Abstract
Key Clinical Message
We present a case of hepatocellular carcinoma located within the pancreas. These tumors occur in the body and tail of the pancreas, with a male predominance, and at a younger age. Tumors with pure hepatocellular histopathology have better survival and recurrence rates and should be offered surgical therapy if possible.
Keywords: Ectopic, hepatoid, pancreas
Introduction and Case Report
A 61-year-old woman undergoing Cat Scan (CT) scan to screen for coronary artery disease was incidentally found to have a 5-cm pancreatic tail mass. This mass did not enhance on arterial phase and had some heterogeneity (Fig.1). She denied any history of abdominal pain. Her past medical history was significant for hypothyroidism, hypertension, and gastroesophageal reflux disease. She had not undergone any previous abdominal surgery. There was no history of liver disease or alcohol abuse. Her mother had pancreatic adenocarcinoma at the age of 87. Examination did not reveal any palpable abdominal masses or evidence of metastatic disease.
Figure 1.
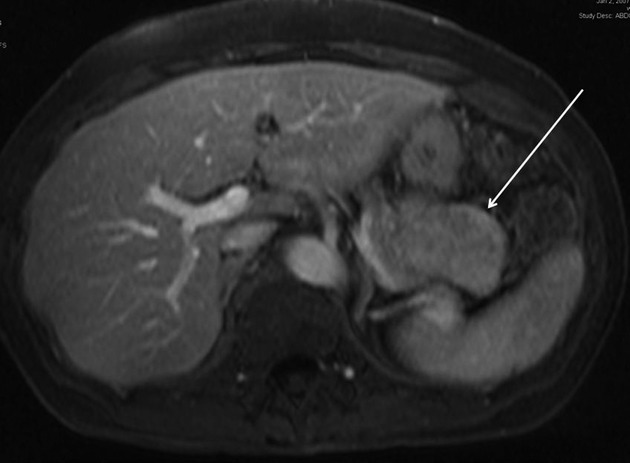
Representative axial CT scan image showing pancreatic tail mass (arrow).
The patient underwent an extensive workup including upper and lower endoscopy. A CT scan of her chest showed multiple small pulmonary nodules that were attributed to an infectious or inflammatory process. A CT scan of her abdomen confirmed the large pancreatic tail mass without evidence of intra-abdominal metastatic disease. A Positron Emission Testing scan showed tracer uptake only in the pancreatic mass, but not as intense as expected for a typical pancreatic adenocarcinoma. Pertinent laboratory values included an alpha-fetoprotein (AFP) of 4.9 (normal #x003C;8.9 mg/mL), cancer 19-9 of 46 (normal #x003C;37 units/mL), and chromogranin A of 45. The neuron-specific enolase (NSE) was slightly elevated at 16.7 (normal 3.7–8.9 μg/L). Other neuroendocrine tumor marker (serotonin, calcitonin, gastrin, glucagon, insulin, somatostatin, pancreatic polypeptide, and substance P) levels were within reference ranges. Protein induced by vitamin K absence or antagonist II (PIVKA-II) was not measured. Endoscopic ultrasonography with fine-needle aspiration (FNA) biopsy, showed a 5.3 × 3.5 cm pancreas tail mass. The FNA cytology showed malignant cells reactive with antibodies directed against cytokeratins but negative for chromogranin and synaptophysin, ruling out lymphoma and neuroendocrine tumor. The diagnosis of hepatoid tumor was not suggested.
Distal pancreatectomy, including splenectomy because the mass involved the splenic vein, was performed. The pancreas proximal to the mass appeared grossly normal. Surrounding tissues were loosely adherent and an adequate plane was identified allowing isolation of the mass and division of the pancreas proximal to the mass with an endo-gastrointestinal anastomosis stapling device. Frozen section of the proximal pancreatic margin was negative for tumor (Fig.2). No evidence of metastatic disease was seen.
Figure 2.
