Abstract
Introduction
Adipose tissue-derived stem cells (ADSCs) herald tremendous promise for clinical application in a wide range of injuries and diseases. Several preclinical reports demonstrate their efficacy in the treatment of cavernous nerve (CN) injury-induced erectile dysfunction in rats. It was recently illustrated that these effects were established as a result of ADSC migration to the major pelvic ganglion (MPG) where these cells induced neuroregeneration in loco.
Aims
The study aims to identify chemotactic factors in the MPG following injury and to match upregulated chemokines to their respective receptors in human ADSC on the genomic, structural, and functional levels.
Methods
Quantitative real-time polymerase chain reaction, fluorescence-activated cell sorting (FACS), intracellular FACS, immunofluorescence microscopy, migration assays, and calcium imaging were used in this study.
Main Outcome Measures
The main outcomes are chemokine expression in the MPG following CN injury, and the functional and structural presence of chemokine receptors in ADSC.
Results
CCR4, CX3CR1, and XCR1 are functionally and structurally present in human ADSC, and are activated by the chemokines CCL2, CX3CL1, and XCL1 respectively, which are upregulated in the MPG following CN injury. CXCR4 and its ligand CXCL12 (SDF1) are likely no major homing factors for ADSC. Expression of chemokine receptor mRNA in ADSC did not necessarily translate into receptor presence at the cell surface and/or functional activation of these receptors. Most of the expressed chemokine receptors were detected in the intracellular compartment of these cells.
Conclusions
We identified the ligand/chemokine receptor pairs CCL2/CCR4, CX3CL1/CX3CR1, and XCL1/XCR1 as potentially responsible for ADSC homing toward the MPG following CN injury. The intracellular localization of various chemokine receptors likely indicates redirecting of chemokine receptors to the cell surface under specific cellular conditions. Furthermore, modification of expression of these receptors at the genomic level may potentially lead to improved migration toward injury sites and thus enhancement of treatment efficacy. Albersen M, Berkers J, Dekoninck P, Deprest J, Lue TF, Hedlund P, Lin C-S, Bivalacqua TJ, Van Poppel H, De Ridder D, and Van der Aa F. Expression of a distinct set of chemokine receptors in adipose tissue-derived stem cells is responsible for in vitro migration toward chemokines appearing in the major pelvic ganglion following cavernous nerve injury. Sex Med 2013;1:3–15.
Keywords: Adipose Tissue-Derived Stem Cells, Cavernous Nerve Injury, Cell Surface, Chemokines, Chemokine Receptors, Erectile Dysfunction, Intracellular, Migration
Introduction
Despite the development of nerve-sparing techniques, neuropraxia of the cavernous nerves (CNs), resulting in erectile dysfunction (ED), remains a highly frequent complication of radical prostatectomy for prostate cancer [1]. Various pharmacological “penile rehabilitation” strategies have been investigated to prevent denervation-induced smooth muscle apoptosis and fibrosis in the erectile tissue during the time frame in which the injured CNs regenerate [2]. While preclinical research was promising, recent clinical trials have not uniformly confirmed the benefit of penile rehabilitation in this setting. Therefore, basic researchers have in the recent years been focusing on stimulating CN regeneration following neuropraxia, rather than aiming at the secondarily damaged erectile tissue as a treatment target [3]. In these studies, the application of multipotent stromal cells (MSC) derived from either bone marrow or adipose tissue has generated promising results [4].
Kendirci and colleagues performed bilateral CN crush injury in rats and injected bone marrow-derived MSC (BM-MSC) and BM-MSC selected for p75-nerve growth factor receptor expression in the corpus cavernosum, resulting in beneficial effects on erectile recovery [5]. These authors suggested paracrine effects on neuroregeneration based on increased in vitro secretion of growth factors by these stem cells. They noted an absence of significant cell incorporation and differentiation into host cell types in the penis. This finding was in line with previous studies regarding cellular therapy for CN injury [4,6,7]. The repeated observation of erectile function improvement in the absence of cell incorporation led to the hypothesis that these cellular therapies may exert their effects in a paracrine fashion [8]. In several consecutive studies, our group has further investigated this “paracrine hypothesis.” In an initiating study, Albersen and coworkers injected either adipose tissue-derived mesenchymal stem cells (ADSCs) or ADSC-derived lysate in the corpus cavernosum of CN-injured rats and observed preservation of neuronal nitric oxide synthase content, diminished smooth muscle apoptosis, and erectile tissue fibrosis in both treated groups [9]. The lysate treatment exposed injured tissues to soluble factors contained in ADSC, without allowing live cells to incorporate in the host tissue, and thus supported paracrine modes of action of stem cell therapy for CN-injury induced ED [10]. This report was followed by an experiment in which the major pelvic ganglion (MPG), the autonomic ganglion from which the CN originates, was cultured in conditioned medium of either ADSC or smooth muscle cells. Strongly enhanced neurite sprouting was observed when these ganglia were cultured in ADSC-conditioned media [11]. Results from the latter experiment indicated that ADSCs secrete substances that induce nerve regeneration. Fandel et al. looked at the fate of EdU-labeled ADSC after intracavernosal injection in the CN-injury rat model, and observed a rapid decrease of cell numbers from the injection site in the corpus cavernosum following injection [12]. Surprisingly, ADSC were located in the MPG in the first days following injury. Furthermore, this cell recruitment proved essential for establishing beneficial effects of cell therapy, including enhanced neuroregeneration and restoration of erectile function. Increased expression of stromal cell-derived factor 1 (SDF1; CXCL12) in the MPG was noted and proposed as a possible homing factor for ADSC following CN injury.
