Abstract
Key Clinical Message
Sorafenib is the standard treatment of hepatocellular carcinoma (HCC). However, fibrolamellar HCC was not included in sorafenib trials. The case is a 26-year-old man with fibrolamellar HCC, who had a cerebrovascular accident (CVA) while being treated with sorafenib. This illustrates a probable relationship between use of sorafenib and CVA in low cardiovascular risk patients.
Keywords: Carcinoma, drug toxicity, fibrolamellar hepatocellular carcinoma, hepatocellular, sorafenib, stroke
Introduction
Sorafenib is a multikinase inhibitor that shares many adverse effects with other tyrosine-kinase inhibitors and antiangiogenic drugs. It has become the standard treatment of advanced hepatocellular carcinoma (HCC) in cirrhotic patients with preserved liver function. Fibrolamellar HCC is a subtype of HCC that most often affects young adults without parenchymal liver disease. However, experience with sorafenib in patients with fibrolamellar HCC is limited. We describe the case of a 26-year-old Caucasian man with fibrolamellar HCC, who was treated with sorafenib monotherapy due to adverse effects to conventional chemotherapy. The patient demonstrated stable disease for a year, but then developed a cerebellar stroke while being treated with sorafenib in the absence of any known risk factors for cerebrovascular accidents (CVA). In addition to the antitumoral efficacy of sorafenib in this subtype, this case illustrates a probable relationship between sorafenib and CVA even in low cardiovascular risk patients. This is contrary to the available evidence that shows that only the high cardiovascular risk patients are susceptible to sorafenib-induced cardiovascular events. Therefore, physicians must be alert to neurological symptoms associated with CVA in sorafenib-treated patients not withstanding their risk factor profile.
Sorafenib (trade name Nexavar; Bayer Pharmaceuticals, Diegem, Belgium) is an orally active multikinase inhibitor that has been approved for the treatment of advanced HCC [1]. Its antineoplastic effect is based on the inhibition of both cell proliferation (by inhibiting Raf kinase) and angiogenesis (by inhibiting vascular endothelial growth factor (VEGF) receptor -2, -3, and platelet-derived growth factor receptor β) [2]. The approval of sorafenib in 2007 was based on the sorafenib HCC assessment randomized protocol (SHARP) study, a multicenter, phase III, double-blind, placebo-controlled trial [1]. The most common adverse effects observed in the trial included fatigue, hand–foot skin syndrome, alopecia, gastrointestinal, and liver dysfunction.
Fibrolamellar HCC represents 0.6–8.6% of all HCCs. In contrast to typical HCC, fibrolamellar HCC most often affects young adults without parenchymal liver disease. However, this subtype was not included in the SHARP study [3] and therefore, experience with sorafenib treatment in this subtype is limited.
We describe the case of a 26-year-old Caucasian man with fibrolamellar HCC who had a cerebellar stroke while being treated with sorafenib. The diagnosis of fibrolamellar HCC with retroperitoneal and paravertebral lymph nodal, pulmonary and left adrenal metastases was made in June 2012 (Fig.1 and 2). Initially, palliative chemotherapy by six cycles of cisplatin and doxorubicin was started and this regimen induced stable disease. However, chemotherapy-induced nausea and asthenia led to a switch to sorafenib 800 mg daily in November 2012. After 5 months of continuous sorafenib, imaging showed no tumor progression. However, the dose needed to be reduced to 200 mg daily because of hand–foot skin syndrome and persistent diarrhea.
Figure 1.
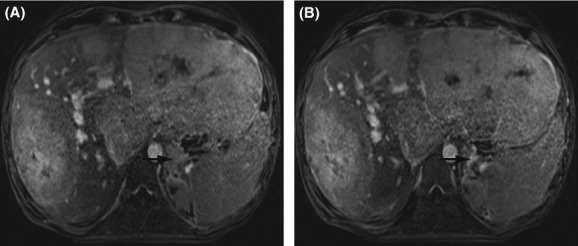
Magnetic resonance imaging of the liver showing hepatomegaly and multiple tumors in the left and right liver lobe before (A) and after 7 months of sorafenib (B). Axial T1-weighted delayed contrast-enhanced images by volumetric interpolated breath-hold examination are shown in portal venous phase of acquisition. Note the left adrenal metastasis (arrows).
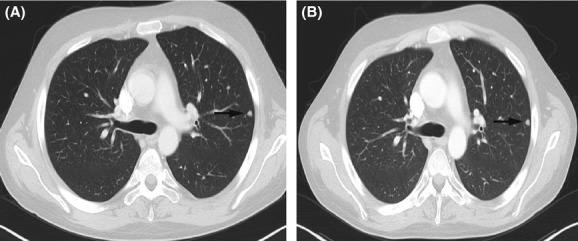
Figure 2.

