Abstract
The apicomplexan intraerythrocytic parasite Babesia microti is an emerging human pathogen and the primary cause of human babesiosis, a malaria-like illness endemic in the United States. The pathogen is transmitted to humans by the tick vector, Ixodes scapularis, and by transfusion of blood from asymptomatic B. microti-infected donors. Whereas the nuclear and mitochondrial genomes of this parasite have been sequenced, assembled and annotated, its apicoplast genome remained incomplete, mainly due to its low representation and high A+T content. Here we report the complete sequence and annotation of the apicoplast genome of the B. microti R1 isolate. The genome consists of a 28.7 kb circular molecule encoding primarily functions important for maintenance of the apicoplast DNA, transcription, translation and maturation of organellar proteins. Genome analysis and annotation revealed a unique gene structure and organization of the B. microti apicoplast genome and suggest that all metabolic and non-housekeeping functions in this organelle are nuclear-encoded. B. microti apicoplast functions are significantly different from those of the host, suggesting that they might be useful as targets for development of potent and safe therapies for the treatment of human babesiosis.
Introduction
Human babesiosis is an emerging infectious disease caused by a select group of intraerythrocytic protozoan parasites defining a new clade in the Apicomplexan phylum distinct from those encompassing Plasmodium species, Theileria species or Babesia bovis [1]. Most babesiosis infections in humans are caused by Babesia microti and are transmitted by Ixodes ticks, the vectors responsible for transmission of several other pathogens including Borrelia, Anaplasma and Ehrlichia species. Human babesiosis is endemic in northeastern and northern midwestern United States but has also been reported in Europe, Asia, Africa, Australia and South America [2], [3]. Depending on their immune status and age, patients with human babesiosis can experience mild, moderate or severe illness with the latter possibly leading to multi-organ system failure and death. In the United States, the mortality rate approaches 9% in hospitalized patients and ∼20% in immunocompromised hosts [3], [4]. As a result babesiosis has been recognized as an emerging health threat [5], and since 2011 has been designated as a nationally notifiable disease by the Center for Disease Control [6].
The ability of B. microti to invade and multiply within human red blood cells, and the lack of effective tools for large-scale screening of blood for B. microti infection from asymptomatic donors make this parasite a major risk to the national blood supply [7], [8]. Accordingly, B. microti is now considered the most commonly reported transfusion-transmitted pathogen in the United States [5], and the number of documented cases of acquired infections by transfusion has substantially increased over the years [9]. Although babesiosis therapy, which consists of combination of atovaquone and azithromycin or clindamycin and quinine [10], is considered generally effective, adverse events and disease failure and relapse can occur in some patients.
Recent efforts aimed to probe the diversity, pathogenicity and metabolism of B. microti and to identify new markers and targets for diagnosis and therapy of human babesiosis have led to the completion of the first genomic sequence of a clinical clone named R1 [11]. Subsequently Whole Genome maps of two B. microti strains R1 and Gray were reported [12]. These genomic analyses revealed that the genome of B. microti is less than 7Mbp, making it the smallest nuclear genome among apicomplexa [11]. Phylogenetic analyses placed B. microti in a new lineage among apicomplexan parasites distinct from B. bovis and Theileria species [11]. The genome effort has also revealed that the parasite has two DNA-containing organelles, the mitochondria and the apicoplast. The apicoplast is a non-photosynthetic plastid that plays an essential role in parasite development and survival [13], [14]. Genetic and biochemical studies in malaria and related parasites have shown that this organelle hosts important metabolic and housekeeping processes, which are critical for parasite survival within host cells (reviewed in [15]). Some of these pathways exist in B. microti and are significantly different from their host counterparts thereby offering new opportunities for the development of selective therapies for treatment of human babesiosis [11].
While the linear mitochondrial genome of B. microti has been fully characterized by two independent studies [12], [16], the apicoplast genome of this parasite remained partially assembled due to its high A+T content and to its low representation. Here we report the completion of the genomic sequence of the apicoplast of the B. microti R1 isolate. We show that this genome consists of a 28.7 kb circular molecule, which encodes genes involved in maintenance of the apicoplast DNA, transcription, translation and maturation of organellar proteins. Sequence analysis of the B. microti apicoplast genome and genome comparisons revealed that major gene alterations and rearrangements occurred in the apicoplast genomes during the evolution of piroplasms.
Materials and Methods
Sequencing, assembly and annotation of the apicoplast genome
Genomic DNA used to complete the sequence of the R1 apicoplast genome was previously described [11], [12]. The apicoplast genome sequence was obtained by primer walking using long PCR reactions. PCR amplification was performed using total genomic DNA and primers designed following sequencing of apicoplast genome contigs obtained after the first genome assembly [11]. Sequencing was performed on both strands and from at least two PCR products using specific primer pairs (Table S1 in File S1). The complete sequence of the apicoplast genome was deposited in the European Nucleotide Archive with Accession Number LK028575. Genome annotation was performed using Artemis (https://www.sanger.ac.uk/resources/software/artemis/) [17] and BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Prediction of tRNA genes was performed using tRNAscanSE 1.21 (http://lowelab.ucsc.edu/tRNAscan-SE/) with the following parameters: search mode: “default”, and source: “Mito/Chloroplast” [18]. Genetic maps were obtained using CGView (http://stothard.afns.ualberta.ca/cgview_server) [19] and GenomeVx (http://wolfe.ucd.ie/GenomeVx) [19]. Genome comparisons were performed using Mauve (http://gel.ahabs.wisc.edu/mauve) [20] and edited using ACT (https://www.sanger.ac.uk/resources/software/act) [21]. Manual editing to correct the above automated analyses was completed on a gene-by-gene basis as needed. The following entries were used for comparative analyses: AAXT01000007 (B. Bovis), HM222968 (Chromera), fusion of X95275 (IRA) and X95276 (IRB) (P. falciparum), AAGK01000009 (T. parva), U87145 (T. gondii). Phylogenetic analyses were performed using Phylogeny.fr with default options [22]. TMpred (http://www.ch.embnet.org/software/TMPRED_form.html) and Pfam (http://pfam.sanger.ac.uk/) were used for transmembrane domain and motif predictions [23], [24].