Aims
The gained results led us to conclude that recruitment of ADSC toward the MPG is an essential step in establishing the beneficial effects of cellular therapy. In this study, we therefore aimed to identify chemotactic factors in the MPG following injury. Furthermore, we matched upregulated chemokines to their respective receptors in human ADSC on the genomic, structural, and functional levels.
Methods
ADSC Isolation
After approval from the local ethical committee, approximately 20 g of subcutaneous human adipose tissue was harvested from three informed and consenting female adult patients undergoing ureteral reimplantation for vesico-ureteral reflux. Adipose tissue was rinsed in sterile calcium/magnesium-free phosphate buffered saline (PBS), minced into small pieces and digested by incubation in a solution containing 0.1% collagenase type IA (Sigma-Aldrich, St. Louis, MO, USA) for 1 hour at 37°C with vigorous shake for 15 seconds in 10-minute intervals. The top lipid layer was removed and the remaining liquid portion was centrifuged at 1,000 × g for 5 minutes at room temperature. The remaining cells were suspended in 10 mL Dulbecco's modified Eagle medium (DMEM) supplemented with streptomycin, Amphotericin-B, penicillin, and 10% fetal bovine serum (FBS). The suspension was filtered through a 70-μm cell strainer, plated at a density of 2–3 × 106 cells in a 75-cm2 dish, and cultured at 37°C in 5% CO2. After 24 hours, the cells were rinsed with PBS to remove floating cells such as blood cells. Cells were cultured until 90% confluence and split in a 1:4 ratio. Cells were then harvested by 3 minutes incubation in trypsin-EDTA solution (Sigma-Aldrich) and used for further study in passage 5.
Fluorescence-Activated Cell Sorting (FACS)
Human ADSC were examined for surface marker expression by FACS. Cells were trypsinized, washed in calcium/magnesium-free PBS containing 1% bovine serum albumin (FACS-buffer) twice, and pelleted. Samples were incubated at 4°C (CD markers) or room temperature (chemokine receptors) for 30 minutes by the appropriate primary anti-human antibody 1:25 in FACS-buffer, followed by a double wash in FACS-buffer, and resuspended in 400 μL PBS for final analysis. A minimimum of 10,000 events were recorded for each analysis using a FACSort flow cytometer (BD Biosciences, Oxford, UK), and analyzed using cell quest software (BD Biosciences). For chemokine receptor expression, ADSCs were first fixed in 4% paraformaldehyde, followed by washing in either standard FACS-buffer (for surface chemokine receptor detection), or by a washing step in permeabilization buffer consisting of FACS-buffer supplemented with 0.1% saponin (for intracellular chemokine receptor detection). FACS was then further conducted as described previously. CCRL1 and CCRL2 expression was assessed by a two-step incubation as no conjugated primary antibodies were available for these human epitopes. As a negative control, cells were incubated with the same species isotype controls as the primary antibodies. Antibodies used included: anti-human CD166-FITC (Acris, Herford, Germany); CD14-PE; CD29-FITC; CD90-FITC (Biosource, Camarillo, CA, USA); HLA-DR-PE; CD35-PE; CD73-PE; HLA-ABC-FITC; CD44-PE (BD Biosciences); CD45-PE; CD105-PE; CCR1-PE; CCR3-FITC; CCR4-FITC; CCR7-FITC; CCR10-FITC; CX3CR1-PE; XCR1-FITC; CXCR4-PE; CXCR6-PE; CXCR7-PE (R&D Systems, Abingdon, Oxon, UK); CCRL1; CCRL2 (1°, Abcam, Cambridge, UK); Gt anti-Rb-IgG-FITC; Gt anti-mouse-IgG-PE (2°, R&D Systems).
ADSC Differentiation
Differentiation capacity of ADSC toward osteogenic lineage was tested by incubation in DMEM supplemented with 10% FBS, 100 nM dexamethasone, 0.2 mM ascorbic acid, and 10 mM β-glycerol phosphate (all by Sigma-Aldrich) for 2.5 weeks. To assess calcium deposit formation; cultures were stained with Alizarin Red. Adipogenic differentiation was tested by culturing confluent ADSC for 2.5 weeks in DMEM, supplemented with 10% FBS, 0.5 mM isobutyl-methylxanthine, 200 μg indomethacin, 1 μM dexamethasone, 10 μg/mL insulin (all by Sigma-Aldrich). After culture, cells were stained with Oil-Red O solution.
Animal Study
Fourteen 12-week-old male Sprague-Dawley rats were subjected to bilateral CN crush injury under isoflurane 2% as previously described [13]. Twenty-four hours following injury, the bilateral MPG was harvested and the animals were sacrificed. Tissues were preserved in RNA-later (Qiagen, Valencia, CA, USA, n = 12) or fixed (n = 2). The institutional animal care and use committee approved the animal studies.
