Abstract
Objectives
We evaluated the effect of riluzole versus placebo added to weekly IM interferon beta-1a in early multiple sclerosis (MS).
Methods
This is a randomized (1:1), double-blind, placebo-controlled trial of riluzole 50 mg twice daily in subjects with MS onset less than 1 year prior. Trial participation was up to 3 years. The primary endpoint was change in percent brain volume change. Secondary endpoints included changes in normalized gray and normal-appearing white matter volumes, retinal nerve fiber layer thickness (RNFL), MS Functional Composite and Symbol Digit Modalities Test scores. Mixed model regression analysis was used to compare the changes over time between groups.
Results
Forty-three subjects were randomized to study drug (22 riluzole, 21 placebo). Baseline characteristics were overall similar between groups except for older age (P = 0.042), higher normalized cerebrospinal fluid volume (P = 0.050), lower normalized gray matter volume (P = 0.14), and thinner RNFL (P = 0.043) in the riluzole group. In the primary analysis, percent brain volume change in the placebo group decreased at a rate of 0.49% per year whereas the riluzole group decreased by 0.86% per year (0.37% more per year; 95% CI −0.78, 0.024; P = 0.065). Although age did not influence the rate of brain volume decline, the difference between groups was attenuated after adjustment for baseline normalized gray matter and lesion volume (0.26% more per year in riluzole group; 95% CI −0.057, 0.014; P = 0.22). Analyses of secondary outcomes showed no differences between groups.
Interpretation
This trial provides class 1 evidence that riluzole treatment does not meaningfully reduce brain atrophy progression in early MS.
Introduction
Tissue injury in multiple sclerosis (MS) occurs through inflammatory pathways but also likely through primary neurodegeneration, which results in permanent disability.1 Mechanisms at play in primary neurodegeneration may be different from these involved in tissue loss secondary to new lesion formation.2 Agents currently approved for the treatment of relapsing MS have only modest, if any, appreciable benefits with regard to neurodegeneration when assessed via conventional measures of disease progression such as Expanded Disability Status Scale (EDSS), MS Functional Composite (MSFC) or whole brain atrophy.1 The inability to detect an effect of these drugs on primary tissue injury may be related to their lack of impact on neurodegeneration or to the lack of sensitivity of standard outcome measures typically used in MS trials. This gap in patient care has prompted efforts to improve the detection of central nervous system (CNS) tissue loss with more specific measures such as normalized white and gray matter atrophy on brain imaging and retinal nerve fiber layer (RNFL) loss with optical coherence tomography (OCT). The relevance and reliability of these measures as markers of primary neuroprotection in clinical trials has not been established as few studies have addressed the longitudinal correlation of these measures with clinical progression independently of relapses.3,4
Several mechanisms may contribute to tissue loss in MS, including excitotoxicity, oxidative damage, and energy deficits. While there are convincing studies suggesting a role for glutamate-induced excitotoxicity,5–8 sodium channels may also play a role in tissue loss.1 Riluzole is a drug that inhibits the release of glutamate from nerve terminals, modulates glutamate kainate and NMDA receptors, and stabilizes voltage-gated sodium channels in an inactivated state.9 It is the only drug with a consistent protective effect on amyotrophic lateral sclerosis progression, a disease which may share common final pathways with MS tissue injury.10 In experimental allergic encephalitis, riluzole was found to attenuate disease severity.11 A crossover study in 13 subjects with primary progressive MS failed to show an effect on clinical progression, although C2 cord atrophy measures suggested a possible benefit of the drug compared to the year prior treatment.12,13
Our goal was to test whether a standard dose of riluzole (50 mg twice daily) as an add-on therapy to weekly intramuscular interferon beta (IFNB)-1a had a neuroprotective effect in very early MS compared with placebo.
