 (10.6) | |
| MSM, n (%) | 0.27 | ||
| Heterosexual | 13 (18.3) | 4 (10.6) | |
| MSM | 48 (67.60) | 29 (76.3) | |
| Missing | 10 (14.1) | 5 (13.1) | |
| Alcohol consumption, n (%) | 0.28 | ||
| Actual | 43 (60.5) | 19 (50) | |
| Previous | 9 (12.7) | 4 (10.6) | |
| No | 19 (26.8) | 14 (36.8) | |
| Missing | 0 (0) | 1 (2.6) | |
| Years of alcohol consumption, mean (SD) | 18.1 (9.8) | 14.6 (8.2) | 0.13 |
| Smoking, n (%) | 0.36 | ||
| Current | 27 (38.05) | 12 (31.6) | |
| Previous | 17 (23.9) | 7 (18.4) | |
| Never | 27 (38.05) | 18 (47.4) | |
| Missing | 0 (0) | 1 (2.6) | |
| Cigarettes/day, mean (SD) | 5.8 (8.4) | 8.7 (14.6) | 0.43 |
| Tobacco years | 15.2 (8.7) | 16.4 (10.5) | 0.69 |
| Drug consumption, n (%) | 0.34 | ||
| Current | 5 (7.0) | 3 (8.9) | |
| Previous | 14 (19.7) | 4 (10.5) | |
| Never | 52 (73.3) | 31 (81.6) | |
| Missing | 0 (0) | 0 (0) |
MSM = men who have sex with men, SD = standard deviation
Mean time since HIV diagnosis and initiation of HAART was 92.7 ± 70.3 and 56.4 ± 45.5 months, respectively. A history of PI use was found in 41.3%. Rates of current use of PIs, NNRTIs, NRTIs, and IIs were 51.4%, 64.2%, 67.0%, and 1.8%, respectively. The prevalence for each group is displayed in Table 2.
Table 2.
HIV-related variables
| Erectile dysfunction | No erectile dysfunction | P | |
|---|---|---|---|
| Months since HIV diagnosis, mean (SD) | 87.4 (64.1) | 102.6 (80.7) | 0.32 |
| CD4 nadir, mean (SD) | 210.5 (189.9) | 190.7 (168.4) | 0.58 |
| Actual CD4, mean (SD) | 421.2 (240.9) | 464.4 (263.7) | 0.40 |
| Viral load, mean (SD) | 4,964.3 (20,807.4) | 225.9 (852.3) | 0.18 |
| Months on HAART, mean (SD) | 55.9 (44.1) | 57.2 (49.8) | 0.90 |
| History of protease inhibitor use, n (%) | |||
| Yes | 28 (39.4) | 17 (39.5) | 0.59 |
| No | 43 (60.6) | 23 (60.5) | |
| Current protease inhibitor use, n (%) | |||
| Yes | 37 (52.1) | 19 (50) | 0.83 |
| No | 34 (47.9) | 19 (50) | |
| Current NNRTI use, n (%) | |||
| Yes | 43 (60.6) | 27 (71.1) | 0.28 |
| No | 28 (39.4) | 11 (28.9) | |
| Current NRTI use, n (%) | |||
| Yes | 46 (64.8) | 27 (71.1) | 0.51 |
| No | 25 (35.2) | 11 (28.9) | |
| Current integrase inhibitor use, n (%) | |||
| Yes | 2 (2.8) | 0 (0) | 0.30 |
| No | 69 (97.2) | 38 (100) |
HIV = human immunodeficiency virus, SD = standard deviation, HAART = highly active antiretroviral therapy, NNRTI = non-nucleoside reverse transcriptase inhibitor, NRTI = nucleoside reverse transcriptase inhibitor
The prevalence rates of DM, dyslipidemia, obesity, and hypertension were 2.8%, 33.0%, 3.7%, and 4.6%, respectively. Depression was present in 11.9% of the patients. Hypertriglyceridemia was present in 15 patients, while 21 suffered hypercholesterolemia. The difference in dyslipidemia between groups was the only variable with statistical significance, with a prevalence of 40.8% in patients with ED and 18.4% in those without ED (P = 0.01). The P values between groups for the rest of the comorbidities are shown in Table 3.
Table 3.