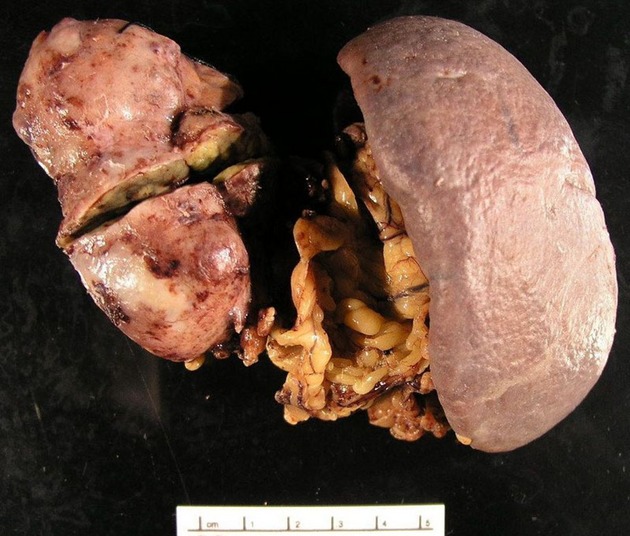
Pancreatic mass (left) and spleen (right).
The histopathologic examination of the specimen revealed extrahepatic hepatocellular cancer (HCC) (“hepatoid” pancreatic adenocarcinoma) that was moderately differentiated, with acinar, trabecular, and solid patterns. The tumor was 7 cm in the greatest dimension with focal venous invasion. The tumor clearly arose in the pancreas and the surrounding pancreatic tissue, including the resection margin, was unremarkable. Tumor cells reacted strongly with a number of immunohistochemical markers indicative of hepatocyte differentiation including Hep Par1, polyclonal carcinoembryonic antigen (pCEA) demonstrating a canalicular pattern, strong staining with antibody against alph-1-antitrypsin and alpha-1-chymotrypsin. Intracellular bile production, pathognomonic for liver cells, and canalicular bile was present (Figs.5). Antibody against albumin was not available and albumin mRNA in situ hybridization was not performed.
Figure 5.
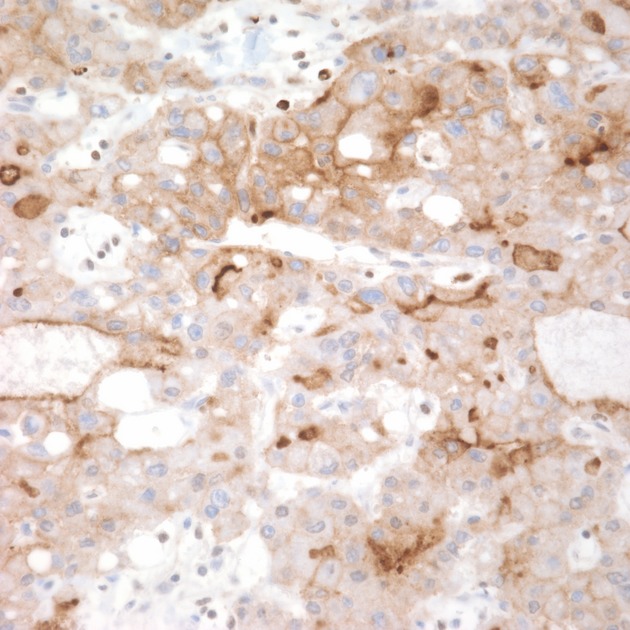
Medium magnification image showing tumor cells after immunohistochemical staining with antibody directed against polyclonal carcinoembryonic antigen (pCEA) with distinct canalicular pattern in some cells (pCEA, 400×).
Figure 3.