Methods
Study design and participants
This is a randomized (1:1), double-blind, placebo-controlled trial. The first half of the randomized subjects was invited to remain under study for a third year while we completed the 2-year core study. Eligible subjects were randomized at baseline to riluzole 50 mg daily or placebo for 1 month. If tolerated after 1 month, the subjects changed to riluzole 50 mg or placebo twice a day. The individual study drug regimen was adjusted during the following 2 months according to tolerability. Three months after initiating the study drug, subjects initiated weekly IFNB-1a. Patients were recruited from two sites: University of California, San Francisco (UCSF) and Oregon Health & Sciences University (OHSU). Recruitment was initiated in January 2007 and completed in May 2010. Eligibility criteria included age 18–55, onset of relapsing-remitting (RR) MS or clinically isolated syndromes (CIS) in the past 12 months,14 at least two silent T2-weighted hyperintense areas on brain or spinal cord magnetic resonance imaging (MRI), no previous exposure to IFNB, natalizumab, or immunosuppressive drugs, no use of glatiramer acetate within 3 months of randomization, no MS relapse or use of glucocorticosteroids within 4 weeks of baseline MRI scan, and no use of hepatotoxic medications such as drugs interfering with CYP 1A2. Patients were excluded if screening aspartate aminotransferase or alanine aminotransferase were more than twice the upper limit of normal.
The study was done in accordance with local regulations, the International Conference on Harmonization Guidelines for Good Clinical Practice, and the Declaration of Helsinki. The protocol and all amendments were approved by the institutional review board at both sites; all patients provided written, informed consent before any study-related procedures were done. An independent Data Monitoring Committee oversaw the study. Investigational New Drug exemption was obtained (#55,357). This trial was registered with clinicaltrial.gov (NCT00501943).
Randomization and masking
Treatment allocation was prepared by a UCSF statistician using randomly permuted blocks, with block sizes of four or six in random order. Subjects and study personnel were blinded to treatment assignment until data were analyzed.
Procedures
Figure S1 shows the study visit schedule. Brain MRI (whole brain T2/T1-weighted images yielding 1 × 1 × 3 mm3 resolution without gap) with injection of single-dose gadolinium was acquired at each site on a 3T scanner according to a standardized protocol as described elsewhere.15 Additionally, a high-resolution inversion recovery spoiled gradient-echo T1-weighted isotropic volumetric sequence (3D-IRSPGR, 1 × 1 × 1 mm2, 180 slices) was acquired for brain volume measurements (TE/TR/TI = 2/7/400 msec, flip angle = 15°, 256 × 256 × 180 matrix, 240 × 240 × 180 mm3 FOV, NEX = 1). Peripapillary RNFL and radial macular volume (MV) were measured using a time domain Stratus OCT machine (Zeiss, Fremont, CA).16 Multifocal visual evoked potentials (mfVEP) were recorded using the Veris-EDI Multi-Focal VEP System (Veris-EDI, Redwood City, CA) employing 128 sectors scaled for cortical magnification under standardized stimulus conditions. The mean latency value of both eyes, both for central vision and the entire field, were used for the analysis.
At the completion of the study period, patients returned for a follow-up visit 3 months after discontinuing study treatment to evaluate whether there was a symptomatic effect of study drug.17
Relapses were evaluated during unscheduled visits and defined as new or recurrent neurologic symptoms lasting for at least 48 h and accompanied by objective neurological findings, not associated with fever or infection. Subjects considered to have a relapse were offered a 3-day course of IV methylprednisolone (1 g daily) or oral equivalent at the discretion of the treating neurologist. The next scheduled MRI was postponed for at least 28 days after the completion of steroid treatment.
The primary endpoint was change in brain volume up to month 36, measured with the use of the structural image evaluation using normalization of atrophy (SIENA), a fully automated method of two-timepoint percentage brain volume change (PBVC) analysis,18 part of the FMRIB Software Library (FSL).19 Changes with SIENA were calculated for all patients between baseline and each subsequent available time points from 3D-IRSPGR images, where the output is converted into PBVC between pairs of scans.
There were five secondary endpoints: normalized gray (nGMV) and normal-appearing white matter (nNAWMV) volumes, MSFC, Symbol Digit Modalities Test (SDMT), and peripapillary RNFL. NGMV and nNAWMV from 3D-IRSPGR images were derived from SIENAX,20 which is part of FSL.19 Prior to running SIENAX, T1 lesion masks were derived from manual segmentation of T1-visible white matter lesions on the 3D-IRSPGR using methods described previously.21 T1 lesion masks were incorporated into the SIENAX program to prevent voxel misclassification errors. The following scans, when available, were used for the brain volume analyses: baseline, and months three, six, 12, 18, 24, and 36.20,22 Tertiary endpoints included MV, EDSS, low contrast vision, relapse rate, new T2 lesions, and total global latency on mfVEP.
