Abstract
Clinical trials revealed that systemic administration of B-cell-depleting anti-CD20 antibodies can hold lesion formation in the early relapsing-remitting phase of multiple sclerosis (MS). Throughout the secondary-progressive (SP) course of MS, pathogenic B cells may, however, progressively replicate within the central nervous system (CNS) itself, which is largely inaccessible to systemic anti-CD20 treatment. Utilizing the murine MS model of experimental autoimmune encephalomyelitis, we show that intrathecal (i.t.) administration of anti-CD20 alone very efficiently depletes meningeal B cells from established CNS lesions. In SP-MS patients, adding i.t. administration of anti-CD20 to its systemic use may be a valuable strategy to target pathogenic B-cell function.
Introduction
Clinical trials testing B-cell-depleting anti-CD20 (rituximab, ocrelizumab) in relapsing-remitting multiple sclerosis (RR-MS) generated encouraging results1,2 and indirectly consolidated the concept that B cells play an important pathogenic role in MS. B cells may contribute to MS pathogenesis as antigen-presenting cells for activation of encephalitogenic T-cells,3 as precursors of antibody-secreting plasma cells,4 and as source of pro-inflammatory cytokines.5 Emerging evidence suggests that throughout the chronic course of MS, pathogenic B-cell function may gradually shift from the periphery into the inflamed central nervous system (CNS). The recent discovery of ectopic B-cell follicle-like structures in the meninges of a proportion of patients with secondary-progressive MS (SP-MS)6,7 suggests that CNS B cells may locally reproduce and differentiate, providing one explanation why in later stages of MS, clinical progression decreasingly correlates with magnetic resonance imaging (MRI)-detectable CNS infiltration. As data indicate that only about 0.1% of systemically infused anti-CD20 reaches the cerebrospinal fluid (CSF),8 intrathecal (i.t.) application of anti-CD20, that may provide a valuable strategy to more efficiently target B cells within the CNS. Based on this assumption, a clinical trial combining i.t. anti-CD20 with systemic B-cell depletion in SP-MS is on its way (ClinicalTrials.gov Identifier: NCT01212094). In order to determine the effectiveness of i.t anti-CD20 by itself in depleting CNS B cells, we investigated this novel therapeutic approach in the murine MS model of experimental autoimmune encephalomyelitis (EAE).
Methods
Female C57BL/6 mice were immunized with 75-μg murine recombinant myelin oligodendrocyte glycoprotein (MOG) 1-117 in Complete Freund's Adjuvant (CFA) subcutaneously and 150ng pertussis toxin intraperitoneal (i.p.) twice. This induction regimen is associated with a high frequency of B cells infiltrating the CNS compared to classical induction regimens like immunization with MOG peptide 35-55.9 EAE severity was scored as follows: 0 = no paralysis, 1 = loss of tail tone, 2 = mild monoparesis or paraparesis, 3 = severe paraparesis, 4 = paraplegia and/or quadriparesis, 5 = moribund or death. At an EAE score ≥2, mice were randomly assigned to one of four groups and concomitantly received 100 μg of murine anti-CD20 or anti-ragweed isotype per week either i.t. (in 10 μL phosphate buffered saline (PBS); percutaneously into the cisterna magna with a 30-gauge needle in 45° anteflexion of the head10) or systemically (i.p.). Anti-CD20 and ragweed isotype control were kindly provided by Genentech Inc (South San Francisco, CA). Immune cells were isolated from blood, spleen, draining lymph nodes, brain, or spinal cord. Regarding the latter two, cells were isolated by Percoll gradient after collagenase/DNAse digestion of CNS tissue for 1 h.11 Cells were stained for CD19, CD3, and CD11b (all BD Biosciences, Heidelberg, Germany). In the brain and spinal cord, additional staining for CD45 (eBioscience, Frankfurt, Germany) identified infiltrating cells of hematopoietic origin. For histological analysis, CNS tissue was sectioned following perfusion with PBS and cryofixation and stained with Luxol fast blue and Periodic acid Schiff (LFB/PAS), hematoxylin and eosin (H&E) or by B220/CD3 immunohistochemistry as described previously.9 For comparison of clinical scores between groups, the Mann–Whitney test was used. For comparison of groups in our Fluorescence-activated cell sorting (FACS) study (Fig.2B), a two-way analysis of variance (ANOVA) with correction for multiple testing (Tukey) was performed. Only near-significant values comparing i.t. and i.p. anti-CD20 groups are shown. This study was approved by the government of Upper Bavaria (protocol number 55.2-1-54-2531-67-09).