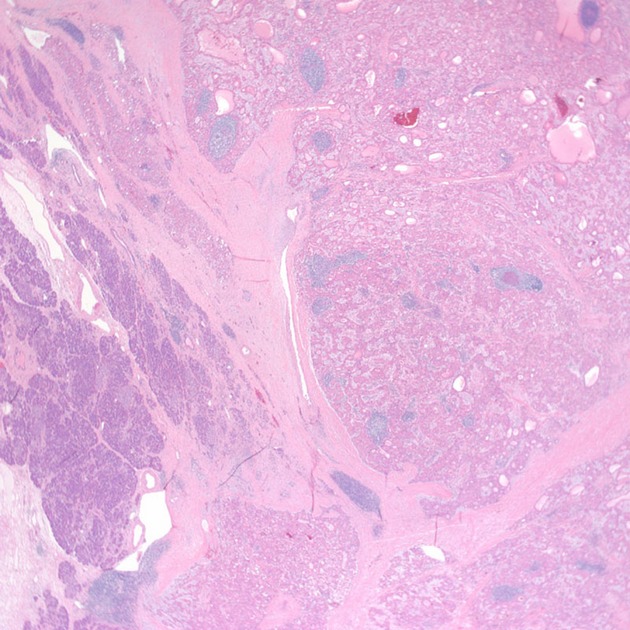
Low-magnification image showing tumor compoased of polygonal cells with abundant eosinophilic cytoplasm and surrounding pancreatic tissue at the lower left (hematoxylin-eosin, 20×).
Figure 4.
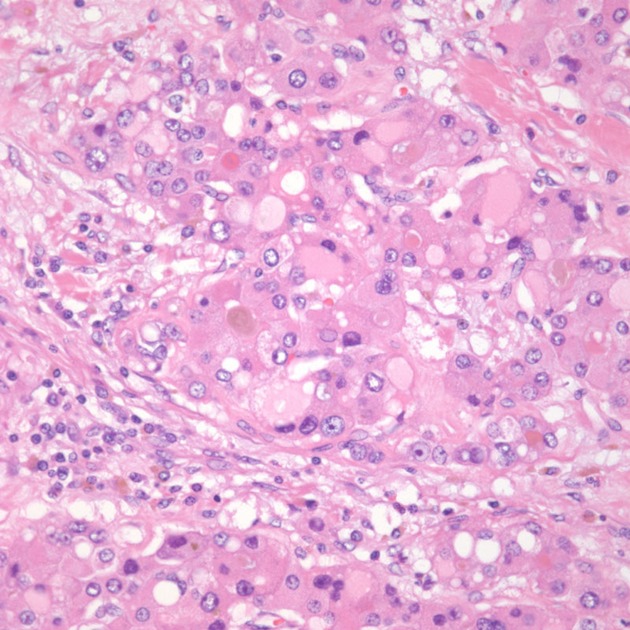
Medium magnification image showing tumor cells with intracellular bile and hyaline inclusions.
The patient's immediate postoperative course was uneventful. A medical oncologist did not recommend adjuvant systemic chemotherapy or local radiation therapy. Follow-up monitoring for recurrence included serial magnetic resonance imaging scans of the abdomen and serum alpha-fetoprotein levels, which remained negative for tumor during 5 years of follow-up.
Discussion
An HCC-like tumor identified in the pancreas may be either a hepatoid carcinoma or hepatocellular carcinoma in ectopic liver tissue. Hepatoid carcinoma arises in an extrahepatic organ but displays hepatic-like differentiation during malignant growth. Thus, it may contain cells of a typical carcinoma admixed with hepatoid tumor cells 1. Hepatoid carcinomas have been described in the gastrointestinal tract, ovary, pancreas, lung, kidney, uterus, gallbladder, urachus, and urinary bladder 2–10. Hepatoid variants of yolk sac tumors have also been described in both gonadal and extragonadal sites 3. Hepatoid tumors are generally associated with an aggressive clinical course and poor prognosis 5,11,12. Pancreatic multipotent cells possess normally repressed genes of hepatocyte differentiation that may be activated during tumorigenesis 13. Mature pancreatic cells can be transformed into true hepatocytes in the animal model 11,14. Pancreatic hepatoid carcinomas have been found admixed with ductal adenocarcinoma, acinar cell carcinoma, and neuroendocrine carcinoma, showing that hepatoid differentiation can arise from ductal, acinar, or islet pancreatic cells 15.