Immunohistochemistry
Human ADSCs were cultured on coverslips under normal circumstances. Cells were washed with PBS, fixed in paraformaldehyde 4% during 10 minutes, and permeabilized by incubation in 0.1% saponin in paraformaldehyde 4% for 10 minutes. Cells were washed, and then stained with CCR4-FITC (R&D Systems) 1:25 in 3% goat serum and 0.1% saponin in PBS at room temperature, after blocking with 3% goat serum and 0.1% saponin in PBS for 30 minutes. Cells were washed twice in PBS supplemented with 0.1% saponin, and actin-filaments were stained using Alexa Fluor 488-conjugated phalloidin 1:500 (Invitrogen, Carlsbad, CA, USA) in 4% paraformaldehyde. Nuclear staining was performed by 2-minute incubation in 4′,6-diamidino-2-phenylindole (DAPI; D-3571, Invitrogen) 1:10,000 in PBS. Coverslips were then mounted on glass slides with fluorescence mounting medium (Vectashield, Vector Laboratories, Burlingame, CA, USA).
Freshly dissected rat MPGs were fixed for 4 hours with cold 2% formaldehyde and 0.002% picric acid in 0.1 M phosphate buffer, followed by overnight immersion in buffer solution containing 30% sucrose. Tissues were frozen in optimum cutting temperature compound (Sakura Finetek, Torrance, CA, USA), and stored at −80°C until use. Sections were cut at 5 μm, adhered to charged slides, air dried for 10 minutes, and rehydrated with PBS. Goat serum 3% in PBS was applied as blocking agent for 30 minutes. Sections were incubated overnight at 4°C with primary antibodies, followed by 1 hour incubation in 1:500 dilution of secondary antibody conjugated with Alexa Fluor 488 (Invitrogen) or Alexa Fluor 594 (Invitrogen). Primary antibodies were: rabbit anti-CCL2 1:400 (Abcam Inc., Cambridge, MA, USA) and mouse anti-4E2 1:400 (the mouse anti-rat4E2 antibody was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA, USA). Nuclear staining was performed by 2-minute incubation in 4′,6-diamidino-2-phenylindole (D-3571, Invitrogen).
Quantitative Real-Time Polymerase Chain Reaction (qPCR)
qPCR was used to identify chemokine expression in the rat MPG after CN injury, and to test the presence of mRNA for 21 currently known chemokine receptors in human ADSC. MPG tissue was lysed in Trizol (Invitrogen), ADSC cells were lysed in RLT buffer (Qiagen, Hilden, Germany) +1% β-mercaptoethanol (Sigma-Aldrich). mRNA was isolated from tissue or cell lysates using the RNeasy mini kit (Qiagen S.A., Courtaboeuf, France) and reverse transcribed using a iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). The amplification was performed using TaqMan™ primer sets in Tables 1 and 2 and the TaqMan™ Universal Fast Master Mix (Applied Biosystems, Carlsbad, CA, USA) on an ABI7900HT System (Applied Biosystems). Normalization of the cycle threshold data was done to reference genes including GADPH, GUSB, 18S, and HPRT1 for ADSC, and beta-actin for the MPG.
Table 1.
Expression of chemokine and cytokine mRNA in the rat MPG in sham and cavernous nerve-injury animals
| Gene (rat) | NCBI RefSeq | Amplicon length | Fold exp. SHAM | Fold exp. CN-injury | t-Test |
|---|---|---|---|---|---|
| TGF-β1 | NM_021578.2 | 65 | 1,262 ± 0,23 | 11,464 ± 2,83 | P = 0.024 |
| TGF-β2 | NM_031131.1 | 95 | 0,059 ± 0,01 | 0,295 ± 0,02 | P < 0.001 |
| TGF-β3 | NM_013174.2 | 61 | 4,120 ± 1,73 | 26,23 ± 11,47 | P = 0.08 |
| TNF-α | NM_012675.3 | 82 | 0,001 ± 0,00 | 0,007 ± 0,00 | P = 0.042 |
| CCL2 | NM_031530.1 | 95 | 0,723 ± 0,03 | 5,278 ± 0,38 | P = 0.002 |
| CCL28 | NM_053700.1 | 66 | 0,001 ± 0,00 | 0,017 ± 0,01 | P = 0.122 |
| CXCL12 | NM_001033882.1 | 60 | 10,037 ± 2,02 | 50,745 ± 10,2 | P = 0.003 |
| CX3CL1 | NM_134455.1 | 74 | 0,193 ± 0,02 | 1,494 ± 0,11 | P < 0.001 |
| XCL1 | NM_134361.1 | 89 | 0,293 ± 0,03 | 0,855 ± 0,16 | P = 0.004 |
Bold font indicates statistically significant values.
MPG = major pelvic ganglion.
Table 2.
Expression pattern of mRNA coding for chemokine receptors in ADSC.
 |
Functional Assays on ADSC
Primers for the investigation of the MPG were based on chemokine receptor expression (qPCR and FACS, see above) in human ADSC. Chemokines that were found to be upregulated in the MPG following injury were acquired in human recombinant form and applied for further functional testing of ADSC: CCL28 (binds to CCR10), CCL19 (CCR7, included as additional negative control based on qPCR and FACS), CCL2 (CCR4), CXCL12 (CXCR4), CX3CL1 (CX3CR1), and XCL1 (XCR1) (R&D Systems). Acquired human recombinant chemokines were then used for functional testing as described next under “ADSC Migration Assay” and “ADSC Calcium Imaging Assay.”
