Abstract
Following surgery of cholesteatoma, a patient developed a chronic infection of the external auditory canal, including extended-spectrum β-lactamase producing Escherichia coli, which caused severe pain. The application of cold atmospheric plasma resulted in a significant reduction in pain and clearance of bacterial carriage, allowing antibiotics and analgesics to be ceased.
Keywords: Cholesteatoma, cold argon plasma, (ESBL+) E. coli, external auditory canal, plasma, tissue tolerable plasma
Research Note
Cold atmospheric plasmas (CAPs) have demonstrated broad bactericidal activity, regardless of the bacterial species and resistance pattern in vitro and in vivo 1–3. Plasma is the fourth state of matter and high temperature plasmas have long been used in the sterilization of medical equipment. The development of CAPs now allows application to viable tissue. The efficacy of a CAP device in reducing bacterial carriage and pain in a patient with chronic nasopharyngeal and external auditory canal infection after cholesteatoma surgery is reported.
A 49-year-old man developed an acquired cholesteatoma of the left tympanic cavity. Cholesteatoma is a rare cyst-like growth formed from keratinizing stratified squamous epithelium in the middle ear cleft or the petrous bone 4. Surgery is usually required because, although benign, cholesteatoma is destructive and potentially life-threatening 5. Under antibiotic prophylaxis (amoxicillin combined with clavulanic acid) the patient underwent tympanoplasty, incus resection and reconstructive surgery of the auditory channel. Subsequently, chronic infection developed in the nasopharynx and external auditory canal involving extended-spectrum β-lactamase-producing (ESBL+) Escherichia coli and other bacteria (Proteus mirabilis and Enterococcus faecalis). The infections were accompanied by severe pain, described as severe radiating headaches.
Over the 3 years post-surgery, repeated courses of systemic co-trimoxazole were required in addition to regular wax removal, topical antiseptics (e.g. octenidine) and hydrogen peroxide 0.3% rinses. Aspirin did not relieve the headaches. Co-trimoxazole was taken continuously for the 6 months prior to presentation for CAP treatment.
After informed consent and with ethics committee approval, daily weekday treatment was commenced to both external auditory canals with 5-min cold atmospheric argon plasma (Fig.1) using the MicroPlaSter β device (built by ADTEC Plasma Technology Co. Ltd., Hiroshima, Japan and London, UK) (Microwave 2.46 GHz, 86 W; Ar 2.2 slm; distance to external auditory meatus 2 cm) 6. The nasopharynx was not treated as at the time respiratory tract CAP exposure had been investigated only in ex vivo models 7.
Figure 1.
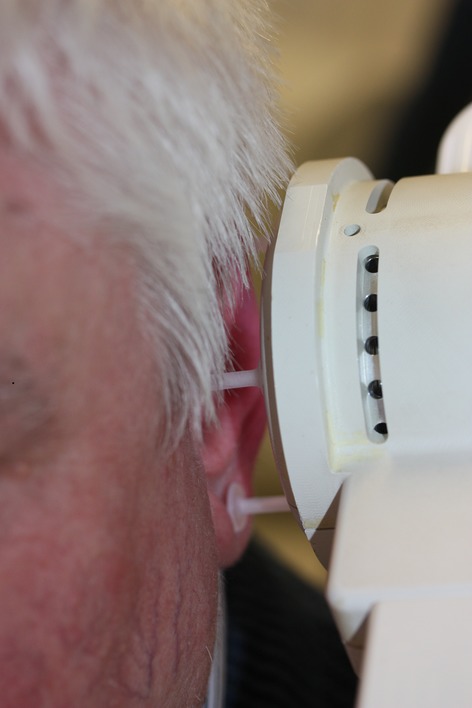
Application of cold atmospheric argon plasma (MicroPlaSter β) to the ear channel.
Plasma treatment was given over 105 days in three blocks of 8, 16 and 19 (total 43) applications, respectively. Pain was recorded using a visual analogue scale (VAS: range 0–10) directly before and immediately after plasma applications. Swabs for bacteriology were taken before, during and after completion of the plasma treatment. Oral co-trimoxazole was continued throughout the initial plasma treatment period, ceasing after the 14th application of plasma. After ceasing the systemic antibiotics, hydrogen peroxide (0.3%) rinsing continued and was performed twice during the final treatment period. No analgesics were required during the treatment period of 105 days. Although the headaches resolved, the patient continued to describe an unpleasant soreness in the ear.
Plasma therapy was well tolerated and no side effects occurred. Immediately after each plasma application there was a mean pain reduction score of 1.1 with highly significant ongoing long-term improvement in pain (Fig.2, p <0.01; Wilcoxon rank t test). Pre-treatment pain was increased on Mondays after the weekend break. One month after completing CAP treatment, the patient remained highly satisfied with the result.
Figure 2.
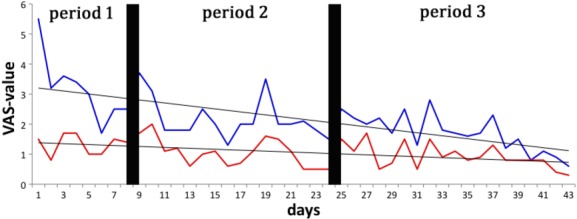
Highly significant reduction of pain after plasma application (43 applications, mean reduction 1.1, p <1.1 × 10−8). Blue line: pain according to VAS (range 0–10, where 0 corresponds to no pain at all and 10 to the most severe pain imaginable), before plasma application; red line: pain after plasma application; grey line: linear trend line. VAS, visual analogue scale.
