Abstract
A 34-year-old woman visited the hospital suffering from enanthema of the tongue, hair loss, and nonproductive cough. Corticosteroid administration slightly resolved the enanthema and hair loss, but not the nonproductive cough. She was transferred to another hospital for the resection of a retroperitoneal mass, which was histopathologically diagnosed as unicentric, hyaline vascular type Castleman’s disease. She was then referred to our hospital due to progressive dyspnea and was diagnosed as having bronchiolitis obliterans based on computed tomography scan findings and a lung function test, while paraneoplastic pemphigus was clinically considered for her enanthema. After her death from respiratory failure in spite of corticosteroid administration, autopsy confirmed constrictive bronchiolitis in the lung. Mucocutaneous lesions, clinically considered as paraneoplastic pemphigus, were histologically different from pemphigus. The constrictive bronchiolitis and other pathological findings, including nonviral chronic hepatitis and interstitial nephritis, confirmed the diagnosis of paraneoplastic autoimmune multiorgan syndrome associated with the Castleman’s disease.
Keywords: Bronchiolitis obliterans, paraneoplastic autoimmune multiorgan syndrome, paraneoplastic pemphigus, unicentric Castleman’s disease
Introduction
Bronchiolitis obliterans manifests as inflammatory and fibrotic changes mainly affecting the small bronchi and membranous bronchioles, leading to occlusion of airway lumens. It is classified into two categories: (1) cellular and destructive bronchiolitis, in which inflammation of bronchial walls destroys normal structural components, including elastic fiber and smooth muscle; or (2) constrictive bronchiolitis [1], in which the structural components of the bronchial walls are preserved with granulation and fibrosis in airway lumens. Constrictive bronchiolitis has been reported to be associated with transplantation, neoplasms, infections, connective tissue diseases, pemphigus, inhalation of toxic gas, and consumption of Sauropus androgynus.
Here, we present a case of unicentric Castleman’s disease associated with paraneoplastic pemphigus-like lesions and constrictive bronchiolitis, which was lethally progressive even after resection of Castleman’s disease and steroid administration.
Case Report
A 34-year-old woman visited the hospital suffering from enanthema of the tongue, hair loss, and nonproductive cough 9 months before admission to our institution. She was diagnosed as having asthma based on wheezing and corticosteroid was prescribed, which slightly improved the enanthema and hair loss but not the nonproductive cough.
Since a retroperitoneal mass was found, she was transferred to another hospital where surgery was performed. The retroperitoneal mass, enclosed within a capsule, was completely resected to reveal unicentric/hyaline vascular type Castleman’s disease. Within multiple lymph follicles, blood vessels with hyalinosis proliferated and reticular cells formed a concentric pattern. Fibrosis between lymph follicles was rich in small round cells but not in plasma cells.
Three months after the operation she was referred to our hospital complaining of progressive dyspnea. She had no significant past medical history and smoked 10 cigarettes a day. Physical examination revealed the following: performance status, 2; modified Medical Research Council Dyspnea Scale (mMRC), 3; oxygen saturation (SpO2), 93% (ambient air); respiration rate, 21/min. Multiple oral mucosal erosions and wheezes in both lung fields were notable. Alkaline phosphatase, γ-glutamyl transpeptidase, white blood cells, platelets, and immunoglobulin M were elevated to 508 IU/L, 147 IU/L, 12,100/μL, 57.5 × 104/μL, and 217 mg/dL, respectively. Blood gas analysis with ambient air showed: pH, 7.417; partial pressure of oxygen (PO2), 68.6 mmHg; PCO2, 48.1 mmHg; and bicarbonates (HCO3), 30.3 mmol/L. Slight hyperinflation and hyperlucency of both lung fields were observed on chest X-ray on admission (Fig. 1A). Chest computer tomography scan showed hyperinflation of both lungs and diminished vascular shadows on peripheral lung fields (Fig. 1B). Severe obstructive ventilatory impairment (forced expiratory volume in 1 sec [FEV1], 0.63 L [22% predicted]; vital capacity [VC], 1.88 L [65% predicted]; FEV1/VC ratio, 33.5%; residual volume/total lung capacity, 59.8%) was observed on a respiratory function test. These findings suggested bronchiolitis obliterans. Biopsy of oral mucosa revealed an ulcer without the typical findings of paraneoplastic pemphigus; that is basal epidermal cell vacuolation, acantholysis, or autoantibody to desmoglein.
Figure 1.
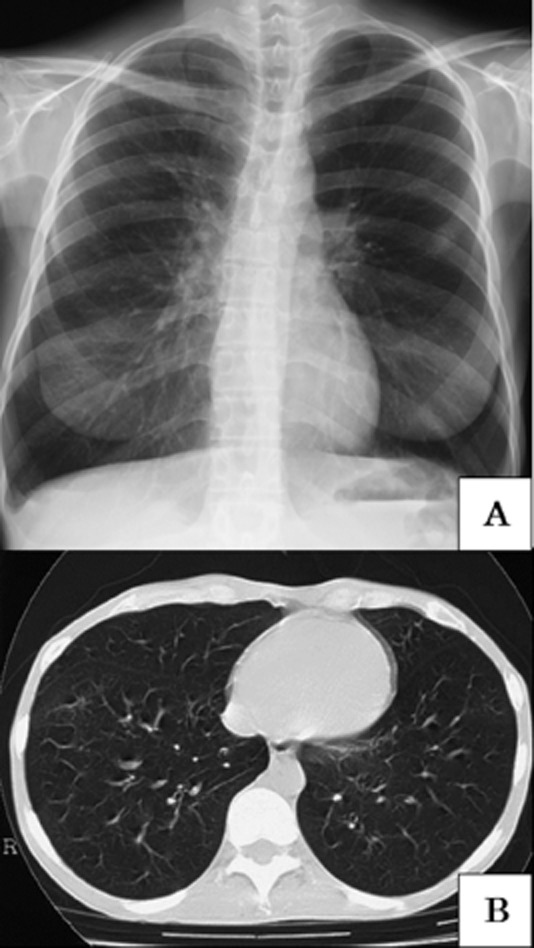
(A) Chest X-ray on admission. Slight hyperinflation and hyperlucency of both lung fields were observed. (B) Chest computed tomography scan in the inspiratory phase. It shows hyperinflation of both lungs and diminished vascular shadows on peripheral lung fields. Other typical signs of bronchiolitis obliterans, that is mosaic perfusion and air trapping sign, were not observed.