Computed tomography of the chest before (A) and after 7 months of sorafenib (B). Note the pulmonary metastasis in the left upper lobe (arrows).
After 7 months, the patient was presented to the emergency department with acute vertigo, nausea, bioccipital headache, slurred speech, and ataxia. In addition, he noticed reduced motor coordination of his left hand. There was no history of passive or active cigarette smoking. There was no significant personal or family history of cardiovascular disease. Clinical exam showed a positive left finger-to-nose test, left dysdiadochokinesis, and a negative Romberg test. He was apyrexial and normotensive; he had a regular pulse and no detectable cardiac murmurs or carotid bruits.
He had a normocytic anemia (hemoglobin 11.2 g/dL, mean corpuscular volume 91.6 fl) and a prolonged erythrocyte sedimentation rate (29 mm/h). Biochemistry including fasting glucose, cholesterol and prothrombin time were within normal limits. The laboratory results also showed known stable elevated liver enzymes (alanine transaminase 74 U/L and γ-glutamyltransferase 176 U/L). There was no serological evidence of a thrombophilic tendency. He was negative for lupus anticoagulant and cryoglobulins and had normal levels of α–galactosidase, protein C and S. He is heterozygous for the MTHFR 677C>T mutation and had normal homocysteine levels.
The chest radiograph, electrocardiogram, echocardiogram, and Holter monitoring were normal. Carotid doppler ultrasonography showed bilateral patent carotid arteries with no evidence of plaques, vessel stenosis, or congenital malformation. At first, these neurological symptoms were considered the result of cerebral metastases. However, magnetic resonance imaging of the brain demonstrated recent left cerebellar infarction with diffusion restriction (Fig.3). In view of this result, aspirin 80 mg was prescribed. During follow-up, the patient did not recover fully from his neurological symptoms.
Figure 3.
Magnetic resonance imaging of the brain showing a semi-recent left cerebellar stroke (arrows) in axial T2-weighted (A) and diffusion-weighted (B) images.
First, it is important to mention that sorafenib induced an antitumor effect on fibrolamellar HCC, a subtype which has never been investigated in any clinical trial.
Second, the assessment of risk factors for CVA, including age, smoking, prior CVA, hypertension, diabetes mellitus, hypercholesterolemia, coronary artery disease, peripheral vascular disease, and atrial fibrillation was negative limiting the differential diagnosis to an adverse effect of the antiangiogenic therapy with sorafenib, which is known to elevate the risk (relative risk = 3.03) to thromboembolic events [4]. However, the pathophysiological mechanisms are yet unknown. A hypothesis to explain this peculiarity is based on the sorafenib-mediated inhibition of the VEGF pathway, which is vital for tumor angiogenesis, but also regulates the survival of endothelial cells which is important in vascular homeostasis, preventing abnormal bleeding or clotting [5]. Furthermore, VEGF increases the production of nitric oxide, which has several vascular-protective effects, such as inhibition of platelet activity and leukocyte adhesion [5]. If the balance is tipped toward clotting, arterial thromboembolic events such as CVA can occur.
Two cases of CVA in high cardiovascular risk patients (age, male, ex-smoker) during sorafenib treatment have been published [6]. We believe that our case illustrates a probable relationship between sorafenib and CVA in low cardiovascular risk patients, according to the Naranjo causality algorithm (score = 6) [7]. This is contrary to the available evidence that shows that only the high cardiovascular risk patients are susceptible to sorafenib-induced CVA [4]. Therefore, physicians must be alert to neurological symptoms associated with CVA in all sorafenib-treated patients.
Conflicts of Interest
None declared.
References
- 1.Llovet J, Ricci S, Mazzaferro V, Hilgard P, Edward G, Blanc J, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 2.Wilhelm S, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 3.Ang C, Kelley R, Choti M, Cosgrove D, Chou J, Klimstra D, et al. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointest. Cancer Res. 2013;6:3–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Choueiri T, Schutz F, Je Y, Rosenberg J, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J. Clin. Oncol. 2010;28:2280–2285. doi: 10.1200/JCO.2009.27.2757. [DOI] [PubMed] [Google Scholar]
- 5.Hoeben A, Landuyt B, Highley M, Wildiers H, Van Oosterom A, De Bruijn E. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 6.Saif M, Isufi I, Peccerillo J, Syrigos K. Cerebrovascular accidents associated with sorafenib in hepatocellular carcinoma. Gastroenterol. Res. Pract. 2011;2011:6160–6180. doi: 10.1155/2011/616080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naranjo C, Busto U, Sellers E, Sandor P, Ruiz I, Roberts E, et al. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]


