Abstract
Prenatal exposure to sodium valproate (VPA) is associated with neurodevelopmental impairments. Cortical thickness was measured in 16 children exposed prenatally to VPA and 16 controls. We found increased left inferior frontal gyrus (IFG; BA45) and left pericalcarine sulcus (BA18) thickness, an association between VPA dose and right IFG thickness, and a close relationship between verbal skills and left IFG thickness. A significant interaction between group and hemispheric IFG thickness showed absence of the normal asymmetry in the IFG region of VPA-exposed children. These data provide preliminary insights into the putative neural basis of difficulties experienced by some VPA-exposed children.
Introduction
Children whose mothers took antiepileptic drugs (AEDs) during pregnancy are at increased risk of poor cognitive and behavioural outcomes. Sodium valproate (VPA) exposure is associated with impaired intellectual1–3 and language ability.4 The probable causal pathway for the longer term effects of fetal AED exposure is the child's brain development during exposure.
Brain changes occur in adults taking VPA, including increased atrophy in dementia5 and reduced parietal lobe cortical thickness in people with epilepsy.6 One study of adults with in utero AED exposure found reduced grey matter volume in lentiform nucleus and hypothalamus,7 but the heterogeneous sample (18 people with 14 different AED combinations) precludes drug-specific interpretations.
Rodent studies suggest that prenatal exposure impacts on brain development. VPA affects neuronal progenitor proliferation,8 increases the number of GABAergic neurons, enhances neuronal differentiation,9 causes morphological changes in hippocampal astrocytes10,11 and impairs synaptic plasticity.12,13 Bittigau et al.14 found widespread and dose-dependent apoptotic neurodegeneration although others report global cerebral volume loss associated with high-dose VPA exposure,15 yet increased cerebral volume with lower doses. VPA-treated adult rats show significant reduction in cell differentiation following VPA-exposure16 without volume changes, suggesting that measures such as cortical thickness may prove useful.
Here, we report imaging findings in children exposed to VPA prenatally. We predicted that widespread reductions in cortical thickness would be present in exposed children compared to age- and sex-matched controls.
Methods and Materials
Participants
Participants were 16 children (seven male, one left-handed) exposed to VPA in utero, recruited to our prospective long-term cognitive outcomes study4,3 and 16 age- and sex-matched controls recruited via hospital and community advertisements whose mothers did not take AEDs or have epilepsy. The average age of cases was 7.2 years (SD = 0.6; range 6–8 years) and in controls was 8.1 (SD = 1.0; range 6–9 years), representing a small but significant difference in age (P < 0.05). There were no differences in ethnicity between groups. One child's mother took VPA and Levetiracetam, and all others were VPA monotherapy. The mean VPA dose across pregnancy was 885 mg (SD = 421). The average IQ of the VPA group was 96.2 (SD = 11.6) and five children's scores fell in the low average or borderline range. There was no significant difference in IQ between VPA exposed children and controls (Control mean = 104.3, SD = 12.4, P > 0.05). Written consent from a parent/guardian and verbal assent from the child was obtained in all cases.
Data acquisition
Full neuropsychological assessment details are reported elsewhere.4,3 The two-subtest short-form of the Wechsler Abbreviated Scale of Intelligence (WASI) was used to measure controls' IQ. Magnetic resonance imaging (MRI) scans were acquired on the 3T Siemens Magnetom Trio at Murdoch Childrens Research Institute, Melbourne, using a 12-channel coil. High-resolution 3D T1-weighted images were acquired (TR = 1900 msec, TE = 2.15 msec, flip angle = 9°, FOV = 256 mm, 176 slices, 1 mm isotropic voxels). As part of our routine research practice, T2-weighted scans are included for clinical reporting by paediatric radiologists.