Less than 5% of hepatocellular carcinomas arise in an ectopic rest of liver tissue 1,7. The most common site is the gallbladder, although ectopic liver tissue also has been reported in suspensory ligaments of the liver, lung, splenic capsule, retroperitoneal space, adrenal gland, greater omentum, placenta, and pancreas 13,16–19. Ectopic liver may be functionally handicapped and more prone to hepatocarcinogenesis than the native liver 8,17. Some authors have postulated that abnormal arterial, venous, or biliary drainage increases the potential for accumulation of toxins and subsequent carcinogenesis, even without the known risk factors of hepatitis or cirrhosis of the native liver 11,16. In only 32% of cases of HCC in ectopic liver does the native liver contain evidence of cirrhosis 8. Residual ectopic normal hepatic tissue is generally not identified and is presumed to have been completely replaced by HCC 11,18. Our patient likely represents hepatocellular carcinoma arising within, and replacing the rest of the ectopic liver as it contained no pancreatic cancer cells and her pancreas contained no evidence of normal liver tissue.
Both hepatoid carcinomas and HCC in ectopic liver show histological features supporting hepatic lineage, such as abundant eosinophilic cytoplasm with round to oval nuclei, clear or finely granular cytoplasm, and intracytoplasmic hyaline globules reactive with and diastase resistant for periodic acid Schiff (PAS) reagent 2,4,9. The morphology is similar to a typical HCC and includes cords of polygonal cells separated by sinusoids, occasionally featuring bile production and/or bile canaliculi formation 10,13,20. HCC and hepatoid carcinomas both show canalicular pattern of staining with polyclonal CEA; by contrast, hepatoid yolk sac tumors show diffuse cytoplasmic staining 3,5,10. Hepatoid carcinomas also share immunohistochemical signatures with HCC, including staining for AFP, anti-albumin, anti-alpha-1-antitrypisine, anti-low molecular weight cytokeratin, and anti-epithelial membrane antigen antibodies 20. Some of these markers are found in many tissue types whereas albumin is made exclusively by the liver and can be used to establish hepatic lineage 4. The immunohistochemical staining for albumin is unreliable, however, and in situ hybridization detection of albumin mRNA is more sensitive and specific 4,10,20,21. Hepatocyte paraffin 1 (Hep Par 1) is a monoclonal antibody that reacts with most hepatic cells and HCC 5,10. An 82% sensitivity and 90% specificity of Hep Par 1 staining in HCC has been reported. Focal Hep Par 1 staining is found in hepatoid carcinomas and hepatoid yolk sac tumors in 33–85% of cases 5,7. Some hepatoid carcinomas may be associated with elevated serum concentrations of protein induced by vitamin K absence or antagonist II (PIVKA-II), a marker used for early diagnosis of HCC; however, only one such case has been identified among patients whose hepatoid carcinoma was in the pancreas, and the elevation was not marked (Table1) 9,15. The production of PIVKA-II by malignant tumors other than HCC is very rare 9.
Table 1.
Literature review and summary of our case report patient.