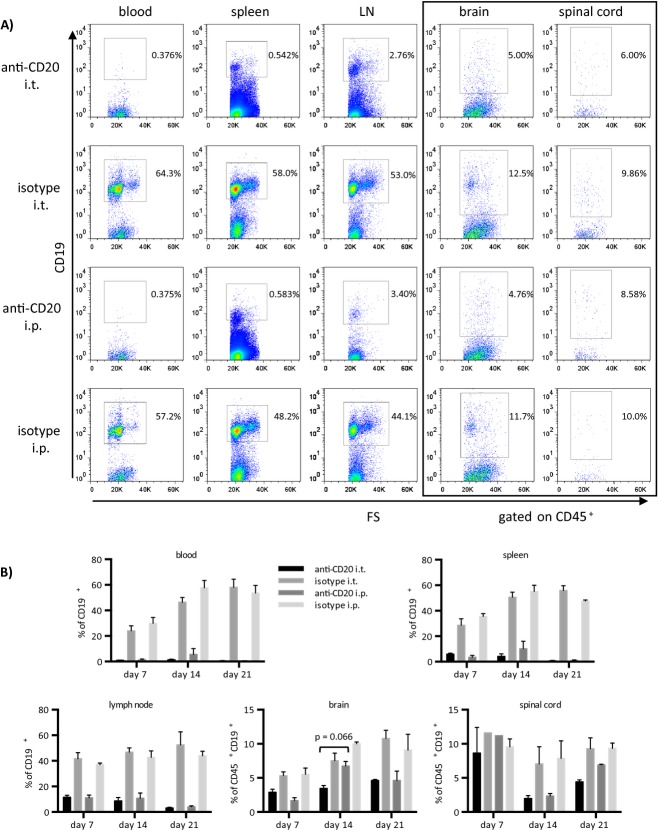
Figure 2.
Intrathecal anti-CD20 alone is modestly superior to its systemic application in depleting infiltrating CNS B cells, while it equally reduces B-cell frequencies in peripheral compartments. Six- to eight-week-old female C57BL/6 mice were immunized with recombinant MOG 1-117. Mice received a total of 1–3 weekly injections of 100 μg of intrathecal (i.t.) anti-CD20, i.t. isotype control, systemic (i.p.) anti-CD20, or i.p. isotype control starting on the day of individual EAE onset (EAE score ≥2). (A) Flow cytometric staining of CD19+ B cells in blood (first column), spleen (second column), lymph node (third column), brain, and spinal cord (fourth and fifth column, both gated on CD45+) on day 21 after treatment onset. Plots shown are representative of the mean values shown below. (B) Bars represent mean percentages of CD19+ B cells in the blood and spleen (upper panel), lymph node, brain, and spinal cord (lower panel; the latter two gated on CD45+), detected by FACS on days 7, 14, and 21 after treatment initiation (n = 3 mice per group per time point). Error bars represent SEM. Statistical analysis (two-way ANOVA with correction for multiple testing) comparing anti-CD20 i.t. versus anti-CD20 i.p. and isotype i.t. versus isotype i.p. groups, respectively, were performed for each time point and near-significant results are indicated in the graph. CNS, central nervous system; MOG, myelin oligodendrocyte glycoprotein; EAE, experimental autoimmune encephalomyelitis; ANOVA analysis of variance.
