Abstract
Introduction
Combined hormonal contraceptives (CHCs) use is becoming an increasingly recognized causes of vestibulodynia.
Aim
This study aims to describe pre- and posttreatment vestibular pain, sex hormone binding globulin (SHBG), and calculated free testosterone levels in women undergoing treatment for vestibulodynia.
Methods
This was a chart review of 50 premenopausal women who presented with vestibular pain while currently using CHCs. Pre- and posttreatment vestibular pain, SHBG, and calculated free testosterone levels were assessed.
Results
There was a statistically significant improvement in posttreatment vestibular pain scores (P = 0.001), SHBG (P = 0.001), and calculated free testosterone (P = 0.001) levels from baseline.
Conclusion
Women with vestibulodynia that began while on CHC may effectively be treated by discontinuing the CHC combined with the application topical hormone therapy. Symptomatic improvement is accompanied by normalization of calculated free testosterone and SHBG values. Burrows LJ and Goldstein AT. The treatment of vestibulodynia with topical estradiol and testosterone. Sex Med 2013;1:30–33.
Keywords: Vestibulodynia, Estradiol, Testosterone
Introduction
Vestibulodynia, also known as provoked localized vulvodynia and formerly termed the “vulvar vestibulitis syndrome,” is characterized by a severe, burning/sharp pain that occurs in response to pressure localized to the vulvar vestibule. Vestibulodynia is a common cause of dyspareunia with an estimated prevalence of 15% among women presenting for general gynecologic care [1]. Painful intercourse (dyspareunia) as well as pain with tampon insertion are hallmarks of this condition. Although there are likely multiple causes of vestibular pain, the relationship to combined hormonal contraceptive (CHC) use is becoming increasingly recognized.
Several studies have demonstrated that CHC use significantly increases the risk of developing vestibulodynia [2–5]. There are several contributing factors that may explain this observation. Due to the fact that CHCs inhibit luteinizing hormone, there is a decreased ovarian production of testosterone. In addition, both the synthetic estrogen and synthetic progestin component of CHCs, which are metabolized in the liver, leads to increased hepatic production of sex hormone binding globulin (SHBG). The combination of lower total testosterone production from the ovaries combined with the increased levels of SHBG in turn lead to decreased levels of circulating free testosterone. It has been suggested that CHC with newer progestins lower free testosterone greater than CHC with the earlier progestins [6].
The vulvar vestibule is embryologically analogous to the male urethra. The mucin-secreting, androgen-dependant Cowper's glands and glands of Litre in men are the embryologic analogs to the Bartholin glands and minor vestibular glands in women. It has been shown that these vestibular glands are rich in androgen receptors [7]. In addition to alterations in serum hormone and SHBG levels, CHCs have been shown to induce changes in hormone receptors as well as alter the morphological pattern in the vestibular mucosa [8]. In addition, CHC have been shown to lower the pain threshold of the vulvar vestibule [9].
Interestingly, the vestibule is not the only female genital structure to sustain detrimental effects from CHCs. A recent study assessed the affects of 30 mg ethinyl estradiol and 3 mg drospirenone (Yasmin™, Bayer, Leverkusen, Germany) on the thickness of the labia minora and vaginal introitus area. After only 3 months of use, the subjects experienced worsening of pain with intercourse, decreased thickness of the labia minora, decreased diameter of the vaginal introitus, and decreased size of the glans clitoris in comparison with the baseline values [10]. Bohm-Starke et al. report that some women with vestibulodynia had resolution of symptoms after discontinuing CHC (personal communication).
In 2008, The National Survey of Family Growth found that 82% of American women age 18–44 had used the oral contraceptive pill at some point in their life, and among women under age 30, a higher percentage of women used the pill than any other method of contraception. Although CHCs are highly reliable in the prevention of pregnancy, like any other medication, they have potential side effects. Clinicians must be aware that (ironically) vestibular pain and dyspareunia are potential side effects of CHCs.
The authors have previously hypothesized that CHC that changes to the vestibular mucosa induced by CHCs are a potential cause of vestibulodynia [11]. Herein, we describe our treatment approach to and clinical outcomes in premenopausal women with vestibulodynia who presented to a vulvar pain clinic while currently taking CHCs.