Comorbidities
| Erectile dysfunction | No erectile dysfunction | P | |
|---|---|---|---|
| IIEF score, mean (SD) | 17.8 (6.1) | 28.3 (1.5) | <0.01 |
| Diabetes mellitus, n (%) | |||
| Yes | 2 (2.8) | 1 (2.7) | 0.95 |
| No | 69 (97.2) | 37 (97.3) | |
| Dyslipidemia, n (%) | |||
| Yes | 29 (40.8) | 7 (18.4) | 0.018 |
| No | 42 (59.2) | 31 (81.6) | |
| Myocardial infarction, n (%) | |||
| Yes | 0 (0) | 2 (5.3) | 0.05 |
| No | 71 (100) | 36 (94.7) | |
| Hypertension, n (%) | |||
| Yes | 4 (5.6) | 1 (2.7) | 0.48 |
| No | 67 (94.4) | 37 (97.3) | |
| Depression, n (%) | |||
| Yes | 11 (15.5) | 2 (5.3) | 0.12 |
| No | 60 (84.50) | 36 (94.7) | |
| Hepatitis B infection, n (%) | |||
| Yes | 2 (2.8) | 0 (0) | 0.30 |
| No | 69 (97.2) | 38 (100) | |
| Obesity, n (%) | |||
| Yes | 3 (4.2) | 1 (2.7) | 0.67 |
| No | 68 (95.8) | 37 (97.3) |
SD = standard deviation, IIEF = International Index of Erectile Function
A multivariate analysis was performed, and dyslipidemia was again the only variable that reached statistical significance (P = 0.01). The variables included in this analysis are shown in Table 4 and were selected based on associations that have been found in previous studies [6].
Table 4.
Multivariate analysis
| Covariate variable | P | Hazard ratio | 95% CI |
|---|---|---|---|
| Age | 0.91 | 1.00 | 0.95–1.06 |
| Protease inhibitor use | 0.46 | 1.42 | 0.56–3.60 |
| Depression | 0.12 | 3.75 | 0.71–19.76 |
| MSM | 0.35 | 1.88 | 0.50–7.10 |
| Dyslipidemia | 0.02 | 3.75 | 1.25–11.28 |
Erectile dysfunction is the dependent variable
MSM = men who have sex with men, CI = confidence interval
Discussion
We found a 64% prevalence of ED among HIV patients, which is consistent with the results reported in other studies (21–86%) 12–17. Stefano Zona et al. compared IIEF scores in patients with and without HIV and found a higher prevalence of ED in those infected with HIV [18]. Even though non-HIV individuals were not included as control groups, the prevalence of ED was higher than the 33.7% found in the general population in Mexico [3].
More recent studies suggest an important association of ED with HAART. However, there are a few studies reporting a higher prevalence of sexual dysfunction in patients before the HAART era [6]. Martínez et al. reported for the first time in 1999 a potential role of protease inhibitors in the development of ED [19]. Several studies have sought for this association, and the results have been variable [6]. PIs are the most commonly associated drugs. In our study, as in other published papers [16,18,20], there was no difference in erectile function between different drug treatment groups.
It has also been suggested that a longer exposure to PI could explain ED [13]. In our study, the duration of HAART was not different between participants with ED and those without ED. In the review by Crum et al., it was found that the time on HAART was associated with ED in a univariate analysis, although it did not reach statistical significance in the multivariate analysis [12]. This association was also found in the univariate analysis in a study by Asboe et al. [20]. They reported in multivariate analysis an association between older age (greater than 40 years) and ED [20]. This relationship has been documented in other studies [13,15,21]. A possible explanation is that survival is increased with current HIV treatment, so these patients may now experience age-related diseases such as ED [12]. We found no differences in age between groups (P = 0.31); however, differences were found when patients were divided into those older than 40 and those younger than 40 (P = 0.038). It is important to highlight that our study sample was mainly composed of young adults, with a mean age of 40 (range 24–64).
In our study, no difference according to sexual preference could be found. This variable was not associated with a higher prevalence of ED in previous studies [18,20]. Sollima et al. found MSM status to be an independent predictive variable of ED, although they compared it with intravenous drug use and not with heterosexuality [22]. It has been suggested by Zona et al. that sexual function in MSM should be documented with a modified IIEF for a more accurate measurement of erectile function in this population [18].Coyne et al. validated the IIEF for MSM in 2010. Our study was performed in 2008; hence, this new index could not be used [23]. In one study of MSM, only those with HIV had a higher prevalence of sexual dysfunction [24]. Sexual preference had no influence over how the questionnaire was applied to the patient. We did not specifically address this issue. However, when a patient answered the questionnaire, one of the authors was always available to answer any doubts regarding its content or meaning.
Depression in HIV-positive patients has been associated with ED [20]. This association was also found with antidepressant therapy in the multivariate analysis of a large study [14]. Moreno-Pérez et al. conducted a sub-analysis and found that lower erectile function as assessed by the IIEF was associated with depression [13].