ADSC Migration Assay
Passage 5 human ADSCs were allowed to reach 90% confluence in DMEM containing 10% FBS in a 96-well Oris cell migration assay plate (Platypus Technologies, Fitchburg, WI, USA) equipped with central stoppers preventing from cells to attach in a central detection area. The medium was then replaced with chemokine-supplemented DMEM in dosages of 1 nM, 100 nM, and 10 μM. Following incubation, the central stopper was removed and the cells were allowed to migrate toward the central detection zone for 24 hours. As a negative control, DMEM lacking FBS was used, while DMEM + 10% FBS was used as a positive control. Cell migration was quantified digitally by Image J software (NIH, Bethesda, MD, USA) on images taken of the central detection area after application of a detection mask.
ADSC Calcium Imaging Assay
Previous to the measurements, cells were incubated with 2 μM Fura-2AM ester (Biotium, Hayward, CA, USA) for 30 minutes at 37°C. Intracellular Ca2+ concentration was measured on a monochromator-based imaging system consisting of a Polychome IV monochromator (Till Photonics, Martinsried, Germany) and a CCD camera (Roper Scientific, Tucson, AZ, USA) connected to a Zeiss Axiovert 200 M inverted microscope (Oberkochen, Germany). Fluorescence was measured during excitation at 340 and 380 nm, and after correction for the individual background fluorescence signals, the ratio of the fluorescence at both excitation wavelengths (F340/F380) was monitored, as a measure for intracellular calcium concentrations. Experiments were performed using standard Krebs solution. All the investigated chemokines were dissolved in Krebs solution at 50 nM and cells were exposed for 2 minutes, followed by a washout period of 3–5 minutes. Ionomycin 10 μM was used as a positive control at termination of the experiment.
Results
Isolation of ADSC, Characterization, and Multilineage Differentiation Capacity
ADSCs were successfully isolated from the harvested adipose tissue of all three donors and formed a monolayer in approximately 1 week to 10 days after initial plating (Figure 1B).
Figure 1.
(A) Flow cytometry analysis on ADSCs isolated from female human donors shows the vast majority of cells express an CD14−, CD34−, CD45−, HLA-DR− and CD29+, CD90+, CD166+, CD73+, CD105+, CD44+ HLA-ABC+ phenotype, consistent with the typical mesenchymal multipotent stromal cell surface marker profile (light gray area: isotype control, black line: sample). (B) Cultured human ADSCs display a typical fibroblast-like morphology and are able to differentiate toward adipogenic and osteogenic lineages in vitro. Figure 1 is adapted from reference [14] with permission. ADSC = adipose tissue-derived stem cell.
For characterization of ADSCs, expression of their cell surface antigens was examined by flow cytometry (Figure 1A). Cells were strongly positive for the typical MSC markers CD29, CD44, CD73, CD90, CD105, CD166, and HLA-ABC. While in the initial passages low positivity for CD34 was detected, cells were typically strictly negative for CD14, CD34, CD45, and HLA-DR above passage 3.
To investigate the differentiation potential of ADSC, these cells were directed toward the osteogenic and adipogenic lineages. The osteogenic differentiation was confirmed by deposition of alizarin red-staining calcium in a mineralized matrix (Figure 1B). Adipogenic differentiation was demonstrated by accumulation of lipid vacuoles in cytoplasm of the cells indicated by positive Oil Red O staining (Figure 1B). All ADSC samples showed both osteogenic and adipogenic differentiation capacities.
Posttraumatic Chemokine Expression in the Major Pelvic Ganglion
Major pelvic ganglia were harvested from rats 24 hours after either sham injury or bilateral CN injury. Following injury, we observed significantly elevated expression of various inflammatory markers including tumor necrosis factor alpha (TNF-α), and transforming growth factor beta (TGF-β)1 and 2, but not the anti-inflammatory isotype TGF-β3. Furthermore, the chemokines CCL2, CXCL12, CX3CL1, and XCL1 were elevated after injury. CCL 28 was elevated but did not reach significant difference compared with sham-operated animals due to large variation in the regulation of this chemokine and low overall expression (Table 1). We located CCL2/MCP-1 expression in the MPG to the glia cells rather than the neurons using immunofluorescence double-staining (Figure 2).
Figure 2.

Representative immunofluorescence image of the rat MPG 24 hours after cavernous nerve injury. Original magnification ×400. 4E2 (red signal) is a rat-specific peripheral glia marker, and MCP1 (monocyte chemoattractant protein) is CCL2, a chemokine. Nuclear staining was performed with DAPI (blue). Note the colocalization of MCP1 and 4E2, indicating this chemokine is mainly expressed in glia rather than neurons. MPG = major pelvic ganglion.
Chemokine Receptor mRNA in ADSCs
With a detection limit of 40 transcription cycles, we detected presence of mRNA for the following chemokines in human ADSC: CCR1, CCR3, CCR4, CCR10, CXCR4, CXCR6, CXCR7, CX3CR1, XCR1, CCRL1, CCRL2 (Table 2).