Statistical analyses
The sample size determination for this study was based on progression of brain atrophy measured with SIENA in a natural history study conducted at UCSF. For PBVC, a sample size of 40 subjects would produce 80% power if riluzole reduced the average worsening by 90% or more. In addition, for all measures except nNAWMV, our proposed sample size would have 80% power for detecting a difference in rates of worsening by 2 years of 92% versus 50% and 75% versus 27% (using Fisher's exact test). As (1) the magnitude of the possible neuroprotective effect of riluzole was unknown on markers such as brain atrophy, (2) best outcome measures of neuroprotection are unclear and (3) this was a pilot study, we felt it was worth testing our hypothesis although the treatment effect we used to design this study was optimistic.
Mixed model regression analysis was used to compare the changes over time between riluzole and placebo while accounting for the longitudinal nature of the data and using the principle of intention-to-treat. The mixed model allowed subject-specific intercepts and slopes. When data were highly non-normal, transformations were used to improve normality, and bootstrapping was used to assess the impact of non-normality on the original scale. We compared the differences in trends over time (Table2). Table1 compared the groups using Fisher's exact test and Wilcoxon rank sum tests. Analyses were conducted using SAS Ver 9.2 (SAS Institute, Cary, NC) and Stata Ver 12 (StataCorp, College Station, TX).
Table 2.
Trends over time between treatment groups for primary and secondary endpoints.
| Number of observations | Monthly change estimate | 95% CI | P value | |
|---|---|---|---|---|
| Primary endpoint | ||||
| % change in brain volume (SIENA) | 192 | −0.031 | −0.065, −0.002 | 0.065 |
| Secondary endpoints | ||||
| nGMV (cm3) | 177 | 0.34 | −1.49, 2.17 | 0.71 |
| nNAWMV (cm3) | 177 | 0.66 | −0.77, 2.09 | 0.36 |
| MSFC scores | 256 | −0.001 | −0.010, 0.009 | 0.86 |
| RNFL (micrometer) | 153 | −0.236 | −0.77, 0.30 | 0.39 |
| SDMT (number correct) | 124 | −0.01 | −0.14, 0.12 | 0.92 |
| Tertiary endpoints | ||||
| EDSS | 282 | −0.005 | −0.02, 0.01 | 0.56 |
| Low contrast vision (mean correct, 1.25%) | 270 | −0.134 | −0.33, 0.06 | 0.19 |
| MV (mm3) | 151 | −0.09 | −0.24, 0.06 | 0.22 |
| mfVEP (total global latency) (msec) | 100 | −0.26 | −0.91, 0.39 | 0.42 |
| nWMV (cm3) | 177 | 0.502 | −0.89, 1.89 | 0.48 |
Analyses are unadjusted for baseline imbalance.
Table 1.
Baseline characteristics.