Dyslipidemia has not been associated with ED in HIV patients. In the studies by Asboe et al. and Moreno-Pérez et al., total cholesterol, triglycerides, and HDL cholesterol were not significantly different between the groups [13,20]. De Rick et al. aimed at finding this link as a qualitative variable, yet found no association [15]. Interestingly, dyslipidemia was the only variable associated with ED in both univariate and multivariate analyses in our study.
Dyslipidemia, DM2, hypertension, and obesity are known risk factors for ED in the non-HIV population [25,26]. All of them contribute to endothelial dysfunction, which is manifested as a reduction in endothelial nitric oxide synthase levels [3,25]. This process alters vascular relaxation, with a subsequent compromise in blood flow. High triglycerides and low HDL cholesterol promote a pro-inflammatory and pro-thrombotic state [3]. Free radical formation and atherosclerotic changes have also been postulated as mechanisms [25,27]. For this reason, ED and cardiovascular disease have been considered as parts of the same spectrum of illness [25,28].
Dyslipidemia and other metabolic disturbances are highly prevalent in HIV patients and even more so in those receiving HAART [29,30]. Adverse effects of certain drugs used in HIV treatment favor metabolic disturbances, but HIV itself is also implicated [31]. There is information that supports a genetic predisposition towards development of dyslipidemia on receiving HAART [3,29,31]. The higher prevalence of ED in our HIV patients might be explained by these factors.
In an observational study performed by Silva Torres et al. in Brazil, the authors found an association between dyslipidemia and increasing age in their HIV population [30]. This supports the fact that, currently, HIV patients are prone to suffer diseases associated with aging. Silva Torres et al. found a prevalence of dyslipidemia of 23%, lower than our finding of 33%. This may be explained by a genetic predisposition to this disorder in our Mexican population. We found dyslipidemia to be significantly related to ED in our population. A possible explanation is that this independent factor was not previously found to be significant due to the low prevalence of this comorbidity. However, in a population where dyslipidemia is prevalent in a third of the subjects, similar to that in our study, this variable may be more strongly associated with ED diagnosis.
Guaraldi et al., in a study of 133 HIV-infected men, were not able to prove an association between ED and the metabolic syndrome or its components, which led them to conclude that another pathogenetic mechanism explains ED in the HIV population [32]. The population assessed and the methods used could explain these differences with the information found in the present study.
One limitation of our study is that hormone levels were not assessed. The prevalence of hypogonadism in the pre-HAART era ranged from 15% to 50%, but this has now been reduced [6,12]. Wasting syndrome, opportunistic infections, drugs, and viral infections have been proposed as the mechanisms underlying low testosterone levels [12,15]. Many other authors have found increased estradiol levels [33]; however, the review by Collazos implies no relationship between high estrogen levels and ED [6]. Amini et al. found statistically significant differences in the erectile function domain between eugonadal and hypogonadal patients [34]; while other studies failed to demonstrate this association [13,20]. Another limitation of the present study is that the use of phosphodiesterase 5 inhibitors and that of statins were not evaluated.
Although anxiety and guilt were not included in our evaluation, they have been previously assessed [35]. We also acknowledge that as the aim of this study was to relate metabolic and clinical variables with ED in patients with HIV, some other confounding variables were not included in our evaluation. In addition, dyslipidemia is unlikely to be modified by psychological factors such as anxiety, depression, or guilt.
Even though our study has the limitations inherent to any cross-sectional study, dyslipidemia could, to some extent, explain ED in this population. Selection bias is acknowledged as well; our sample is taken from an HIV clinic, and the results may be different if compared with an open population sample. Our findings are supported by the fact that dyslipidemia is a common risk factor for ED in the general population, the increasing age and age-associated comorbidities in HIV individuals, and the occurrence of dyslipidemia as an adverse effect of HAART and HIV itself. According to different studies, it seems that multifactorial components could be present. As a precise link between ED and HIV has not been found, all factors should be studied individually.