Image analysis
Data were analysed using Freesurfer, an automated segmentation and cortical reconstruction tool. For imaging processing details, see http://surfer.nmr.mgh.harvard.edu/fswiki/FreeSurferMethodsCitation. Voxelwise group comparisons were conducted, correcting for multiple comparisons (P < 0.05, Monte Carlo method). Only differences that survived this stringent correction step are presented here. Analyses were repeated excluding data from one child whose mother took VPA and Levetiracetam. The findings were unchanged and the total group results are reported here. Values of significantly different regions were then used in subsequent statistical analyses (SPSS 21, IBM Corp, 2011). Freesurfer volumes were used to examine whole brain group differences.
Results
Increased cortical thickness was found in the left inferior frontal gyrus (IFG) (Pars Opercularis; x, y, z: −52.5, 23.8, 11.3) and left medial occipital cortex (Pericalcarine; x, y, z: −12.3, −80.6, 14.1). The differences remained when age was included as a covariate. Total cortical white and grey matter did not differ between groups (both P > 0.1). Asymmetry of cortical thickness in the inferior frontal lobes was seen in controls but not cases; a significant interaction between group and hemispheric thickness in the inferior frontal region, controlling for age, was detected (F(1, 29) = 12.38, P = 0.001; Fig.1). Post-hoc analysis showed greater right than left inferior frontal cortical thickness in controls but no such right-left asymmetry in VPA cases. VPA dose across pregnancy did not correlate significantly with left inferior frontal thickness (r = −0.17, P = 0.53), however, there was a significant relationship with the homologous right hemisphere region (r = 0.57, P = 0.02, two-tailed; Fig.2A). A trend towards poorer verbal abilities (Wechsler Intelligence Scale for Children – 4th Edition WISC Verbal Comprehension Index Scores) and greater thickness was observed in VPA cases (r = −0.42, P = 0.12, two-tailed; Fig.2B). No abnormalities were detected on visual inspection of the data by a paediatric radiologist.
Figure 1.
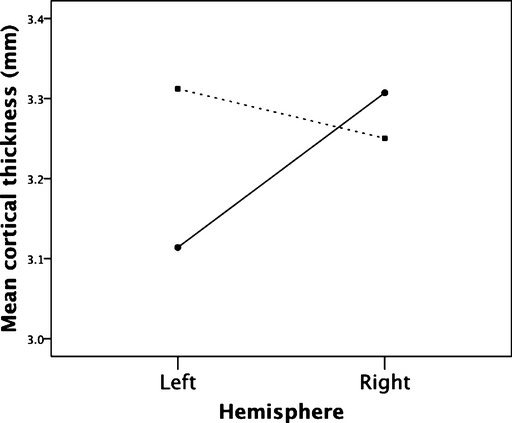
A significant interaction between hemispheric inferior frontal gyrus cortical thickness in cases (hashed line, square end) and controls (solid line, round end).
Figure 2.
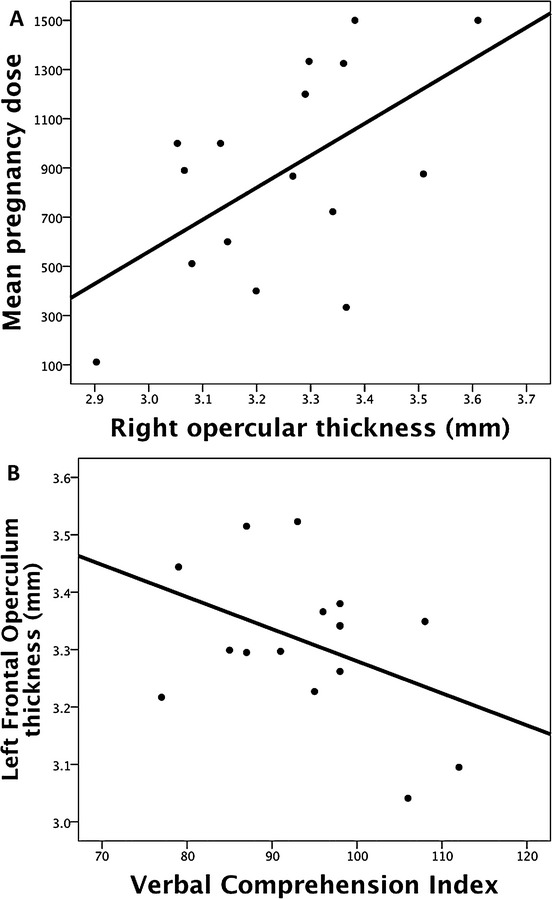
(A) Valproate (VPA) dose in pregnancy correlates significantly with right inferior frontal gyrus thickness (B) Left inferior frontal gyrus thickness is associated with poorer verbal abilities.