Results
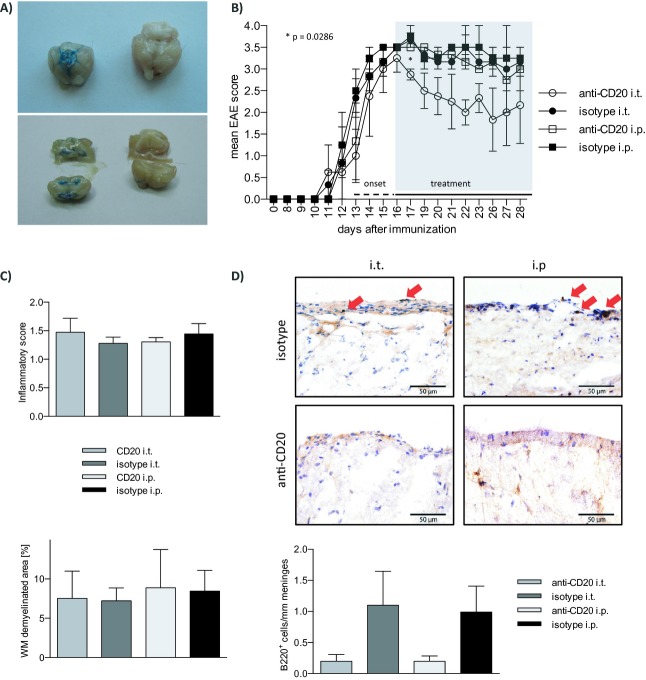
A first experiment intended to validate the method of i.t. injection in mice. As shown in Figure1A, 10-μL methylene blue dye injected into the cisterna magna stained inner and outer CSF spaces, while no leakage of the dye to the periphery was detected. Anti-CD20 or the respective control was then injected i.t. or i.p. into mice with established rMOG-induced EAE. As indicated in Figure1B, weekly i.t. anti-CD20 moderately ameliorated disease severity, whereas systemic anti-CD20 had a mild, but not significant effect. After three weekly injections, all four groups showed a comparable extent of inflammatory CNS infiltration and demyelination (Fig.1C). In contrast, i.t. application of anti-CD20 as well as its systemic use strongly reduced the number of B220+ B cells within meningeal lesions (Fig.1D), where the vast majority of CNS B cells are located in this model.9 In order to quantify the frequency of B cells more precisely (including the entire CNS with adjacent meninges) and to assess the kinetic of their depletion, B cells were isolated from blood, spleen, lymph nodes, brain, and spinal cord 7 days after 1 (day 7), 2 (day 14), or 3 (day 21) weekly injections (Fig.2). Paralleling the histologic analysis, i.t. as well as systemic anti-CD20 treatment was associated with a marked decline in B cells in the brain and spinal cord, while there was a trend that i.t. anti-CD20 may have been slightly superior to its systemic use (Fig.2A and B). Of note, depletion of B cells from the brain, but in particular from the spinal cord showed a delayed kinetic with the most prominent effect occurring after the second injection. Interestingly, particularly in i.p. anti-CD20, in some instances B cells accumulated in the CNS under continuous anti-CD20 treatment (brain – day 7 to day 14; spinal cord – day 14 to day 21). Unexpectedly, weekly injections of anti-CD20 into the cisterna magma also led to a virtually complete depletion of B cells in all peripheral compartments analyzed (Fig.2A and B). Already one i.t. injection of anti-CD20 (day 7) was followed by a broad depletion of B cells from blood, spleen, and lymph nodes in its extent indistinguishable from systemic anti-CD20 treatment. In order to evaluate the immunological consequences of anti-CD20-mediated B-cell depletion, we included an analysis of CD3+ T cells and CD11b+ myeloid cells. As indicated in Figure S1A and B, all peripheral compartments showed an expected compensatory increase in the frequency of T cells and myeloid cells upon depletion of B cells. In contrast, frequency of T cells and myeloid cells remained roughly stable in brain and spinal cord. In these compartments, B cells only represented 5–12% of infiltrating cells. Thus, it would be expected that their depletion may only lead to a mild compensatory frequency increase of T cells and myeloid cells. To quantify T-cell infiltration by absolute numbers, CD3 immunohistochemistry was performed. While systemic anti-CD20 had no dampening effect on T-cell infiltration into the CNS, i.t. application of anti-CD20 was indeed associated with a trend toward a decline of CNS T cells (Fig. S1C).
Figure 1.
Intrathecal anti-CD20 effectively depletes meningeal B cells and moderately ameliorates chronic EAE. (A) 10 μL methylene blue dye suboccipitally injected into the cisterna magna accurately distributes in basal cisterns (upper left panel) and ventricles (lower left panel, 1 h post injection; right panels: identical anatomical regions in an untreated control). (B–D) Six- to eight-week-old female C57BL/6 mice were immunized with recombinant MOG 1-117. After individual EAE onset (EAE score ≥2) mice were randomized to receive weekly intrathecal (i.t.) or intraperitoneal (i.p.) injections with 100 μg of murine anti-CD20 or isotype control antibody. (B) Clinical EAE score of n = 12 mice. *P = 0.0286 (anti-CD20 i.t. vs. isotype i.t.; Mann–Whitney test), error bars represent SEM. (C) One week after the last of three weekly injections, the CNS of n = 12 mice were dissected for histologic assessment. For analysis of CNS inflammation and demyelination, brains and spinal cords were sectioned following cryofixation and stained with Luxol fast blue and Periodic acid Schiff (LFB/PAS) or hematoxylin and eosin (H&E). Inflammatory infiltration (H&E; top) was scored as follows: 0 = no inflammation, 1 = little inflammation, 2 = moderate inflammation, 3 = strong inflammation. Demyelination (LFB/PAS; bottom) was evaluated as % of demyelinated area of the entire white matter. Error bars represent SEM. (D) For quantification of meningeal B cells, brain and spinal cord sections were stained by B220 immunohistochemistry. Representative slides are shown. Infiltrating B cells (B220) were counted and graphed as cells per mm meninges. Error bars represent SEM. EAE, experimental autoimmune encephalomyelitis; MOG, myelin oligodendrocyte glycoprotein; CNS, central nervous system.