FACS of ADSCs
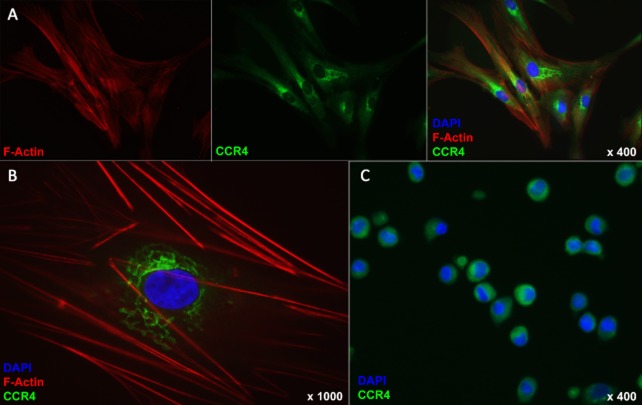
Other groups have noticed low surface marker expression of chemokine receptors in MSC despite their ability to migrate toward a gradient of the ligands of these receptors. In standard FACS analysis, surface marker expression of any chemokine receptor was very low, with minor expression for CCR4, CCR10, CXCR6, CXCR7, CX3CR1, and XCR1. CCR1, CCR3, CCR7, CXCR4, CCRL1, and CCRL2 were not expressed on the cell surface of human ADSC. CCR7 was included in this analysis as a negative control based on absence of mRNA expression for this receptor (Figure 3A). After cell permeabilization, however, detection of CCR4, CCR10, CX3CR1, and XCR1 was strongly positive (signal : noise (S : N) > 5). CCR1, CCR3, CXCR4, CXCR6, and CXCR7 were detected but displayed lower expressions (S : N < 5). CCR7, CCRL1 and CCRL2 were not detected in the intracellular compartment (Figure 3B). By far, the strongest intracellular expression was noted for CCR4. We confirmed the intracellular presence of this receptor by immunofluorescence microscopy on attached and suspended cells (Figure 4). This investigation showed rather low membranous and cytoplasmic expression, and strong expression of the receptor in the perinuclear endoplasmic reticulum, indicating active production of this receptor in attached ADSC in culture.
Figure 3.
(A) FACS of nonpermeabilized cells shows low expression of chemokine receptors at the cell surface. (B) After cell permeabilization, it becomes evident that cells express most of the receptors that were detected by qPCR. CCR4, CCR10, CX3CR1, and XCR1 are strongly expressed, while expression of the remaining chemokine receptors is rather low. (Light gray: isotype control, black line: sample.) FACS = fluorescence-activated cell sorting; qPCR = quantitative real-time polymerase chain reaction.
Figure 4.
Immunofluorescence staining of human attached and suspended ADSC. (A, B) Cells were cultured on coverslips, permeabilized using saponin, and stained against f-actin (red), CCR4 (green), and DAPI (blue). Note that the staining pattern of CCR4 is perinuclear and morphologically consistent with expression in the endoplasmic reticulum. (C) Cells in suspension were permeabilized using saponin and stained CCR4 (green) and DAPI (blue). Note the intracellular staining of CCR4. ADSC = adipose tissue-derived stem cell.
ADSC Migration Assay
Chemokines generally induced migration in a dosage-dependent fashion. FBS-supplemented DMEM induced migration, while in the serum-free DMEM incubated wells only few cells migrated into the central detection zone. In the 100 nM and 10 μM concentrations, significantly more cells were counted in the detection zone when cells were treated with CCL28, CCL2, XCL1, and CX3CL1 than when treated with serum-free DMEM. CCL19 did not induce an increase in cell migration compared with serum-free medium. For CXCL12, only in the highest concentration there was a relatively small increase (1.71 ± 0.2-fold) of number of migrated cells compared with serum-free medium (Figure 5A, B).
Figure 5.

Functional activation of chemokine receptors in human ADSC. (A, B) Chemotaxis assay: ADSCs were incubated with increasing concentrations of chemokines in standard medium. Serum-free medium was used as a negative control. FBS-supplemented medium was used as a positive control (green bar). Migration was assessed by counting the number of cells migrated toward the middle area of a well after removal of a central stopper. Values are expressed as ratio of migrated cells over migrated cells in negative control medium. anova: +P < 0.05 compared with other concentration of same chemokine, *P < 0.05 compared with negative control, §P < 0.05 compared with all other samples. (C) Live cell calcium imaging: ADSCs were plated on a coverslip and loaded with FURA2. Coverslips were mounted in a perfusion chamber and cells were exposed to DMEM medium supplemented with chemokines at a concentration of 50 nM for 2 minutes each. The ratio of light emission during 340/380 nM excitation was recorded as a measure of intracellular calcium concentrations. At conclusion of the experiment, cell viability and calcium influx were assessed using ionomycin as a positive control. n = 20–30. ADSC = adipose tissue-derived stem cell; DMEM = Dulbecco's modified Eagle medium; FBS = fetal bovine serum.