| Reference | Age | Gender | Pathology | PIVKA-II (nl #x003C;0.034 AU/mL) | AFP (nl #x003C;7 ng/mL) | Method of diagnosis | Location within pancreas | Outcome |
|---|---|---|---|---|---|---|---|---|
| Hruban 14 | 53 | F | Acinar cell carcinoma with hepatoid features | NA | 10 | Autopsy | Body/tail | 3 months, died |
| Obara 4 | NA | NA | Duct cell carcinoma with hepatoid features | NA | NA | NA | NA | NA |
| Tanno 22 | 59 | F | Mucin-producing carcinoma with hepatoid features | NA | 181 | Laparotomy, resection | Head | 24 months, NED |
| Yano 1999 #x005B;27#x005D; | 57 | M | Duct cell carcinoma with hepatoid features | NA | 177 | Laparotomy, resection | Head | 3 months, died |
| Paner 4 | 57 | M | Islet cell carcinoma with hepatoid features | NA | NA | Laparotomy, resection | Body/tail | 102 months, died |
| Paner 4 | 28 | M | Duct cell carcinoma with hepatoid features | NA | NA | Laparotomy, biopsy | Body/tail | 14 months, died |
| Lam 25 | 64 | F | Islet cell carcinoma with hepatoid features | NA | 2119 | Laparotomy, resection | Body/tail | 22 months, died |
| Cuilliere 13 | 70 | M | Serous microcystic adenoma with hepatoid features | NA | Normal | Laparotomy, resection | Body/tail | 12 months, NED |
| Hughes 26 | 51 | M | Hepatocellular carcinoma features primarily | NA | NA | Laparotomy, resection | Body/tail | 14 months, NED |
| Matsueda 9 | 49 | F | Hepatocellular carcinoma features primarily | 1.63 | 623 | Laparotomy, resection | Total gland | 48 months, NED |
| Shih 2006 #x005B;28#x005D; | 32 | M | Hepatocellular carcinoma features primarily | Normal | NA | Laparotomy, resection | Body/tail | 18 months, NED |
| Oh 2006 #x005B;29#x005D; | 21 | M | Neuroendocrine carcinoma with hepatoid features | NA | Elevated | Laparotomy, resection | Head | 7 months, NED |
| Kubota 18 | 56 | M | Hepatocellular carcinoma features primarily | NA | NA | Laparotomy, resection | Body/tail | 36 months, NED |
| Cardona 16 | 58 | M | Hepatocellular carcinoma features primarily | NA | NA | Laparotomy, resection | Body/tail | 15 months, NED |
| Liu 24 | 80 | M | Hepatocellular carcinoma features primarily | NA | Normal | Laparotomy, resection | Uncinate | 8 months, NED |
| Hameed 10 | 41 | F | Neuroendocrine carcinoma with hepatoid features | NA | 2714 | Laparotomy, resection | Head | 26 months, died |
| Jung 15 | 46 | M | Neuroendocrine carcinoma with hepatoid features | 0.015 | 262 | Laparotomy, resection | Head | 4 months, NED |
| Kelly 11 | 53 | F | Hepatocellular carcinoma features primarily | NA | 148 | Laparotomy, resection | Body/tail | 22 months, AWD |
| Huang 2012 #x005B;30#x005D; | 52 | M | Hepatocellular carcinoma features primarily coexisting with separate neuroendocrine tumor | NA | NA | Laparotomy, resection | Head | 15 months, AWD |
| Kai 2012 #x005B;31#x005D; | 79 | F | Hepatocellular carcinoma features primarily | NA | NA | Laparotomy, resection | Body/tail | 2 months, died |
| Current case | 61 | F | Hepatocellular carcinoma features primarily | NA | 4.9 | Laparotomy, resection | Body/tail | 72 months, NED |
NED, no evidence of disease; nl, normal.
AFP is a fetal protein expressed by fetal liver, yolk sac, and some gastrointestinal cells 1,22. After birth, the serum level of this protein rapidly drops because the gene is silent in the adult liver 2. The AFP gene is reactivated in liver regeneration and hepatocellular cancer 2,23. AFP production has been reported in many hepatoid carcinomas and in non-hepatoid tumors of the lung, pancreas, colon, bladder, ovary, and stomach 1,9,13,20,22. In Western countries, serum levels of AFP are elevated in only 0–24% of patients with pancreatic cancer, and elevated levels are usually associated with liver metastases 22. Thus, the presence of AFP in the tumor or in the serum is not a diagnostic criterion for hepatoid tumor 7,10,12,24–26 unless the tumor also has concanavalin A (Con A)-binding affinity, is len culinaris agglutin-(LCA) reactive, and contains histopathologic features consistent with HCC 3,4,7,10,12,24,25.