During the total 105 day plasma-treatment period, bacteriology swabs from the external auditory canals and nasopharynx were all negative. Specifically no ESBL-producing E. coli were detected. However 3 weeks after the final plasma application, Propionibacterium acnes was cultured from the external auditory canal and ESBL(+) E. coli from the nasopharynx.
Despite antibiotic prophylaxis, surgical site infections occur in head and neck cancer surgery in over 30% of cases 8. The battle against antibiotic-resistant bacteria is one of the greatest challenges in medicine in the 21st century and new approaches are required to manage this problem 9,10. CAPs may be an option as they can achieve high antibacterial activity without the development of resistance (neither showing primary nor secondary resistance development) or harmful effects on surrounding human tissue 11–13. The effects are probably due to a synergy of physical–chemical features resulting in cell wall permeabilization, penetration of reactive oxygen and nitrogen species, and subsequent chemical reactions and actions inside the cell 13.
The broad antimicrobial benefit of CAP has been demonstrated in randomized controlled clinical trials in patients with chronic wounds 6,14. CAP application in Hailey-Hailey disease resulted in successful clearance of Proteus mirabilis and Candida albicans, and reduction in pain and burning sensations due to the impetiginization 15.
In this case report, plasma application was limited by the diffusion and penetration of the plasma into the external auditory canal, and the inability to treat the nasopharynx. However, even in a set-up not primarily developed for the treatment of the external auditory canal, CAP treatment resulted in the clearance of bacteria and relief of symptoms of infection during treatment. Long-term antibiotics could be ceased and quality of life improved to a patient’s satisfaction.
The current report will be advanced by future studies demonstrating the efficacy of CAP sources designed for treat regions that are difficult to treat, such as the external auditory canal, and safety of use in the oral cavity.
Acknowledgments
We are indebted to Delwyn Dyall-Smith, FACD, for proofreading the manuscript. We thank our medical colleagues for the referral and care of the patient, and evaluation of the bacterial specimens. We thank ADTEC Plasma Technology Co. Ltd., Hiroshima, for the allocation of the MicroPlaSter β device.
Transparency Declaration
The MicroPlaSter beta device was provided by the Max Planck Institute for Extraterrestrial Physics. The following authors are designated inventors and patent-holders of the plasma applicator and corresponding method: G.I., T.S., G.E.M. and W.S.
References
- Klampfl TG, Isbary G, Shimizu T, et al. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl Environ Microbiol. 2012;78:5077–5082. doi: 10.1128/AEM.00583-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfill GE, Shimizu T, Steffes B, Schmidt HU. Nosocomial infections: a new approach towards preventive medicine using plasmas. New J Phys. 2009;11:1–10. [Google Scholar]
- Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaan MT, Megerian CA. The pathophysiology of cholesteatoma. Otolaryngol Clin North Am. 2006;39:1143–1159. doi: 10.1016/j.otc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Stark T, Gurr A, Sudhoff H. Principles of cholesteatoma surgery. HNO. 2011;59:393–399. doi: 10.1007/s00106-011-2265-4. [DOI] [PubMed] [Google Scholar]
- Isbary G, Heinlin J, Shimizu T, et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. Br J Dermatol. 2012;167:404–410. doi: 10.1111/j.1365-2133.2012.10923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welz C, Becker S, Li Y-F, et al. Effects of cold atmospheric plasma on mucosal tissue culture. J Phys D Appl Phys. 2013;46:1–9. [Google Scholar]
- Penel N, Lefebvre JL, Cazin JL, et al. Additional direct medical costs associated with nosocomial infections after head and neck cancer surgery: a hospital-perspective analysis. Int J Oral Maxillofac Surg. 2008;37:135–139. doi: 10.1016/j.ijom.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Piddock LJV. The crisis of no new antibiotics—what is the way forward? Lancet Infect Dis. 2012;12:249–253. doi: 10.1016/S1473-3099(11)70316-4. [DOI] [PubMed] [Google Scholar]
- Wise R. The urgent need for new antibacterial agents. J Antimicrob Chemother. 2011;66:1939–1940. doi: 10.1093/jac/dkr261. [DOI] [PubMed] [Google Scholar]
- Isbary G, Shimizu T, Li YF, et al. Cold atmospheric plasma devices for medical issues. Expert Rev Med Devices. 2013;10:367–377. doi: 10.1586/erd.13.4. [DOI] [PubMed] [Google Scholar]
- Isbary G, Zimmermann J, Shimizu T, et al. Non-thermal plasma: more than five years of clinical experience. Clin Plasma Med. 2013;1:19–23. [Google Scholar]
- Zimmermann JL, Shimizu T, Schmidt HU, Li Y-F, Morfill G, Isbary G. Test for bacterial resistance build-up against plasma treatment. New J Phys. 2012;14:1–14. [Google Scholar]
- Isbary G, Morfill G, Schmidt HU, et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol. 2010;163:78–82. doi: 10.1111/j.1365-2133.2010.09744.x. [DOI] [PubMed] [Google Scholar]
- Isbary G, Morfill G, Zimmermann J, Shimizu T, Stolz W. Cold atmospheric plasma: a successful treatment of lesions in Hailey-Hailey disease. Arch Dermatol. 2011;147:388–390. doi: 10.1001/archdermatol.2011.57. [DOI] [PubMed] [Google Scholar]