Discussion
Our data demonstrate regional structural cortical brain changes in humans exposed to VPA in utero. Increases in cortical thickness were identified in children exposed prenatally to VPA, which is a potent and effective anti-epileptic drug, widely considered the treatment of choice for genetic generalized epilepsies. Our findings provide the first direct insight into its central neural impact on the developing human brain.
Differences in left IFG cortical thickness and absence of the usual hemispheric asymmetry in this region are compelling given the known association between language impairments and prenatal VPA exposure. Atypical hemispheric language specialization in VPA-exposed children was predicted by Meador and colleagues2 on the basis of the pattern of neuropsychological impairment in their cohort of children. Several groups, including our own, report significantly reduced verbal intellectual function and we also showed that specific language impairments occur in VPA-exposed children.4 Meador's group also had a higher than usual rate of left handedness, which is associated with an increased likelihood of atypical (i.e. bilateral or right-sided) language representation in the brain. Taken together, these data led to the conclusion that VPA exposure in utero impacts on the normal functional specialization for language. An alternative explanation is that the atypical hemispheric specialization is inherited from the child's mother. Epilepsy is associated with a higher than usual rate of atypical language representation.17,18 Nevertheless, this seems unlikely to account for Meador and colleagues' hypothesis and our findings because atypical language dominance is typically a feature of lesional epilepsy, and VPA is used most often for idiopathic generalized epilepsy. Second, although the mechanism underlying atypical language in epilepsy is not well characterized, it is assumed to reflect a shift away from pathological eloquent cortex19,20 rather than a genetic factor. Our findings support the idea that there may be altered functional hemispheric specialization in children exposed to VPA and future studies will examine this hypothesis directly.
The minor age discrepancy between our groups is not the basis for differences in cortical thickness. The findings were not altered when age was covaried was and the pattern of results is opposite to the expected developmental processes; greater cortical development in the controls would in fact minimize the cortical differences found here. Although there is an overall reduction in grey matter volume and cortical thickness during childhood and adolescence,21,22 thickness in specific regions such as left IFG increases with age.21 Thus, controls would show greater cortical thickness in controls. We found the opposite, suggesting that either the VPA-exposed cases have enhanced postnatal cortical development relative to their peers or that the increased thickness represents perturbed prenatal neurodevelopmental processes.
The association between poorer verbal abilities and increased cortical thickness in the left IFG suggests that our results cannot be accounted for by “enhanced” cortical development in the children exposed to VPA. Positive correlations between IQ and cortical thickness are reported in adults23; this relationship is not evident in young children (i.e. similar to our cohort) but emerges later.24 Indeed, the rate of change in cortical thickness is the salient factor in the relationship with intellectual skills, with higher levels of ability associated with a later onset of thinning.24 Thus, existing data that link the magnitude and developmental trajectory of cortical thickness to intellectual abilities are inconsistent with our findings. Instead, our findings suggest that there may be a direct relationship between the structural changes in this region and cognitive deficits.