Calcium Imaging
Attached ADSCs were subjected to calcium imaging to confirm the functional activation of chemokine receptors by binding of their specific chemokines. Positive responses to administration of 50 nM of CCL28, CCL2, XCL1, and CX3CL1 were noted. CXCL12 elicited an insignificant response (increase in ratio fura 340/380 from baseline <0.2). Binding of CCL19 to its receptor CCR7 was not observed as indicated by a complete absence of calcium influx upon administration of this chemokine (Figure 5C).
Discussion
MSCs were first described by Friedenstein and colleagues in 1968 as fibroblast-like cells derived from the bone marrow which generated colonies when plated at low densities [15]. Of the various tissues in which MSC have since been isolated, the fat tissue has been of particular interest for ED researchers investigating cellular therapy [16]. ADSCs are a distinct population of MSC residing at the perivascular niche in adipose tissue [17]. They share many characteristics with BM-MSC pertaining to morphology, phenotype, ex vivo differentiation ability, and they have a highly similar gene expression profile [18]. However, the frequency of these cells is 100 to 500-fold higher in adipose tissue compared with the frequency of MSC in bone marrow [4,19]. Furthermore, while bone marrow is obtainable in the gram range by a painful marrow aspiration, hundreds of grams of adipose tissue can be obtained with minimally invasive procedures under local anesthesia.
ADSCs have been shown to be capable of recruitment to injured tissues after systemic administration [20–23]. In particular, we have recently observed ADSC recruitment toward the MPG in the early days after injury to the CNs [12]. Likely, this recruitment process is essential, as those rats that received ADSC injection in the corpus cavernosum (a highly perfused system) showed improvement of functional and structural outcomes. In contrast, those rats in which ADSCs were injected in the dorsal penile perineural space, which is rather static in terms of blood flow, did not recover erectile function and showed erectile tissue deterioration similar to untreated rats. Strikingly, only in the group treated with intracavernosal injection, ADSCs were found in the MPG early after infliction of the nerve injury. Upregulation of the chemokine CXCL12 (SDF1) was observed and it was initially assumed that this was an important recruitment factor for ADSC in the MPG [12].
While CXCL12 binds to both CXCR4 and CXCR7, the affinity of CXCR4 for CXCL12 is high and the interaction between CXCL12 and CXCR4 has been extensively investigated and has been a focus of cell migration research in various disease models [22–27]. However, the expression of this receptor on the cell surface of MSC, and ADSC in particular, has been debated, and has repeatedly been shown to be very low, and either or not functional [25,28]. This led us to design the current study as to investigate which chemokine receptors may be play an important role in the observed ADSC recruitment to the MPG following injury of the CNs. CXCL12 was, as we reported before [12], indeed expressed in the rat MPG following CN injury as illustrated by an upregulation of its mRNA in the MPG 1 day after injury. There was a low but present intracellular expression of CXCR4 in all three ADSC cell lines, and no expression on the surface of the cells. This structural finding was reflected in the functional experiments in which CXCL12 did not elicit significant calcium influx at the applied concentration, and induced ADSC migration in vitro only at the highest concentration of CXCL12. It is therefore not likely an important recruitment factor for ADSC at lower concentrations. Based on the observations made, we have been able to match expression of CCL28, CCL2, CX3CL1, and XCL1 in the MPG to genomic, structural, and functional expression and activation of their respective receptors in ADSC by their ligands. These data help us understand how ADSC recruitment takes place and may identify targets for improvement of stem cell recruitment and thus potentially also treatment efficacy.
Several studies have reported the structural expression of various chemokine receptors on human ADSC or MSC in general; some of the results are inconsistent between research groups, and many studies have not looked at the full panel of chemokine receptors [28–34]. The low cell surface expression of various chemokine receptors has been reported before in MSC [31]. This has been attributed to the use of trypsin-EDTA during cell detachment [32], and of certain receptors it has been shown that they are at least in part residing in intracellular compartments. We now observed that this holds true for most of the functional receptors in ADSC [31]. The intracellular localization does not appear to affect either migratory capacity or activation of these receptors results in calcium influx in the cell, representing an important step in signaling of most G-protein coupled receptors. It should however be kept in mind that in the functional experiments the cells were attached and thus no trypsinization was performed. The latter issue indicates that receptors at the cell surface may still be the effectors for the observed functional activation. Our immunofluorescence study of attached ADSC for CCR4 localization revealed expression mainly in the endoplasmic reticulum. Previously, a similar observation has been made pertaining to the intracellular expression of CXCR7 in cultured neurons [35]. While the implications of this finding are to date not clear, it is known that the endoplasmic reticulum is part of the secretory pathway implicated in protein translocation to the cell surface, and it is commonly considered a default pathway [35]. These data suggest that chemokine receptors may be redirected to the cell surface under specific cellular conditions. It may further imply that these receptors are continuously produced, even when cells remain in culture for several weeks. Some authors have proposed that ADSCs, when cultured in hypoxic conditions or in presence of TNF-α, display a higher number of chemokine receptors at their cell surface [25,34,36]. The findings in our study imply that increased cell surface expression in these conditions may not only be the result of elevated transcription and/or translation, but may be due to receptor translocation to the surface of the cell when the cell is under noxious conditions. Further research pertaining to receptor cycling and translocation is currently in progress in our laboratories to better understand these processes.