We started treatment with prednisolone 40 mg/day and four courses of high-dose corticosteroid administration (methylprednisolone 1 g/day) for 3 days, which were not effective. She was discharged with home oxygen therapy after prednisolone was tapered to 15 mg/day. Six months later, she died from respiratory failure and carbon dioxide (CO2) narcosis. Autopsy confirmed the diagnosis of constrictive bronchiolitis. Multifocal obliteration and marked stenosis of the small bronchi and bronchioles (sixth to ninth generation branches) were observed macroscopically (Fig. 2A). Granulation with foamy macrophages caused stenosis of the bronchiolar lumen, whereas smooth muscle of the wall was preserved (Fig. 2B). The lumen of the small bronchus came to be stenotic by hydropic fibrous tissue. In contrast, the structures of the wall (smooth muscle and elastic fibers) were spared (Fig. 2C). These findings were compatible with constrictive bronchiolitis. We detected no abnormalities at the respiratory bronchioles or alveoli. Nonviral chronic hepatitis and interstitial nephritis were also observed as well as constrictive bronchiolitis.
Figure 2.

(A) Macroscopic reconstruction of the bronchi. Multifocal obliteration of marked stenosis of the small bronchi and bronchioles (third–sixth generation peripheral to the segmental bronchi) was observed. (B) Constrictive bronchiolitis. Granulation tissue with foamy macrophages caused stenosis of the bronchiolar lumen, but smooth muscle of the wall was preserved (hematoxylin and eosin stain, ×20). (C) Constrictive bronchiolitis. The lumen of the small bronchus came to be stenotic by hydropic fibrous tissue. In contrast, the structure of the wall (smooth muscle and elastic fibers) was spared (elastic van Gieson stain, ×4).
Discussion
Most cases of Castleman’s disease with constrictive bronchiolitis are accompanied by paraneoplastic pemphigus, with only one reported case lacking paraneoplastic pemphigus [2]. On the other hand, a broader pathological concept, paraneoplastic autoimmune multiorgan syndrome, has been proposed instead of classical paraneoplastic pemphigus [3]. Paraneoplastic autoimmune multiorgan syndrome includes paraneoplastic pemphigus as one manifestation of a heterogeneous autoimmune syndrome encompassing impairment of multiple organs and a spectrum of different mucocutaneous variants and humoral and cellular autoimmunity responses. Based on the autopsy findings, including mucocutaneous lesions, nonviral chronic hepatitis, and interstitial nephritis in addition to constrictive bronchiolitis, this case was compatible with paraneoplastic autoimmune multiorgan syndrome.
Whereas paraneoplastic pemphigus tends to improve after resection of unicentric Castleman’s disease, constrictive bronchiolitis is lethally progressive. Deposition of autoantibodies in respiratory epithelia and basal membranes [4] might play a role in causing inflammation in the early phase of constrictive bronchiolitis. This injury and inflammation of epithelial cells and subepithelial structures of small airways may lead to excessive fibroproliferation, matrix deposition, and vascular remodelling [5]. Considering an irreversible pathological change leading to constrictive bronchiolitis, early treatment on inflammation phase seems important. Like post-lung-transplantation monitoring by lung function test, neutrophils and matrix metalloproteinase (MMP)-9 in bronchoalveolar lavage, etc. [5], establishing a new follow-up methodology for the early detection of constrictive bronchiolitis in cases of paraneoplastic autoimmune multiorgan syndrome is essential.
Disclosure Statements
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
References
- Colby TV. Bronchiolar pathology. In: Epler GR, editor. Diseases of the bronchioles. New York: Raven Press; 1994. pp. 77–100. [Google Scholar]
- Wang J, Zhu X, Li R, et al. Paraneoplastic pemphigus associated with Castleman tumor: a commonly reported subtype of paraneoplastic pemphigus in China. Arch. Dermatol. 2005;141:1285–1293. doi: 10.1001/archderm.141.10.1285. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Ndoye A, Bassler KD, et al. Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: a reappraisal of paraneoplastic pemphigus. Arch. Dermatol. 2001;137:193–206. [PubMed] [Google Scholar]
- Nousari HC, Deterding R, Wojtczack H, et al. The mechanism of respiratory failure in paraneoplastic pemphigus. N. Engl. J. Med. 1999;340:1406–1410. doi: 10.1056/NEJM199905063401805. [DOI] [PubMed] [Google Scholar]
- Nicod LP. Mechanisms of airway obliteration after lung transplantation. Proc. Am. Thorac. Soc. 2006;3:444–449. doi: 10.1513/pats.200601-007AW. [DOI] [PubMed] [Google Scholar]