The alteration of cortical development in our cohort is consistent with perturbed prenatal brain development. The specific basis for this is difficult to discern on the basis of our findings and the various mechanisms of action of VPA. For example, VPA increases the availability of GABA, which plays a critical role in cortical development.25,26 Increased bioavailability in the fetus could affect the normal execution of GABA-related processes such as neurite branching and dendrite outgrowth. The trophic function of GABA is well recognized,25 and regionally-increased cortical thickness is seen in fetal alcohol disorder,27 in which GABA is implicated. Alternative mechanisms may also be relevant to understanding the effects of VPA exposure during brain development. For example, VPA impacts on the activity of histone deacetylase and these changes have been shown to influence social behavior in animal models28 and neural plasticity.29
Patterns of morphological changes may vary in relation to the timing of AED exposure,7 and dose may also play a role. VPA-exposed rodents show abnormalities of cortical development including increased apoptosis,8,14 however, volume changes depend on dose, with volume loss at high dose, yet increased volume at medium doses.15 Our cohort was exposed to a relatively low average VPA dose; future studies should include a range of doses to better understand this effect in humans. In conclusion, this study demonstrates regional structural brain changes in children exposed to VPA in utero, and highlights a need for additional research.
Acknowledgments
The authors thank the staff of the Australian Pregnancy Register for their support in recruitment, the families who travelled across Australia to participate and the radiographers at Murdoch Childrens Research Institute's MRI facility. Financial support for the study was provided by MCRI Theme funds, APEX Foundation for Intellectual Disabilities, Australian Research Council (LP0669648) and Pearson Pls. A. W. received research fellowships from NHMRC (251755) and Rotary Health Australia (Geoffrey Betts Award). C. N. and S. B. were supported by Australian Postgraduate Award Scholarships.
Conflict of Interest
Dr. Wood reports grants from Australian Research Council, Apex Foundation for Intellectual Disabilities, Pearson Pls, Murdoch Childrens Research Institute, during the conduct of the study.
Dr. Reutens reports grants from Australian Research Council, Apex Foundation for Intellectual Disabilities, during the conduct of the study.
Dr. Vajda reports grants from Australian Research Council, Apex Foundation for Intellectual Disabilities, during the conduct of the study; grants from RMH Neuroscience Foundation, UCB Pharma, Jansen-Cilag, Sanofi, Epilepsy Society of Australia, outside the submitted work.
Dr. O'Brien reports grants from NHMRC, RMH Neuroscience Foundation, UCB Pharma, Jansen-Cilag, Sanofi-Genzyme, Scigen, Epilepsy Society of Australia, outside the submitted work.
References
- Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ, Baker GA, Browning N, et al. Foetal antiepileptic drug exposure and verbal versus non-verbal abilities at three years of age. Brain. 2011;134:396–404. doi: 10.1093/brain/awq352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadebaum C, Anderson V, Vajda F, et al. The Australian brain and cognition and antiepileptic drugs study: IQ in school-aged children exposed to sodium valproate and polytherapy. J Int Neuropsychol Soc. 2011;17:133–142. doi: 10.1017/S1355617710001359. [DOI] [PubMed] [Google Scholar]