Discussion
I.t. administration of anti-CD20 efficiently reduced the frequency of CNS meningeal B cells at an EAE disease stage, when CNS lesions are largely established and infiltration of immune cells into the CNS gradually declines.12 This finding supports the concept that effector mechanisms of anti-CD20-mediated eradication of B cells are sufficiently provided within the CNS and that accordingly anti-CD20 is generally capable of locally depleting CNS B cells. Our study was neither designed nor sufficiently powered to detect clinical effects of i.t. or i.p. anti-CD20 compared to isotype control antibody. Yet, in Figure1B, there was a trend that suggested that i.p. anti-CD20 may be beneficial compared to isotype i.p., which, although in our current study a lower dose was used, would be in agreement with our previously published results.9 Furthermore, there was a trend that i.t. anti-CD20 may be slightly superior to its systemic counterpart. Additional studies are needed to verify this observation and to elucidate the underlying mechanisms, for example, the generation of plasma cells with their various effector functions and other factors. In the model used, the majority of CNS B cells are found within meningeal areas (Fig.1D and 9). Given the immediate access of i.t. administered agents to the meninges, it is plausible that injecting anti-CD20 into the CSF may result in its highest concentration in the vicinity of meningeal B cells. In our FACS analysis, there was indeed a trend that i.t. anti-CD20 may be superior to its systemic use in depleting infiltrated B cells from the brain or spinal cord. This finding is of particular relevance, as ectopic lymphoid follicles presumably containing and replicating pathogenic B cells are found in meningeal regions adjacent to the subarachnoid space in patients with SP-MS.6 Interestingly, we observed in some occasions, more pronounced when anti-CD20 was applied systemically, that B cells accumulated in the CNS despite almost complete peripheral B-cell depletion. This finding may reflect local expansion of B cells, which would render targeting B cells locally in the CNS even more important.
Unexpectedly, anti-CD20 injected into the cisterna magna depleted peripheral B cells in a manner comparable to systemic anti-CD20, indicating that the compound did not remain within the CNS. Paralleling these findings, in a recent trial in subjects with CNS B-cell lymphoma serial i.t. application of rituximab showed an unexpected short CSF half-life of the compound, whereas it accumulated in the serum.13 While the CSF is drained primarily into the venous system where transmission of the larger anti-CD20 molecule should be restricted, an alternative route of CSF drainage leads into deep cervical lymphatics,14 which may represent the link to the periphery. In the clinical context, the subsequent depletion of peripheral B cells following i.t. administration of anti-CD20 should enhance the projected benefit in treatment of MS. In our preclinical model, however, this finding makes it difficult to distinguish primary i.t. effects from secondary systemic effects of i.t. anti-CD20. Concomitantly, this observation indicates, however, that selective targeting of CNS B cells, which may be desirable in some settings to reduce systemic side effects, may not be feasible by this approach.
Taken together, our mechanistic data provide evidence that CNS B cells may be targeted by anti-CD20 and that, herein, its i.t. application is at least as potent as its systemic application. In the context of treating patients, where there is evidence of poor accessibility of anti-CD20 to the CNS compartment, adding i.t. anti-CD20 administration to its systemic use may indeed be the most promising strategy to eradicate CNS-established pathogenic B-cell function. Results from the on-going clinical trial applying this regimen to patients with SP-MS will determine whether such facilitated elimination of CNS B cells may translate into clinical benefit.