Hepatoid carcinomas tend to have extensive hematogenous metastasis to the liver and early and frequent involvement of lymph nodes 5,24. There can be confusion about the origins of hepatoid tumors as metastatic nodules in the liver are microscopically similar to primary HCC and no immunohistochemical test can distinguish the two 1,2,5,19. The first reported a case of hepatoid tumor of the pancreas by Hruban had concurrent hepatic metastases. The authors reasoned that the presence of peripancreatic metastatic nodes in the absence of additional nodal disease supported a pancreatic origin 14. The presence of mucin excludes metastatic HCC because mucin production has never been described in a true HCC 3. The absence of risk factors for hepatocellular cancer, such as hepatitis or cirrhosis, also decreases the likelihood that a tumor has arisen from liver tissue 1,19. A combination of clinical and histopathologic findings can aid in determining the origins of a hepatoid tumor found simultaneously in the liver and extrahepatic organs.
A review of the world literature reveals only 20 previous reports of hepatoid carcinoma of the pancreas (Table1). Most reported lesions were in the body and tail of the pancreas; only seven of 21 cases (33%) arose solely within the head or uncinate process. Most (62%) patients were men. The average age at diagnosis was 53, younger than the typical patient with pancreatic adenocarcinoma. Of the 10 patients whose tumors were typical of hepatocellular carcinoma, seven had no evidence of recurrence at an average postoperative follow-up of 30 months. Two additional patients were alive with disease at an average follow-up of 18 months, and one patient with a large poorly differentiated tumor died within 2 months after surgery. In comparison, of the 11 patients whose hepatoid tumors were mixed with other carcinoma types (acinar = 1, duct cell = 3, mucin = 1, neuroendocrine = 5, serous microcystic adenoma = 1), six died at an average of 41 months after diagnosis. Patients whose tumors had pure hepatoid or hepatocellular histopathology have better survival and recurrence rates than patients with mixed-type tumors 11,26. This suggests that surgical resection of pancreatic hepatoid tumors can provide good control of disease, particularly when histology shows pure hepatocellular carcinoma.
In our patient, there was no evidence of non-hepatoid carcinoma and no evidence of normal non-neoplastic hepatic tissue remaining in the tumor. There was no evidence of germ cell tissues excluding a hepatoid variant of a yolk sac tumor. We believe that this patient's hepatocellular carcinoma arose in ectopic liver tissue located within the pancreas, and eventually replaced all original ectopic liver tissue. Five years without recurrence, in the absence of chemo- or radiotherapy, negates the possibility of this tumor representing a metastasis. Although only 21 hepatoid tumors of the pancreas have now been reported, the frequency may be higher because large amounts of tissue and usually surgical exploration are needed for diagnosis. Two previously reported cases of preoperative endoscopic ultrasound with fine-needle aspiration of hepatoid pancreatic tumors have shown that the diagnosis was not even considered based on such a small sample – similar to what we found with our preoperative FNA 10,26. It is possible that many patients with widely metastatic tumors of extrahepatic hepatoid origin may go undiagnosed based on inadequate samples. If the diagnosis of hepatoid tumor is suspected, serum markers such as AFP or PIVKA-II may be of assistance. The pathologist must be cognizant of the wide array of testing necessary to establish this diagnosis.
Conclusions
In conclusion, awareness of this rare tumor type is important to allow accurate diagnosis and proper treatment. Aggressive surgical extirpation should be attempted if possible and may lead to long-term cure.
Conflict of Interest
None declared.
Funding Information
No funding information provided.