- Nadebaum C, Anderson V, Vajda F, et al. Language skills of school-aged children prenatally exposed to antiepileptic drugs. Neurology. 2011;76:719–726. doi: 10.1212/WNL.0b013e31820d62c7. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Schneider LS, Cummings J, et al. Chronic divalproex sodium to attenuate agitation and clinical progression of Alzheimer disease. Arch Gen Psychiatry. 2011;68:853–861. doi: 10.1001/archgenpsychiatry.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe HR, Berg AT, Jackson GD. Sodium valproate use is associated with reduced parietal lobe thickness and brain volume. Neurology. 2013;80:1895–1900. doi: 10.1212/WNL.0b013e318292a2e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Sheer I, Wilhelm T, et al. Brain morphology alterations in the basal ganglia and the hypothalamus following prenatal exposure to antiepileptic drugs. Eur J Paediatr Neurol. 2007;11:297–301. doi: 10.1016/j.ejpn.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Finnell RH, Waes JG, Eudy JD, Rosenquist TH. Molecular basis of environmentally induced birth defects. Annu Rev Pharmacol Toxicol. 2002;42:181–208. doi: 10.1146/annurev.pharmtox.42.083001.110955. [DOI] [PubMed] [Google Scholar]
- Laeng P, Pitts R, Lemire A, et al. The mood stabilizer valproic acid stimulates GABA neurogenesis from rat forebrain stem cells. J Neurochem. 2004;91:238–251. doi: 10.1111/j.1471-4159.2004.02725.x. [DOI] [PubMed] [Google Scholar]
- Fennrich S, Ray D, Nau H, Schlosshauer B. Radial astrocytes: toxic effects induced by antiepileptic drug in the developing rat hippocampus in vitro. Eur J Cell Biol. 1998;77:142–150. doi: 10.1016/S0171-9335(98)80082-4. [DOI] [PubMed] [Google Scholar]
- Sobaniec-Lotowska M. Ultrastructure of astrocytes in the cortex of the hippocampal gyrus and in the neocortex of the temporal lobe in experimental valproate encephalopathy and after valproate withdrawal. Int J Exp Pathol. 2003;84:115–126. doi: 10.1046/j.1365-2613.2003.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Brown L, Teyler T. The effects of anticonvulsant drugs on long-term potentiation (LTP) in the rat hippocampus. Brain Res Bull. 1996;39:39–42. doi: 10.1016/0361-9230(95)02041-1. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yu K, Xiao C, Ruan D. The influence of developmental periods of sodium valproate exposure on synaptic plasticity in the CA1 region of rat hippocampus. Neurosci Lett. 2003;351:165–168. doi: 10.1016/j.neulet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch C, Husch K, Angenstein F, et al. Dose-dependent memory effects and cerebral volume changes after in utero exposure to valproate in the rat. Epilepsia. 2009;50:1432–1441. doi: 10.1111/j.1528-1167.2008.01943.x. [DOI] [PubMed] [Google Scholar]
- Umka J, Mustafa S, ElBeltagy M, et al. Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience. 2010;166:15–22. doi: 10.1016/j.neuroscience.2009.11.073. [DOI] [PubMed] [Google Scholar]
- Loring DW, Meador KJ, Lee GP, King DW. Amobarbital effects and lateralized brain function: the Wada test. New York: Springer-Verlag; 1992. [Google Scholar]
- Helmstaedter C, Kurthen M, Linke DB, Elger C. Patterns of language dominance in focal left and right hemisphere epilepsies: relation to MRI findings, EEG, sex, and age of onset of epilepsy. Brain Cogn. 1997;33:135–150. doi: 10.1006/brcg.1997.0888. [DOI] [PubMed] [Google Scholar]
- Sass KJ, Silberfien CM, Platis I, et al. Right hemisphere mediation of verbal learning and memory in acquired right hemisphere speech dominant patients. J Int Neuropsychol Soc. 1995;1:554–560. doi: 10.1017/s1355617700000680. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Kurthen M, Linke DB, Elger CE. Right hemisphere restitution of language and memory functions in right hemisphere language-dominant patients with left temporal lobe epilepsy. Brain. 1994;117:729–737. doi: 10.1093/brain/117.4.729. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, et al. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, et al. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Yang Y, Roussotte F, Kan E, et al. Abnormal cortical thickness alterations in fetal alcohol spectrum disorders and their relationships with facial dysmorphology. Cereb Cortex. 2012;22:1170–1179. doi: 10.1093/cercor/bhr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldrich RX, Leanage G, She D, et al. Inhibition of histone deacetylase in utero causes sociability deficits in postnatal mice. Behav Brain Res. 2013;257:253–264. doi: 10.1016/j.bbr.2013.09.049. [DOI] [PubMed] [Google Scholar]
- Taniura H, Sng JCG, Yoneda Y. Histone modifications in the brain. Neurochem Int. 2007;51:85–91. doi: 10.1016/j.neuint.2007.04.018. [DOI] [PubMed] [Google Scholar]