Acknowledgments
The authors wish to thank Veronika Husterer for excellent technical support, Uwe Ködel (Ludwig-Maximilians-University, Munich) for sharing his expertise on i.t. injections in mice and Anne Winkler for sharing her expertise on CNS histology. K. L.-H. is supported by the Kommission für Klinische Forschung (KKF) of the Technische Universität München and is a fellow of the Deutsche Forschungsgemeinschaft (DFG; Le 3079/1-1). M. S. W. is supported by the National Multiple Sclerosis Society (NMSS; PP 1660), the Deutsche Forschungsgemeinschaft (DFG; WE 3547/4-1), and the ProFutura Programm of the Universitätsmedizin Göttingen. B. H. is supported by a grant from the German Ministry for Education and Research (BMBF, “German Competence Network Multiple Sclerosis” (KKNMS), Control-MS, 01GI0917) and the DFG (He2386/7-1). C. C. A. B. is supported by grants from the National Health and Medical Council of Australia.
Conflict of Interest
None declared.
Supporting Information
Anti-CD20 B-cell depletion leads to a compensatory increase of T-cell and myeloid cell frequency in the periphery but not in the CNS. Six- to eight-week-old female C57BL/6 mice were immunized with recombinant MOG 1-117. Mice received a total of 1–3 weekly injections of 100 μg of intrathecal (i.t.) anti-CD20, i.t. isotype control, systemic (i.p.) anti-CD20, or i.p. isotype control starting on the day of individual EAE onset (EAE score ≥2). Bars represent mean percentages of (A) CD11b+ myeloid cells and (B) CD3+ T cells in the blood, spleen, lymph node, brain, and spinal cord detected by FACS on days 7, 14, and 21 after treatment initiation (n = 3 mice per group per time point). Error bars represent SEM. (C) For quantification of absolute T-cell numbers infiltrating brain and spinal cord, sections were stained by CD3 immunohistochemistry. CD3+ T cells were counted and graphed as cells per mm meninges. Error bars represent SEM.
References
- Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378:1779–1787. doi: 10.1016/S0140-6736(11)61649-8. [DOI] [PubMed] [Google Scholar]
- Weber MS, Hemmer B. Cooperation of B cells and T cells in the pathogenesis of multiple sclerosis. Results Probl Cell Differ. 2010;51:115–126. doi: 10.1007/400_2009_21. [DOI] [PubMed] [Google Scholar]
- Weber MS, Hemmer B, Cepok S. The role of antibodies in multiple sclerosis. Biochim Biophys Acta. 2011;1812:239–245. doi: 10.1016/j.bbadis.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Barr TA, Shen P, Brown S, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med. 2012;209:1001–1010. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Combs D, Rosenberg J, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101:466–468. doi: 10.1182/blood-2002-06-1636. [DOI] [PubMed] [Google Scholar]
- Weber MS, Prod'homme T, Patarroyo JC, et al. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol. 2010;68:369–383. doi: 10.1002/ana.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht TA, Angele B, Klein M, et al. Complement C1q and C3 are critical for the innate immune response to Streptococcus pneumoniae in the central nervous system. J Immunol. 2007;178:1861–1869. doi: 10.4049/jimmunol.178.3.1861. [DOI] [PubMed] [Google Scholar]
- Weber MS, Prod'homme T, Youssef S, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- Steinman L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron. 1999;24:511–514. doi: 10.1016/s0896-6273(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Fridlyand J, Abrey L, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol. 2007;25:1350–1356. doi: 10.1200/JCO.2006.09.7311. [DOI] [PubMed] [Google Scholar]
- Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19:480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anti-CD20 B-cell depletion leads to a compensatory increase of T-cell and myeloid cell frequency in the periphery but not in the CNS. Six- to eight-week-old female C57BL/6 mice were immunized with recombinant MOG 1-117. Mice received a total of 1–3 weekly injections of 100 μg of intrathecal (i.t.) anti-CD20, i.t. isotype control, systemic (i.p.) anti-CD20, or i.p. isotype control starting on the day of individual EAE onset (EAE score ≥2). Bars represent mean percentages of (A) CD11b+ myeloid cells and (B) CD3+ T cells in the blood, spleen, lymph node, brain, and spinal cord detected by FACS on days 7, 14, and 21 after treatment initiation (n = 3 mice per group per time point). Error bars represent SEM. (C) For quantification of absolute T-cell numbers infiltrating brain and spinal cord, sections were stained by CD3 immunohistochemistry. CD3+ T cells were counted and graphed as cells per mm meninges. Error bars represent SEM.