References
- Inagawa S, Shimazaki J, Hori M, et al. Hepatoid adenocarcinoma of the stomach. Gastric Cancer. 2001;4:43–52. doi: 10.1007/s101200100016. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Nagai Y, Kato K, Ozaki D. Ishikura H. Hepatoid adenocarcinoma: a new clinicopathological entity and the hypotheses on carcinogenesis. Med. Electron Microsc. 2000;33:57–63. doi: 10.1007/s007950070002. [DOI] [PubMed] [Google Scholar]
- Gopaldas R, Kunasani R, Plymyer MR. Bloch RS. Hepatoid malignancy of unknown origin–a diagnostic conundrum: review of literature and case report of collision with adenocarcinoma. Surg. Oncol. 2005;14:11–25. doi: 10.1016/j.suronc.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Paner GP, Thompson KS. Reyes CV. Hepatoid carcinoma of the pancreas. Cancer. 2000;88:1582–1589. doi: 10.1002/(sici)1097-0142(20000401)88:7<1582::aid-cncr12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Terracciano LM, Glatz K, Mhawech P, et al. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am. J. Surg. Pathol. 2003;27:1302–1312. doi: 10.1097/00000478-200310000-00002. [DOI] [PubMed] [Google Scholar]
- Nakashima H, Nagafuchi K, Satoh H, et al. Hepatoid adenocarcinoma of the gallbladder. J. Hepatobiliary Pancreat. Surg. 2000;7:226–230. doi: 10.1007/s005340050181. [DOI] [PubMed] [Google Scholar]
- Koswara T, Marwoto W, Siregar NC, et al. Hepatoid carcinoma of the gallbladder. Acta Med. Indones. 2007;39:179–182. [PubMed] [Google Scholar]
- Shigemori M, Kondo M, Azechi H, et al. A case of ectopic hepatocellular carcinoma in the jejunum. J. Gastroenterol. 2006;41:913–918. doi: 10.1007/s00535-006-1872-4. [DOI] [PubMed] [Google Scholar]
- Matsueda K, Yamamoto H, Yoshida Y. Notohara K. Hepatoid carcinoma of the pancreas producing protein induced by vitamin K absence or antagonist II (PIVKA-II) and alpha-fetoprotein (AFP) J. Gastroenterol. 2006;41:1011–1019. doi: 10.1007/s00535-006-1889-8. [DOI] [PubMed] [Google Scholar]
- Hameed O, Xu H, Saddeghi S. Maluf H. Hepatoid carcinoma of the pancreas: a case report and literature review of a heterogeneous group of tumors. Am. J. Surg. Pathol. 2007;31:146–152. doi: 10.1097/01.pas.0000213370.79300.e1. [DOI] [PubMed] [Google Scholar]
- Kelly PJ, Spence R, Dasari BV, Burt AD, Taylor M. Loughrey MB. Primary hepatocellular carcinoma of the pancreas: a case report and review of the heterogeneous group of pancreatic hepatoid carcinomas. Histopathology. 2012;60:1012–1015. doi: 10.1111/j.1365-2559.2011.04129.x. [DOI] [PubMed] [Google Scholar]
- Lee MW, Lee JY, Kim YJ, et al. Gastric hepatoid adenocarcinoma: CT findings. Abdom. Imaging. 2007;32:293–298. doi: 10.1007/s00261-006-9073-4. [DOI] [PubMed] [Google Scholar]
- Cuilliere P, Lazure T, Bui M, et al. Solid adenoma with exclusive hepatocellular differentiation: a new variant among pancreatic benign neoplasms? Virchows Arch. 2002;441:519–522. doi: 10.1007/s00428-002-0683-0. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Molina JM, Reddy MN. Boitnott JK. A neoplasm with pancreatic and hepatocellular differentiation presenting with subcutaneous fat necrosis. Am. J. Clin. Pathol. 1987;88:639–645. doi: 10.1093/ajcp/88.5.639. [DOI] [PubMed] [Google Scholar]
- Jung JY, Kim YJ, Kim HM, et al. Hepatoid carcinoma of the pancreas combined with neuroendocrine carcinoma. Gut Liver. 2010;4:98–102. doi: 10.5009/gnl.2010.4.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona D, Grobmyer S, Crawford JM. Liu C. Hepatocellular carcinoma arising from ectopic liver tissue in the pancreas. Virchows Arch. 2007;450:225–229. doi: 10.1007/s00428-006-0353-8. [DOI] [PubMed] [Google Scholar]