Three limitations inherent to the research may restrict the conclusions of this study. First, while we have studied the chemokine regulation in the neural tissue of rats, the chemokine receptor characterization was done in human cells. This is due to limited availability for rat-specific chemokine receptor primers and antibodies, as well as rat recombinant proteins with commercial suppliers. Furthermore, for evident ethical reasons, it is not possible to harvest human hypogastric and pelvic ganglia following radical prostatectomy. On the other hand, it has been demonstrated that rodent and human chemokine receptor profiles in stem cells are highly similar, and our conclusions may thus be extended, with caution, to the rat in vivo situation [32]. A second limitation encompasses the fact that no in vivo recruitment experiments with blockade or knockdown of certain ligands or receptors were included in this study. We chose not to perform these studies, as it is not likely that blocking one single ligand/receptor pair would significantly impair recruitment as we have shown that there are several receptors involved in these migratory processes. Third, both chemokines and receptors are promiscuous. We chose those chemokines that have no or very limited known cross-reactivity with other receptors present in the ADSC for functional activation assays, to limit this bias. However, although unlikely, previously unknown cross-reactivity may occur.
In spite of these limitations, our study provides a qPCR screening of the full panel of the 21 currently known chemokine receptors, and an in-depth structural and functional characterization of the expressed receptors. We are, to our knowledge, the first to assess the complete chemokine receptor expression profile in human ADSC in this detail. Furthermore, we performed calcium-imaging experiments with chemokine stimulation on adult stem cells to validate migration data, and to bypass the possible bias of manual cell counting in migration assays as a measure for functional activation of chemokine receptors. Our findings may further help in tailoring stem cell sources in the function of pathophysiology of different diseases with different chemokine expression profiles. In addition, upregulation of chemokine receptor expression, or translocation of known intracellular receptors to the cell surface, may very well improve treatment efficacy of cellular therapy for ED [34].
Conclusions
We identified the ligand/chemokine receptor pairs CCL2/CCR4, CX3CL1/CX3CR1, and XCL1/XCR1 as potentially responsible for ADSC homing toward the MPG following CN injury. While CCL28 was expressed in a low level in the MPG before and after injury, this ligand also induced functional activation of ADSC and its receptor CCR10 was highly expressed by ADSC. Surprisingly, CXCR4-CXCL12 (SDF1) interaction is not likely a major homing factor for ADSC. Modification of expression of these receptors in ADSC could improve migration toward injury sites and thus treatment efficacy.
Acknowledgments
M.A. is a fellow of the Research Foundation-Flanders (FWO). D.D.R. is a fundamental-clinical researcher of the FWO. This work was sponsored by the Federico Foundation and the Research Foundation-Flanders (FWO). We would like to thank Lieve Coorevits, Carl Van Haute. and the Laboratory of Ion-channel Research at the Catholic University of Leuven for excellent technical assistance.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Boorjian SA, Eastham JA, Graefen M, Guillonneau B, Karnes RJ, Moul JW, Schaeffer EM, Stief C, Zorn KC. A critical analysis of the long-term impact of radical prostatectomy on cancer control and function outcomes. Eur Urol. 2012;61:664–675. doi: 10.1016/j.eururo.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 2.Albersen M, Joniau S, Claes H, Van Poppel H. Preclinical evidence for the benefits of penile rehabilitation therapy following nerve-sparing radical prostatectomy. Adv Urol. 2008 doi: 10.1155/2008/594868. doi: 10.1155/2008/594868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bella AJ, Lin G, Lin C-S, Hickling DR, Morash C, Lue TF. Nerve growth factor modulation of the cavernous nerve response to injury. J Sex Med. 2009;6(suppl 3):347–352. doi: 10.1111/j.1743-6109.2008.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albersen M, Kendirci M, Van der Aa F, Hellstrom WJG, Lue TF, Spees JL. Multipotent stromal cell therapy for cavernous nerve injury-induced erectile dysfunction. J Sex Med. 2011;9:385–403. doi: 10.1111/j.1743-6109.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- 5.Kendirci M, Trost L, Bakondi B, Whitney MJ, Hellstrom WJG, Spees JL. Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J Urol. 2010;184:1560–1566. doi: 10.1016/j.juro.2010.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fall PA, Izikki M, Tu L, Swieb S, Giuliano F, Bernabe J, Souktani R, Abbou C, Adnot S, Eddahibi S, Yiou R. Apoptosis and effects of intracavernous bone marrow cell injection in a rat model of postprostatectomy erectile dysfunction. Eur Urol. 2009;56:716–725. doi: 10.1016/j.eururo.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 7.Bochinski D, Lin GT, Nunes L, Carrion R, Rahman N, Lin CS, Lue TF. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int. 2004;94:904–909. doi: 10.1111/j.1464-410X.2003.05057.x. [DOI] [PubMed] [Google Scholar]