| Riluzole (n = 22) | Placebo (n = 21) | P value | |
|---|---|---|---|
| Age (years) at disease onset (mean ± SD) | 38.2 ± 9.84 | 32.4 ± 7.85 | 0.042 |
| Disease duration (months) (mean ± SD) | 6.9 ± 4.5 | 8.2 ± 5.4 | 0.54 |
| % Females | 77.3% | 66.7% | 0.44 |
| % Nonwhites | 4.5% | 0% | 0.32 |
| % Hispanics | 9.1% | 14.3% | 0.60 |
| Median EDSS (range) | 2.0 (0, 4.0) | 2.0 (0, 5.5) | 0.36 |
| Normalized brain parenchymal volume (mean ± SD) | 1620 ± 119 | 1660 ± 117 | 0.27 |
| Normalized cerebrospinal fluid volume (mean ± SD) | 439 ± 39.1 | 412 ± 37.9 | 0.050 |
| Normalized gray matter volume (mean ± SD) | 893 ± 67.3 | 924 ± 72.2 | 0.14 |
| Normalized normal-appearing white matter volume (mean ± SD) | 724 ± 68.3 | 736 ± 55.8 | 0.54 |
| % with enhancing scans | 36.4% | 23.8% | 0.62 |
| Normalized lesion volume (mean ± SD) | 7.56 ± 9.37 | 4.01 ± 4.67 | 0.21 |
| T2 lesion load (mean ± SD) | 5.7 ± 7.15 | 2.84 ± 2.92 | 0.25 |
| MSFC (mean ± SD) | 0.161 ± 0.392 | −0.138 ± 1.03 | 1.00 |
| Total RNFL (mean ± SD, micrometers) | 183 ± 22.1 | 199 ± 24.2 | 0.043 |
| SDMT (mean ± SD correct) | 59 ± 9 | 56.8 ± 9.93 | 0.55 |
Results
Study population
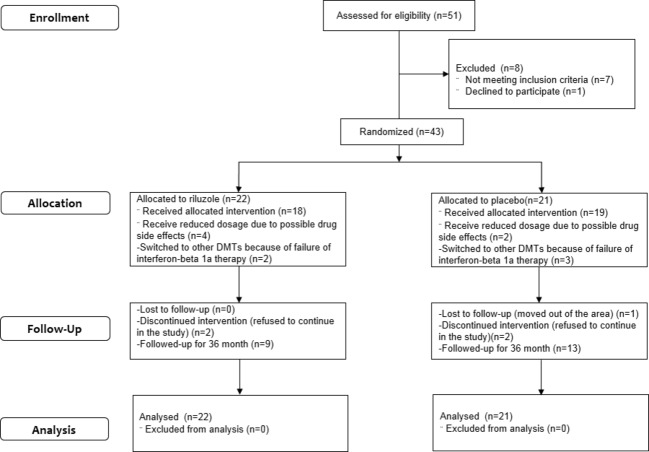
Thirty-six subjects at UCSF and seven subjects at OSHU were randomized to study drug (22 riluzole, 21 placebo). Five (11.2%) did not complete the core 24-month study and were lost to follow-up, respectively, after months 3, 9, 15, 18, and 21 (Fig.1). Their data were used for analyses up to study discontinuation. Only five subjects switched to other disease-modifying therapy during the study after the treating physicians determined breakthrough on weekly IFNB-1a by clinical or MRI criteria. In the riluzole group, one switched to subcutaneous IFNB-1a three times a week (month 13), and one to natalizumab (month 20). In the placebo group, one switched to glatiramer acetate (month 17), one switched to subcutaneous IFNB-1b every other day (month 19), and then natalizumab (month 32), and another switched to glatiramer acetate (month 10), and then to fingolimod (month 16). There were no differences in baseline values (Table1) between groups for most characteristics except that riluzole subjects were older (P = 0.045), had higher normalized cerebrospinal fluid volume (P = 0.050), thinner RNFL (P = 0.043), and tended to have lower nGMV (P = 0.14).
Figure 1.
Consort diagram. This figure represents subject numbers for randomization, treatment decrease or discontinuation and loss of follow-up.
Primary endpoint
A total of 192 scans coming from 42 subjects were available for the primary intent-to-treat analysis. The active group had a yearly change of −0.86% (95% CI −1.17% to −0.56%) whereas the placebo group had a change of −0.49% (CI −0.77% to −0.21%). Taking into account all available time points, brain volume in the placebo group decreased at a rate of 0.49% per year whereas in the active group, it decreased at a rate of 0.86% per year (0.37% more per year in the riluzole group, 95% CI −0.78, 0.024; P = 0.065, Fig.2). Although age was not significantly associated with the rate of brain volume decline (Fig.3), the difference between treatment groups was attenuated when analyses were adjusted, as preplanned for key baseline imbalances, for baseline nGMV and baseline normalized lesion volume (0.26% more per year in the riluzole group; 95% CI −0.057, 0.014; P = 0.22).
Figure 2.