Methods
After obtaining approval from the Anne Arundel Medical Center Institutional Review Board, the database for The Center for Vulvovaginal Disorders was utilized to identify 50 consecutive premenopausal women with vestibulodynia who were using CHCs at the time they presented for care. All women had previously had pain-free intercourse and had subsequently developed vestibular pain (i.e., secondary vestibulodynia). As is our standard practice, all patients were asked to discontinue CHCs, and patients were asked to apply a compounded preparation of topical estradiol 0.03% and testosterone 0.01% to the vestibule twice daily. Testosterone was used in treatment as a recent study examining the impact of vaginal testosterone alone on vaginal atrophy demonstrated improvement in vaginal atrophy symptoms [12].
Patients who had other causes of vestibular pain such as pelvic floor muscle hypertonus, infection, and vulvar dermatoses were excluded from this review; thus, only patients in whom CHCs were the only identifiable cause of their vestibular pain were included. Data abstracted included age, average time taking CHCs, baseline and posttreatment vestibular pain scores, SHBG, and calculated free testosterone levels as well as treatment time (in weeks). Vestibular pain was assessed using a moist cotton swab. If the vestibular pain in a given patient varied at different locations on the vestibule, the highest score was used when calculating the average pre- and postvestibular pain scores. Descriptive statistics and t-tests were applied as appropriate.
Results
The average age was 26 years, and the average duration of CHC use was 7 years. The most commonly used CHHs were LoEstrin (Duramed, Tikva, Israel) (n = 6), Yasmin (n = 6), and Yaz (Bayer) (n = 5). Vestibular pain scores decreased from 7.5 to 2 (P = 0.001, see Figures 1 and 2). Baseline and posttreatment SHBG levels were 154 nmol/L and 64 nmol/L, respectively (P = 0.001), and calculated free testosterone levels were 0.193 ng/dL and 0.813 ng/dL, respectively (P = 0.001, see Figures 3 and 4). The average duration of treatment was 20 weeks.
Figure 1.
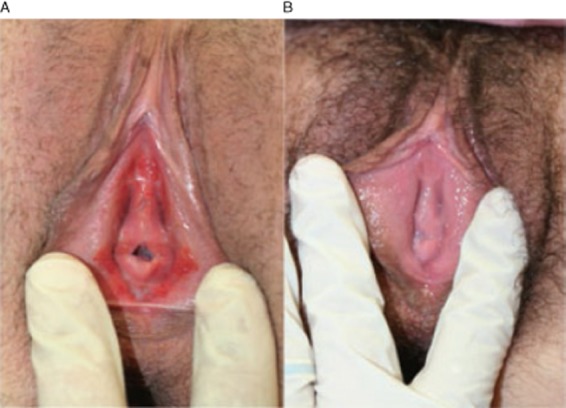
(A) Before estradiol and testosterone. (B) After estradiol and testosterone
Figure 2.
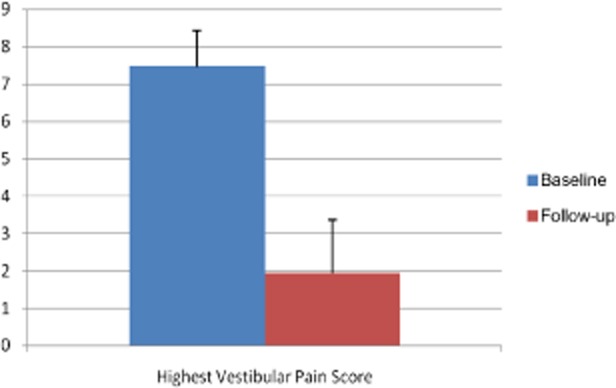
Pre- and posttreatment vestibular pain scores
Figure 3.
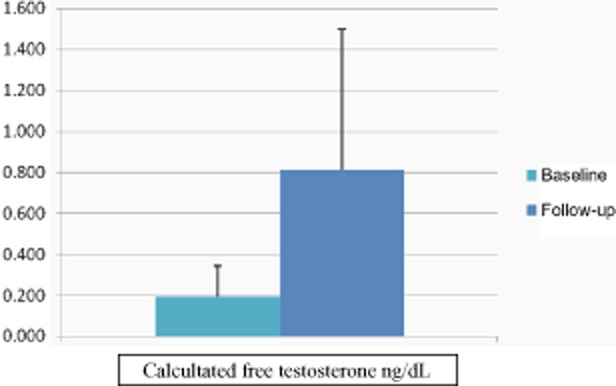
Pre- and posttreatment calculate free testosterone
Figure 4.