- 8.Meirelles LdaS, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin C-S, Lue TF. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–3340. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeghiazarians Y, Zhang Y, Prasad M, Shih H, Saini SA, Takagawa J, Sievers RE, Wong ML, Kapasi NK, Mirsky R, Koskenvuo J, Minasi P, Ye J, Viswanathan M, Angeli FS, Boyle AJ, Springer ML, Grossman W. Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol Ther. 2009;17:1250–1256. doi: 10.1038/mt.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Yang R, Wang Z, Lin G, Lue TF, Lin C-S. Adipose tissue-derived stem cells secrete CXCL5 cytokine with neurotrophic effects on cavernous nerve regeneration. J Sex Med. 2011;8:437–446. doi: 10.1111/j.1743-6109.2010.02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fandel TM, Albersen M, Lin G, Qiu X, Ning H, Banie L, Lue TF, Lin CS. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol. 2011;61:201–210. doi: 10.1016/j.eururo.2011.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albersen M, Fandel TM, Zhang H, Banie L, Lin G, De Ridder D, Lin CS, Lue TF. Pentoxifylline promotes recovery of erectile function in a rat model of postprostatectomy erectile dysfunction. Eur Urol. 2011;59:286–296. doi: 10.1016/j.eururo.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castiglione F, Hedlund P, Van der Aa F, Bivalacqua TJ, Rigatti P, Van Poppel H, Montorsi F, De Ridder D, Albersen M. Intratunical injection of human adipose tissue-derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of peyronie's disease. Eur Urol. 2012;63:551–560. doi: 10.1016/j.eururo.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 16.Zhang H, Albersen M, Jin X, Lin G. Stem cells: Novel players in the treatment of erectile dysfunction. Asian J Androl. 2012;14:145–155. doi: 10.1038/aja.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin C-S, Xin Z-C, Deng C-H, Ning H, Lin G, Lue TF. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–815. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- 18.Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, Bae YC, Jung JS. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 19.Casteilla L, Planat-Benard V, Laharrague P, Cousin B. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J Stem Cells. 2011;3:25–33. doi: 10.4252/wjsc.v3.i4.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamfers M, Idema S, van Milligen F, Schouten T, van der Valk P, Vandertop P, Dirven C, Noske D. Homing properties of adipose-derived stem cells to intracerebral glioma and the effects of adenovirus infection. Cancer Lett. 2009;274:78–87. doi: 10.1016/j.canlet.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 21.Kim U, Shin D-G, Park J-S, Kim Y-J, Park S-I, Moon Y-M, Jeong K-S. Homing of adipose-derived stem cells to radiofrequency catheter ablated canine atrium and differentiation into cardiomyocyte-like cells. Int J Cardiol. 2011;146:371–378. doi: 10.1016/j.ijcard.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Lin G, Wang G, Banie L, Ning H, Shindel AW, Fandel TM, Lue TF, Lin CS. Treatment of stress urinary inconOtinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12:88–95. doi: 10.3109/14653240903350265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bobis-Wozowicz S, Miekus K, Wybieralska E, Jarocha D, Zawisz A, Madeja Z, Majka M. Genetically modified adipose tissue-derived mesenchymal stem cells overexpressing CXCR4 display increased motility, invasiveness, and homing to bone marrow of NOD/SCID mice. Exp Hematol. 2011;39:686–96.e4. doi: 10.1016/j.exphem.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Cho HH, Kyoung KM, Seo MJ, Kim YJ, Bae YC, Jung JS. Overexpression of CXCR4 increases migration and proliferation of human adipose tissue stromal cells. Stem Cells Dev. 2006;15:853–864. doi: 10.1089/scd.2006.15.853. [DOI] [PubMed] [Google Scholar]
- 25.Hung S-C, Pochampally RR, Hsu S-C, Sanchez C, Chen S-C, Spees J, Prockop DJ. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PloS ONE. 2007;2:e416. doi: 10.1371/journal.pone.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Duan B, Cheng Z, Jia X, Mao L, Fu H, Che Y, Ou L, Liu L, Kong D. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein & Cell. 2011;2:845–854. doi: 10.1007/s13238-011-1097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin G, Yang R, Banie L, Wang G, Ning H, Li L-C, Lue TF, Lin CS. Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate. 2010;70:1066–1073. doi: 10.1002/pros.21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thangarajah H, Vial IN, Chang E, El-Ftesi S, Januszyk M, Chang EI, Paterno J, Neofytou E, Longaker MT, Gurtner GC. IFATS collection: Adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells. 2009;27:266–274. doi: 10.1634/stemcells.2008-0276. [DOI] [PubMed] [Google Scholar]
- 29.Baek SJ, Kang SK, Ra JC. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med. 2011;43:596–603. doi: 10.3858/emm.2011.43.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmadian Kia N, Bahrami AR, Ebrahimi M, Matin MM, Neshati Z, Almohaddesin MR, Aghdami N, Bidkhori HR. Comparative analysis of chemokine receptor's expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. J Mol Neurosci. 2011;44:178–185. doi: 10.1007/s12031-010-9446-6. [DOI] [PubMed] [Google Scholar]
- 31.Brooke G, Tong H, Levesque J-P, Atkinson K. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev. 2008;17:929–940. doi: 10.1089/scd.2007.0156. [DOI] [PubMed] [Google Scholar]
- 32.Chamberlain G, Wright K, Rot A, Ashton B, Middleton J. Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: Comparison with human. PloS ONE. 2008;3:e2934. doi: 10.1371/journal.pone.0002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 34.Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow meseSnchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu S, Brown M, Sengupta R, Penfold ME, Meucci O. CXCR7 protein expression in human adult brain and differentiated neurons. PloS ONE. 2011;6:e20680. doi: 10.1371/journal.pone.0020680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]