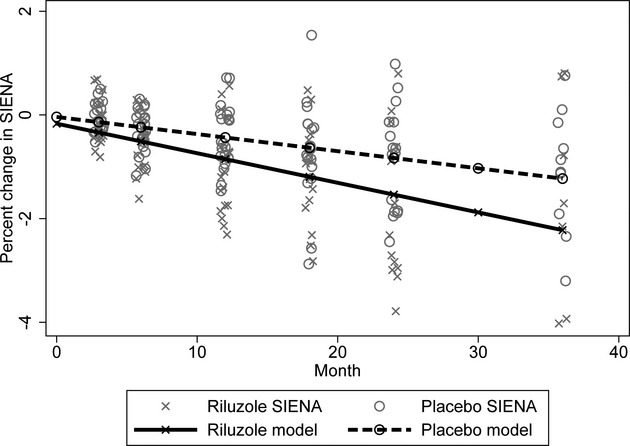
Whole brain atrophy progression. This figure shows percent brain volume changes (SIENA) during the various epics of the study. Dots represent individual data while lines represent model findings. The riluzole group (X) tends to have a faster rate of progression than the placebo group (circles).
Figure 3.
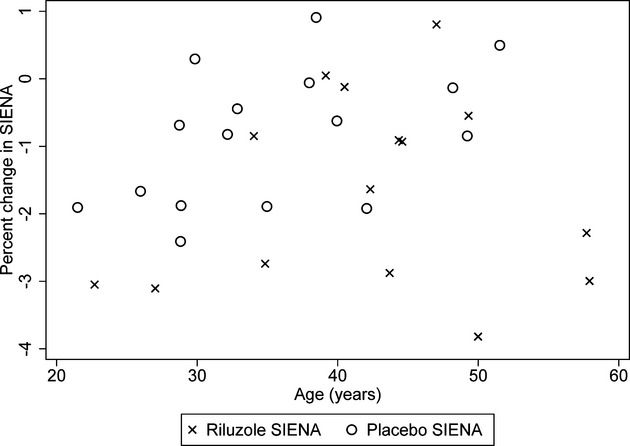
Association between age and progression of percent brain atrophy at month 24. The Xs (riluzole) tend to be a bit shifted to the right compared to the circles (placebo) showing the older average age in the riluzole group. Although there are some older patients with more severe decline in the riluzole group, which could partially explain the differences, the largest declines in percent brain volume change (worse than 2%) were mostly in the treatment group (seven riluzole vs. one placebo subject) and were irrespective of age. In addition, a worsening in percent brain volume change worse than 1% at month 24 was seen almost equally in the placebo (n = 6) and the riluzole (n = 8) groups.
Secondary endpoints
Four subjects did not contribute data for SIENAX analysis as their scans did not meet quality standards. In the intent-to-treat analyses, the rates of change in nGMV, nNAWMV, MSFC, SDMT, and RNFL were similar in the riluzole and placebo groups during the entire study (Table2). Yearly mean nGMV change was −14.37 cm3 in the riluzole group (95% CI −30.57, 1.83) versus −18.44 in the placebo group (95% CI −34.43, −2.45). Yearly mean nNAWM change in the active group was −1.75 cm3 (95% CI −14.71, 11.21) while change in the placebo group was −9.69 cm3 (95% CI −22.82, 2.82). Yearly mean MSFC change was 0.041 in the active group (95% CI −0.04, 0.13) versus 0.05 in the placebo group (95% CI −0.03, 0.13). Yearly mean SDMT change was 0.34 in the riluzole group (95% CI −0.77, 1.46) versus −0.42 in the placebo group (95% CI −0.70, 1.53). Mean total RNFL change per year was −4.6 micrometers in the riluzole group (95% CI −9.31, −0.03) versus −1.84 in the placebo group (95% CI −6.69, 3.01).
Tertiary endpoints
Subjects on riluzole developed more new T2-bright lesions during the study than those on placebo (P = 0.047; analysis using a bootstrap to accommodate the non-normality). To reduce the influence of a few large values, an analysis of the log-transformed values was performed for T2 (P-value for the interaction = 0.114). The number of gadolinium-enhancing lesions was similar in both groups (P = 0.53). No differences were seen for EDSS, low contrast vision, MV, mfVEP, and normalized white matter volume (nWMV) across groups (Table2). Relapse rate was 0.22 per year in the placebo and 0.15 in the riluzole group (P = 0.27).