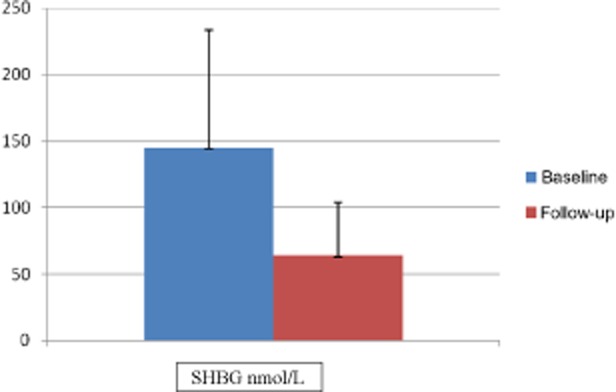
Pre- and posttreatment sex hormone binding globulin (SHBG) levels
Discussion
The potential for negative side effects from CHCs on female sexuality in general is a subject of debate. Perhaps the most widely studied aspect of female sexuality as it relates to CHC use is libido. To date, studies have yielded conflicting results with some studies showing improvement, others a decrease in, and others showing no effect on female desire with CHC use [13]. Interestingly, the proposed explanation of decreased libido in CHC users is the same as what we are hypothesizing causes vestibular pain: elevated SHBG and decrease calculated free testosterone levels.
Although several studies have shown that CHCs increase a woman's risk of developing vestibulodynia, other studies have not demonstrated this effect [14,15]. Unfortunately, these studies did not control for the type of CHC used or length of use. Although some studies have assessed hormone receptor expression in the vulvar vestibule and found no difference between women with vestibulodynia and pain-free controls, CHC use was not controlled for [16]. Nevertheless, thousands of women suffer from vestibular pain and are seeking relief. Although there are undoubtedly many causes of vestibular pain, this study provides further evidence to support a hormonally mediated cause, specifically one induced by CHCs. This study has demonstrated that women with vestibulodynia who have no other identifiable cause of their pain that began while taking CHCs are effectively treated by discontinuing the CHCs combined with the application topical hormone therapy. Furthermore, subjective improvement is accompanied by normalization of calculated free testosterone and SHBG values.
Panzer et al. found that in women with sexual dysfunction, SHBG levels in women who discontinued CHCs did not decrease to values of women who never used CHCs [17]. Although the present study noted a marked decrease in SHBG levels, it is unknown if the patients decreased to their baseline level. The hypothesis put forth by Panzer et al. suggesting that prolonged exposure to the synthetic estrogens in CHCs induced gene imprinting and increased gene expression of SHBG in the liver is an interesting one and merits further investigation.
There are many limitations of this study, including those inherent to a retrospective review, the lack of a standardized questionnaire, lack of a standardized instrument to assess vestibular pain (such as a vulvalgesiometer), lack of placebo control, and testosterone levels that were drawn randomly during the menstrual cycle. In addition, subjects effectively underwent three concurrent interventions: cessation of CHC, application of topical estradiol, and the application of topical testosterone. As such, we cannot know the relative importance of each of these three interventions in the patients' improvement in symptoms. A prospective, randomized, placebo-controlled trial is needed to ascertain if all three components of this treatment regimen are needed to treat women with hormonally mediated vestibulodynia. Therefore, until these trial are completed and despite the aforementioned limitations of this study, we suggest that women with secondary vestibulodynia that developed while on CHC be considered for the treatment approach described in this article.
Conflict of Interest
The authors report no conflict of interest.