- Kim KA, Park CM, Kim CH, et al. Hepatocellular carcinoma in an ectopic liver: CT findings. Eur. Radiol. 2003;13(Suppl. 4):L45–L47. doi: 10.1007/s00330-003-1908-6. [DOI] [PubMed] [Google Scholar]
- Kubota K, Kita J, Rokkaku K, et al. Ectopic hepatocellular carcinoma arising from pancreas: a case report and review of the literature. World J. Gastroenterol. 2007;13:4270–4273. doi: 10.3748/wjg.v13.i31.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga S. Takahashi Y. Primary hepatocellular carcinoma and hepatoid adenocarcinoma of the stomach with liver metastasis: an unusual association. Jpn. J. Clin. Oncol. 1996;26:258–263. doi: 10.1093/oxfordjournals.jjco.a023225. [DOI] [PubMed] [Google Scholar]
- Lopez-Beltran A, Luque RJ, Quintero A, Requena MJ. Montironi R. Hepatoid adenocarcinoma of the urinary bladder. Virchows Arch. 2003;442:381–387. doi: 10.1007/s00428-003-0772-8. [DOI] [PubMed] [Google Scholar]
- Liu X, Cheng Y, Sheng W, et al. Analysis of clinicopathologic features and prognostic factors in hepatoid adenocarcinoma of the stomach. Am. J. Surg. Pathol. 2010;34:1465–1471. doi: 10.1097/PAS.0b013e3181f0a873. [DOI] [PubMed] [Google Scholar]
- Tanno S, Obara T, Shudo R, et al. Alpha-fetoprotein producing mucin-producing carcinoma of the pancreas: a case report with immunohistochemical study and lectin-affinity profile. Dig. Dis. Sci. 1997;42:2513–2518. doi: 10.1023/a:1018812628541. [DOI] [PubMed] [Google Scholar]
- Galvez-Munoz E, Gallego-Plazas J, Gonzalez-Orozco V, Menarguez-Pina F, Ruiz-Macia JA. Morcillo MA. Hepatoid adenocarcinoma of the stomach - a different histology for not so different gastric adenocarcinoma: a case report. Int. Semi. Surg. Oncol. 2009;6:13. doi: 10.1186/1477-7800-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CZ, Hu SY, Wang L, et al. Hepatoid carcinoma of the pancreas: a case report. Chin. Med. J. 2007;120:1850–1852. [PubMed] [Google Scholar]
- Lam K, Lo C, Wat M. Fan ST. Malignant insulinoma with hepatoid differentiation: a unique case with alpha-fetoprotein production. Endocr. Pathol. 2001;12:351–354. doi: 10.1385/ep:12:3:351. [DOI] [PubMed] [Google Scholar]
- Hughes K, Kelty S. Martin R. Hepatoid carcinoma of the pancreas. Am. Surg. 2004;70:1030–1033. [PubMed] [Google Scholar]
- Yano T, Ishikura H, Wada T, et al. Hepatoid adenocarcinoma of the pancreas. Histopathology. 1999;35:90–92. doi: 10.1046/j.1365-2559.1999.0728f.x. [DOI] [PubMed] [Google Scholar]
- Shih NN, Tsung JS, Yang AH, Tsou MH. Cheng TY. A unique pancreatic tumor with exclusive hepatocytic differentiation. Ann Clin Lab Sci. 2006;36:216–221. [PubMed] [Google Scholar]
- Oh HJ, Cheung DY, Kim TH, et al. A case of hepatoid carcinoma of the pancreas. Korean J Gastroenterol. 2006;47:389–393. [PubMed] [Google Scholar]
- Huang SC, Chang HC, Yeh TS, Ng KF. Chen TC. Hepatoid microcarcinoma of the pancreas: a case report and review of the literature. Chang Gung Med. J. 2012;35:285–291. doi: 10.4103/2319-4170.106142. [DOI] [PubMed] [Google Scholar]
- Kai K, Nakamura J, Ide T, et al. Hepatoid carcinoma of the pancreas penetrating into the gastric cavity: a case report and literature review. Pathol. Int. 2012;62:485–490. doi: 10.1111/j.1440-1827.2012.02814.x. [DOI] [PubMed] [Google Scholar]