Blinding evaluation
The treating physician guessed treatment assignment correctly in 6/14 riluzole and 12/15 placebo subjects, with an agreement rate of 62.1% (95% CI 42.3, 79.3) (κ = 0.23, asymptotic standard error [ASE] 0.17, 95% CI −0.10, 0.56). EDSS physicians guessed treatment assignment in 3/10 on riluzole and 0/6 on placebo, with an agreement rate of 18.8% (95% CI 4.0, 45.6) (κ < 0.001, ASE 0.07, 95% CI −0.14, 0.14).
Adverse events
Two pregnancies (at months 3 and 21, one in each group) and one miscarriage (at month 12, placebo group) occurred during the study. Both subjects elected to discontinue their pregnancies: one remained in the study while the one who was pregnant at month 3 was lost to follow-up. The subject who miscarried remained in the study.
Nine subjects (21%) reported transient nausea (two riluzole, seven placebo) and 14 reported (32.5%) transient dizziness (nine riluzole, five placebo). Three (7%) subjects developed increased liver function tests (>3× upper limit of normal) on two consecutive tests (two riluzole, one placebo). One subject (2.3%) on placebo reported dysuria on study medication.
Six patients reported at least one side effect possibly related to study medication, five of whom decreased the dose until study completion (four in riluzole, two in placebo).
Thirty-two subjects (74.4%) returned for follow-up 3 months after last treatment visit to evaluate for the possibility of a symptomatic effect with study drug.17 No clinical changes were reported (data not shown).
Discussion
This study provides class I evidence that riluzole does not meaningfully prevent progression of brain atrophy in early RR MS. It remains unclear whether this might also be generalizable to progressive forms or more advanced stages of MS. Although in the unadjusted primary analysis, there was a trend for faster brain atrophy in riluzole recipients, the preplanned analyses adjusting for baseline imbalance demonstrated this difference was likely due to these imbalances. In addition, there was no detectable impact of riluzole treatment on any other clinical or imaging outcomes, thus suggesting riluzole is unlikely to negatively impact MS. Our results emphasize the importance of taking into account key baseline measures that may reflect a more severe or active preexisting disease, such as seen with the riluzole group.
Ours is the only randomized clinical trial of neuroprotection in early RR MS. However, two randomized trials have evaluated the neuroprotective potential of lamotrigine and simvastatin in secondary progressive (SP) MS.23,24 Another investigated neuroprotection in acute optic neuritis with erythropoietin.25 Assessing neuroprotection in early MS is challenging due to the necessary combination of study drug with standard of care treatment to prevent relapses. However, given their preserved baseline neurological function, early MS patients form an important group to study, as they have more CNS tissue at risk and potentially less tissue already committed to death due to prior accumulated injuries. Furthermore, in progressive MS trials clinical fluctuations of baseline measurements can interfere with the reliability of some outcome measures.23,26 In the setting of acute optic neuritis, prompt enrollment in trials after symptom onset is difficult and may preclude timely rescue of injured tissue.
In the lamotrigine trial,23 active treatment recipients displayed more brain atrophy progression. The processes underlying this finding remain unclear. While the failure to detect a treatment effect might be due to studying patients with advanced deficits and substantial preexisting irreversible neuroaxonal tissue loss, it might also relate to limited experience in neuroprotection trial design.
The fact that our trial did not show an effect of study drug on progression of brain atrophy could be related to randomization imbalance or lack of power. Conversely, it could relate to the use of a drug that does not target key disease processes leading to tissue loss at the early stage of the disease. Although riluzole inhibits the release of glutamate from nerve terminals and modulates glutamate kainate and NMDA receptors, its effect on the glutamate pathway is modest. It remains unclear based on animal models and pathology data what would be the best target for prevention of neurodegeneration in MS.1
Some have questioned whether “pseudoatrophy,” as possibly seen in the lamotrigine trial, may confound the results of neuroprotection trials. We believe “pseudoatrophy” was not seen in this study for three reasons. First, study drug did not demonstrate an effect on new lesion formation, thus study drug unlikely had an anti-inflammatory effect, which could lead to a “pseudoatrophy” effect as previously suggested with natalizumab.27 Second, weekly interferon treatment has not been reported to be associated with “pseudoatrophy,” and there was no acceleration of brain volume loss after interferon initiation at month 3 in the study compared to first 3 months. Finally, we do not believe there was “pseudoatrophy” related to study drug as the slope of changes with gray matter and normal-appearing white matter volumes were similar in both the riluzole and the placebo groups.