References
- 1.Goetsch MF. Vulvar vestibulitis: Prevalence and historic features in a general gynecologic practice population. Am J Obstet Gynecol. 1991;164(6 pt 1):1609–1614. doi: 10.1016/0002-9378(91)91444-2. discussion 1614–6. [DOI] [PubMed] [Google Scholar]
- 2.Bazin S, Bouchard C, Brisson J, Morin C, Meisels A, Fortier M. Vulvar vestibulitis syndrome: An exploratory case-control study. Obstet Gynecol. 1994;83:47–50. [PubMed] [Google Scholar]
- 3.Bouchard C, Brisson J, Fortier M, Morin C, Blanchette C. Use of oral contraceptive pills and vulvar vestibulitis: A case-control study. Am J Epidemiol. 2002;156:254–261. doi: 10.1093/aje/kwf037. [DOI] [PubMed] [Google Scholar]
- 4.Greenstein A, Ben-Aroya Z, Fass O, Militscher I, Roslik Y, Chen J, Abramov L. Vulvar vestibulitis syndrome and estrogen dose of oral contraceptive pills. J Sex Med. 2007;4:1679–1683. doi: 10.1111/j.1743-6109.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- 5.Berglund AL, Nigaard L, Rylander E. Vulvar pain, sexual behavior and genital infections in a young population: A pilot study. Acta Obstet Gynecol Scand. 2002;81:738–742. doi: 10.1034/j.1600-0412.2002.810809.x. [DOI] [PubMed] [Google Scholar]
- 6.Pitashny M, Martinez de Morentin H, Brenner S. Oral contraceptives: Their mode of action and dermatologic applications. Skinmed. 2005;4:101–106. doi: 10.1111/j.1540-9740.2005.03955.x. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein A, Goldstein I, Pukall C, editors. Female Sexual Pain Disorders: Evaluation and Management. 1st edition. Hoboken, NJ: Wiley-Blackwell; 2009. [Google Scholar]
- 8.Johannesson U, Sahlin L, Masironi B, Hilliges M, Blomgren B, Rylander E, Bohm-Starke N. Steroid receptor expression and morphology in provoked vestibulodynia. Am J Obstet Gynecol. 2008;198:311 e1–3116. doi: 10.1016/j.ajog.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 9.Bohm-Starke N, Johannesson U, Hilliges M, Rylander E, Torebjörk E. Decreased mechanical pain threshold in the vestibular mucosa of women using oral contraceptives: A contributing factor in vulvar vestibulitis? J Reprod Med. 2004;49:888–892. [PubMed] [Google Scholar]
- 10.Battaglia C, Battaglia B, Mancini F, Busacchi P, Paganotto MC, Morotti E, Venturoli S. Sexual behavior and oral contraception: A pilot study. J Sex Med. 2012;9:550–557. doi: 10.1111/j.1743-6109.2011.02597.x. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein A, Burrows L, Goldstein I. Can oral contraceptives cause vestibulodynia? J Sex Med. 2009;7:1585–1587. doi: 10.1111/j.1743-6109.2009.01685.x. [DOI] [PubMed] [Google Scholar]
- 12.Witherby A. 2011. Topical testosterone for breast cancer patients with vaginal atrophy related to aromatase inhibitors: A phase I/II study.
- 13.Burrows LJ, Basha M, Goldstein AT. The effects of hormonal contraceptives on female sexuality: A review. J Sex Med. 2010;9:2213–2223. doi: 10.1111/j.1743-6109.2012.02848.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee M, Morgan M, Rapkin A. Clitoral and vulvar vestibular sensation in women taking 20 mcg ethinyl estradiol combined oral contraceptives: A preliminary study. J Sex Med. 2011;8:213–218. doi: 10.1111/j.1743-6109.2010.02074.x. [DOI] [PubMed] [Google Scholar]
- 15.Edgardh K, Abdelnoor M. Vulvar vestibulitis and risk factors: A population-based case-control study in Oslo. Acta Derm Venereol. 2007;87:350–354. doi: 10.2340/00015555-0250. [DOI] [PubMed] [Google Scholar]
- 16.Goetsch MF, Morgan TK, Korcheva VB, Li H, Peters D, Leclair CM. Histologic and receptor analysis of primary and secondary vestibulodynia and controls: A prospective study. Am J Obstet Gynecol. 2010;202:614 e1–6148. doi: 10.1016/j.ajog.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Panzer C, Wise S, Fantini G, Kang D, Munarriz R, Guay A, Goldstein I. Impact of oral contraceptives on sex hormone-binding globulin and androgen levels: A retrospective study in women with sexual dysfunction. J Sex Med. 2006;3:104–113. doi: 10.1111/j.1743-6109.2005.00198.x. [DOI] [PubMed] [Google Scholar]