Conclusions
Erectile dysfunction was highly prevalent (65.1%) in HIV patients undergoing HAART. None of the variables that have been previously associated with ED in HIV patients were found to be relevant in our study. However, we found an association with dyslipidemia that should be further investigated. As dyslipidemia was the only variable associated with ED in our study, it ought to be considered as a risk factor for ED in HIV patients.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.National Institutes of Health Consensus Development Panel on Impotence. Impotence. NIH Consens Statement. 1992;10:1–31. [PubMed] [Google Scholar]
- 2.Miner M, Billups KL. Erectile dysfunction and dyslipidemia: Relevance and role of phosphodiesterase type-5 inhibitors and statins. J Sex Med. 2008;5:1066–1078. doi: 10.1111/j.1743-6109.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 3.González-Cuenca E, Villeda-Sandoval CI, Sotomayor de Zavaleta M, Ibarra-Saavedra R, Calao-Perez MB, Quijada-Carlton H, Feria-Bernal G, Castillejos-Molina RA. Prevalencia de disfuncion erectil en una muestra de población joven en Mexico. Rev Mex Urol. 2012;73:245–249. [Google Scholar]
- 4.World Health Organization. HIV/AIDS. WHO Fact Sheet No. 360. Geneva: WHO, 2013. Available at: http://www.who.int/mediacentre/factsheets/fs360/en (accessed September 3, 2013)
- 5.Centro Nacional para la Prevención el y Control del VIH/SIDA. El VIH/SIDA en Mexico 2012. Mexico City: CENSIDA, 2012.
- 6.Collazos J. Sexual dysfunction in the highly active antiretroviral therapy era. AIDS. 2007;9:237–24. [PubMed] [Google Scholar]
- 7.Rosen RC, Riley A, Wagner G, OsterLoh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 8.Zegarra L, Loza C, Perez V. Validación psicométrica del Instrumento Indice Internacional de Función Eréctil en pacientes con disfunción eréctil en Perú. Rev Perú Med Exp Salud Pública. 2011;28:477–483. doi: 10.1590/s1726-46342011000300011. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31(suppl 1):S62. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 10.Adult Treatment Panel III. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 11.Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure; National High Blood Pressure Education Program. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 12.Crum NF, Furtek KJ, Olson PE, Amling CL, Wallace MR. A review of hypogonadism and erectile dysfunction among HIV-infected men during the pre- and post-HAART eras: Diagnosis, pathogenesis, and management. AIDS Patient Care STDS. 2005;19:655–671. doi: 10.1089/apc.2005.19.655. [DOI] [PubMed] [Google Scholar]
- 13.Moreno-Pérez O, Escoín C, Serna-Candel C, Pico A, Alfayate R, Merino E, Reus S, et al. Risk factors for sexual and erectile dysfunction in HIV-infected men: The role of protease inhibitors. AIDS. 2010;24:255–264. doi: 10.1097/QAD.0b013e328334444b. [DOI] [PubMed] [Google Scholar]
- 14.Hart TA, Moskowitz D, Cox C, Li X, Ostrow DG, Stall RD, Gorbach PM, et al. The cumulative effects of medication use, drug use, and smoking on erectile dysfunction among men who have sex with men. J Sex Med. 2012;9:1106–1113. doi: 10.1111/j.1743-6109.2011.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Ryck I, Van Laeken D, Apers L, Colebunders R. Erectile dysfunction, testosterone deficiency, and risk of coronary heart disease in a cohort of men living with HIV in Belgium. J Sex Med. 2013;10:1816–1822. doi: 10.1111/jsm.12175. [DOI] [PubMed] [Google Scholar]
- 16.Lallemand F, Salhi Y, Linard F, Giami A, Rozenbaum W. Sexual dysfunction in 156 ambulatory HIV infected men receiving HAART combination with and without protease inhibitors. J Acquir Immune Defic Syndr. 2002;30:187–190. doi: 10.1097/00042560-200206010-00007. [DOI] [PubMed] [Google Scholar]
- 17.Vansintejan J, Janssen J, Van de Vijver E, Vandevoorde J, Devroey D. The Gay Men Sex Studies: Prevalence of sexual dysfunctions in Belgian HIV+ gay men. HIV/AIDS-Res Palliat Care. 2013;5:89–96. doi: 10.2147/HIV.S43962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zona S, Guaraldi G, Luzi K, Beggi M, Santi D, Stentarelli C, Madeo B, et al. Erectile dysfunction is more common in young to middle-aged HIV-infected men than in HIV-uninfected men. J Sex Med. 2012;9:1923–1930. doi: 10.1111/j.1743-6109.2012.02750.x. [DOI] [PubMed] [Google Scholar]