Our biostatistical analytic strategy is novel. Primary and secondary analyses took into consideration all time points collected rather than only data available at month 24 study visit. This allows for identification of trends over time with higher reliability and avoids the loss of precious information in case patients do not remain in the study until completion or the last study measure does not pass quality control. Furthermore, analyzing the trend over time allows for taking baseline differences into account and for retaining all data from subjects, regardless how long they remained in the study. For example, although differences were identified at month 24 for progression of atrophy with SIENA and RNFL changes, these differences were not confirmed in the analysis taking baseline values and all data into account. Indeed, differences in brain atrophy appeared to be related to preexisting rate of brain atrophy and lesion accumulation while differences in RNFL changes were entirely explained by baseline differences across groups for that measure. Using statistical analyses that take in account possible baseline differences is critical as these differences may otherwise confound detection of treatment effect, especially in small studies that are more prone to randomization imbalances.
Several limitations of this study are acknowledged. First, as a small trial, it may have been underpowered to detect a treatment effect and been more prone to randomization imbalance. It is also possible that by including subjects with such early MS, some may have had a more benign course, which has been associated with lower rates of brain atrophy.28 Second, the add-on design may have led to lesser disease progression and decreased power. However, no consistent effect of IFNB-1a on measures of brain atrophy, RNFL, or SDMT has ever been reported. While missing data may have decreased our power or biased the results, the statistical analyses allowed making use of all available time points, thereby maximizing the contributions of all subjects to analyses. It is also possible that this study was too short to fully capture the effects of the medication on the outcomes of interest. Finally, our primary and secondary imaging outcome measures were limited to percent brain atrophy, nGMV and nNAWMV. These atrophy measures were chosen as they have been consistently used to monitor brain tissue loss in MS studies.29 We cannot exclude the fact that other imaging metrics of tissue loss such as thalamic atrophy, cortical thickness, and cross-sectional area at C2 may have been more sensitive to pick up relevant changes.
As a definitive surrogate marker for disease progression has not been established, this trial combined state-of-the-art comprehensive testing of various outcomes considered relevant to the study of neuroprotection. The only measure showing trend for a difference in unadjusted analyses was percent brain atrophy, suggesting this outcome may be more sensitive than the secondary and tertiary outcome measures used in this study. We are currently analyzing longitudinal correlations between specific imaging and clinical measures to identify the most sensitive imaging outcome of disease progression in early MS. This study will no doubt help to improve the design of future neuroprotection studies.
Acknowledgments
We are thankful to the patients who participated in this study. We are grateful for the careful oversight of the data and safety monitoring board composed of Gary Cutter, Mariko Kita and Nancy Sicotte. We thank CTSI at UCSF for its support. The study was funded by the National MS Society, the MS International Federation (Maghzi), and the Race to Erase MS (Waubant). Study drug and interferon beta-1a were provided by Sanofi Aventis and Biogen Idec.
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Trial design. This figure recapitulates the timing of study visits and treatment initiation.