- 19.Martínez E, Collazos J, Mayo J, Blanco MS. Sexual dysfunction with protease inhibitors. Lancet. 1999;353:810–811. doi: 10.1016/S0140-6736(99)00593-0. [DOI] [PubMed] [Google Scholar]
- 20.Asboe D, Catalan J, Mandalia S, Dedes N, Florence E, Schrooten W, et al. Sexual dysfunction in HIV-positive men is multi-factorial: A study of prevalence and associated factors. AIDS Care. 2007;19:955–965. doi: 10.1080/09540120701209847. [DOI] [PubMed] [Google Scholar]
- 21.Schrooten W, Colebunders R, Youle M, Molenberghs G, Dedes N, Koitz G, et al. Sexual dysfunction associated with protease inhibitor containing highly active antiretroviral treatment. AIDS. 2001;15:1019–1023. doi: 10.1097/00002030-200105250-00010. [DOI] [PubMed] [Google Scholar]
- 22.Sollima S, Osio M, Muscia F, Gambaro P, Alciati A, Zucconi M, et al. Protease inhibitors and erectile dysfunction. AIDS. 2001;15:2331–2333. doi: 10.1097/00002030-200111230-00020. [DOI] [PubMed] [Google Scholar]
- 23.Coyne K, Mandalia S, McCullough S, Catalan J, Noestlinger C, Colebunders R, et al. International Index of Erectile Function: Development of an adapted tool for use in HIV-positive men who have sex with men. J Sex Med. 2010;7:769–774. doi: 10.1111/j.1743-6109.2009.01579.x. [DOI] [PubMed] [Google Scholar]
- 24.Mao L, Newman CE, Kidd MR, Saltman DC, Rogers GD, Kippax SC. Self-reported sexual difficulties and their association with depression and other factors among gay men attending high HIV-caseload general practices in Australia. J Sex Med. 2008;6:1378–1385. doi: 10.1111/j.1743-6109.2008.01160.x. [DOI] [PubMed] [Google Scholar]
- 25.Ryan JG, Gajraj J. Erectile dysfunction and its association with metabolic syndrome and endothelial function among patients with type 2 diabetes mellitus. J Diabetes Complications. 2012;26:141–147. doi: 10.1016/j.jdiacomp.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Mulhall J, Telojen P, Brock G, Kim E. Obesity, dyslipidemias and erectile dysfunction: A report of a subcommittee of the Sexual Medicine Society of North America. J Sex Med. 2006;3:778–786. doi: 10.1111/j.1743-6109.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- 27.Chugtai B, Lee RK, Te AE, Kaplan SA. Metabolic syndrome and sexual dysfunction. Curr Opin Urol. 2011;21:514–518. doi: 10.1097/MOU.0b013e32834b8681. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez JJ, Dashti RA, Schwarz ER. Linking erectile dysfunction and coronary artery disease. Int J Impot Res. 2005;17:12–18. doi: 10.1038/sj.ijir.3901424. [DOI] [PubMed] [Google Scholar]
- 29.Barbaro G. Metabolic and cardiovascular complications of highly active antiretroviral therapy for HIV infection. Curr HIV Res. 2006;4:79–85. doi: 10.2174/157016206775197664. [DOI] [PubMed] [Google Scholar]
- 30.Silva-Torres T, Wagner- Cardoso S, De Souza-Velasque L, Spindola Marins LM, Santini de Oliveira M, Goncalves Veloso V, Grinsztejn B. Aging with HIV: An overview of an urban cohort in Rio de Janeiro (Brazil) across decades of life. Braz J Infect Dis. 2013;17:324–331. doi: 10.1016/j.bjid.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dube MP, Cadden JJ. Lipid metabolism in treated HIV infection. Best Pract Res Clin Endocrinol Metab. 2011;25:429–442. doi: 10.1016/j.beem.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Guadarili G, Beggi M, Zona S, Luzi K, Orlando G, Carli F, et al. Erectile dysfunction is not a mirror of endothelial dysfunction in HIV-infected patients. J Sex Med. 2012;9:1114–1121. doi: 10.1111/j.1743-6109.2011.02243.x. [DOI] [PubMed] [Google Scholar]
- 33.Richardson D, Goldmeier D, Frize G, Lamba H, De Souza C, Kocsis A, Scullard G. Letrozole versus testosterone. A single-center pilot study of HIV-infected men who have sex with men on highly active anti-retroviral therapy (HAART) with hypoactive sexual desire disorder and raised estradiol levels. J Sex Med. 2007;4:502–508. doi: 10.1111/j.1743-6109.2007.00451.x. [DOI] [PubMed] [Google Scholar]
- 34.Amini M, Parsa N, Marzban M, Shams M, Faramarzi H. Depression, testosterone concentration, sexual dysfunction and methadone use among men with hypogonadism and HIV infection. AIDS Behav. 2012;16:2236–2243. doi: 10.1007/s10461-012-0234-x. [DOI] [PubMed] [Google Scholar]
- 35.Perez I, Moreno T, Navarro F, Santos J, Palacios R. Prevalence and factors associated with erectile dysfunction in a cohort of HIV-infected patients. Int J STD AIDS. 2013;24:712–715. doi: 10.1177/0956462413482423. [DOI] [PubMed] [Google Scholar]