References
- Maghzi AH, Minagar A, Waubant E. Neuroprotection in multiple sclerosis: a therapeutic approach. CNS Drugs. 2013;27:799–815. doi: 10.1007/s40263-013-0093-7. [DOI] [PubMed] [Google Scholar]
- Ellwardt E, Zipp F. Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp Neurol. 2014 doi: 10.1016/j.expneurol.2014.02.006. ; doi: 10.1016/j.expneurol.2014.02.006 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247–254. doi: 10.1002/ana.21423. [DOI] [PubMed] [Google Scholar]
- Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol. 2008;64:255–265. doi: 10.1002/ana.21436. [DOI] [PubMed] [Google Scholar]
- Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61:1113–1120. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- Werner P, Pitt D, Raine CS. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann Neurol. 2001;50:169–180. doi: 10.1002/ana.1077. [DOI] [PubMed] [Google Scholar]
- Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- Smith T, Groom A, Zhu B, Turski L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat Med. 2000;6:62–66. doi: 10.1038/71548. [DOI] [PubMed] [Google Scholar]
- Peluffo H, Estevez A, Barbeito L, Stutzmann JM. Riluzole promotes survival of rat motoneurons in vitro by stimulating trophic activity produced by spinal astrocyte monolayers. Neurosci Lett. 1997;228:207–211. doi: 10.1016/s0304-3940(97)00384-4. [DOI] [PubMed] [Google Scholar]
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Panet H, Melamed E, Offen D. Riluzole suppresses experimental autoimmune encephalomyelitis: implications for the treatment of multiple sclerosis. Brain Res. 2003;989:196–204. doi: 10.1016/s0006-8993(03)03343-2. [DOI] [PubMed] [Google Scholar]
- Kalkers NF, Barkhof F, Bergers E, et al. The effect of the neuroprotective agent riluzole on MRI parameters in primary progressive multiple sclerosis: a pilot study. Mult Scler. 2002;8:532–533. doi: 10.1191/1352458502ms849xx. [DOI] [PubMed] [Google Scholar]
- Killestein J, Kalkers NF, Polman CH. Glutamate inhibition in MS: the neuroprotective properties of riluzole. J Neurol Sci. 2005;233:113–115. doi: 10.1016/j.jns.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- Waubant E, Pelletier D, Mass M, et al. Randomized controlled trial of atorvastatin in clinically isolated syndrome: the STAyCIS study. Neurology. 2012;78:1171–1178. doi: 10.1212/WNL.0b013e31824f7fdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villoslada P, Cuneo A, Gelfand J, et al. Color vision is strongly associated with retinal thinning in multiple sclerosis. Mult Scler. 2012;18:991–999. doi: 10.1177/1352458511431972. [DOI] [PubMed] [Google Scholar]
- Ristori G, Romano S, Visconti A, et al. Riluzole in cerebellar ataxia: a randomized, double-blind, placebo-controlled pilot trial. Neurology. 2010;74:839–845. doi: 10.1212/WNL.0b013e3181d31e23. [DOI] [PubMed] [Google Scholar]
- Smith SM, De Stefano N, Jenkinson M, Matthews PM. Normalized accurate measurement of longitudinal brain change. J Comput Assist Tomogr. 2001;25:466–475. doi: 10.1097/00004728-200105000-00022. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Mowry EM, Beheshtian A, Waubant E, et al. Quality of life in multiple sclerosis is associated with lesion burden and brain volume measures. Neurology. 2009;72:1760–1765. doi: 10.1212/WNL.0b013e3181a609f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Furby J, Hayton T, et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9:681–688. doi: 10.1016/S1474-4422(10)70131-9. [DOI] [PubMed] [Google Scholar]
- Chataway J, Alsanousi A, Chan D, et al. The MS-STAT trial: high dose simvastatin demonstrates neuroprotection without immune-modulation in secondary progressive multiple sclerosis (SPMS) – a phase II trial. Mult Scler. 2012;18(S4):509. [Google Scholar]
- Suhs KW, Hein K, Sattler MB, et al. A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann Neurol. 2012;72:199–210. doi: 10.1002/ana.23573. [DOI] [PubMed] [Google Scholar]
- Zhang J, Waubant E, Cutter G, et al. EDSS variability before randomization may limit treatment discovery in primary progressive MS. Mult Scler. 2013;19:775–781. doi: 10.1177/1352458512459685. [DOI] [PubMed] [Google Scholar]
- Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68:1390–1401. doi: 10.1212/01.wnl.0000260064.77700.fd. [DOI] [PubMed] [Google Scholar]
- Gauthier SA, Berger AM, Liptak Z, et al. Rate of brain atrophy in benign vs early multiple sclerosis. Arch Neurol. 2009;66:234–237. doi: 10.1001/archneurol.2008.567. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Airas L, Grigoriadis N, et al. Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs. 2014;28:147–156. doi: 10.1007/s40263-014-0140-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial design. This figure recapitulates the timing of study visits and treatment initiation.