Results and Discussion
Sequence analysis of the circular apicoplast genome of B. microti
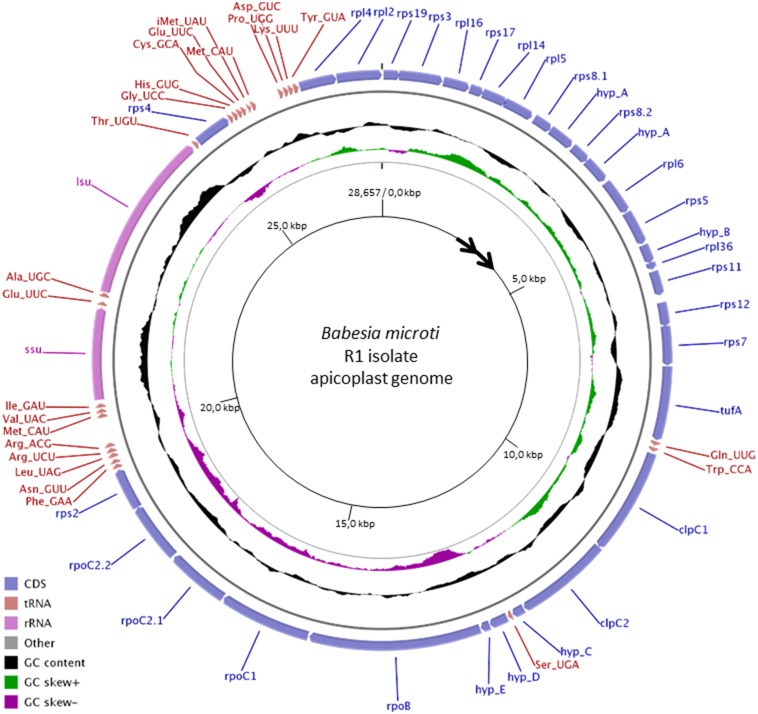
Previous efforts aimed to sequence the nuclear and organellar genomes of B. microti identified several contigs representing partial sequences of the apicoplast genome of this parasite. In order to generate a complete sequence of the apicoplast genome, we performed primer walking using total genomic DNA and specific primers derived from the complete sequencing of the individual contigs. Sequencing and assembly revealed that the apicoplast genome of B. microti is composed of a circular molecule of 28.7 kbp. It is the smallest apicoplast genome found in apicomplexan parasites. The genome is 86% A+T rich and has a coding density of over 98%. It encodes SSU and LSU rRNAs, 18 ribosomal proteins, an EF-Tu elongation factor, three subunits of the RNA polymerase, 2 copies of the ClpC chaperone, 24 tRNAs and five hypothetical proteins ranging in size between 49 (Hyp-E) and 140 amino acids (Hyp-A) (Fig. 1). All genes encoded in the apicoplast genome of B. microti are transcribed in the same orientation (Fig. 1). AUG or AUA serve as initiators for 18 and 13 of the 31 codon sequences (CDSs), respectively, and are preceded by an A-rich region that may play an important role in the recruitment of the ribosome. Consistent with this unusual translation initiation, a single Met-itRNA with an UAU anticodon was found in the apicoplast genome. Termination codons used in the CDSs of apicoplast genome include UAA (25 of the 31 CDSs) and UGA (6 of the 31 CDSs). This finding is consistent with the annotation of the B. microti nuclear genome, which identified an apicoplast targeting motif in the RF2 release factor [11]. No UAG stop codons were found in the apicoplast genome of B. microti consistent with the lack of an apicoplast targeting sequence in the RF1 release factor encoded by the nuclear genome [11].
Figure 1. Graphical circular map of the apicoplast genome of B. microti R1 isolate.
The map was designed using CGview and GenomeVx. From outside to center: coding sequence (CDS), % G+C, GC skew and base coordinates. hypA-E refer to five hypothetical protein encoding genes found in the apicoplast genome of B. microti.
The majority of CDSs in the apicoplast genome of B. microti do not overlap, and only four coding sequences were found to overlap over one to three codons, making the start of one CDS part of the stop codon of the previous CDS. tRNAscanSE analyses identified 24 tRNAs. In addition to the Met-itRNA, other tRNAs with a U at the first position of the anticodon have been found but the Wobble pairing U - G represents less than 1% of codons in the B. microti apicoplast genome. Half of the tRNAs known to decode codons ending with a U or a C were not detected by tRNAscanSE; these tRNAs might have a sequence that is too divergent from others to be recognized by tRNAscanSE. Similar to P. falciparum, CGU is the only codon found in the B. microti apicoplast genome used for arginine in the CGN group.
Detailed analysis of the ribosomal proteins and RNAs predicted from the B. microti apicoplast genome revealed significant similarity with other apicomplexa. The genome encodes proteins of the small (11 rps proteins) and large (7 rpl proteins) ribosomal subunits. Additional ribosomal proteins are encoded by the nuclear genome and targeted to the apicoplast [11]. Association of nuclear and apicoplast encoded ribosomal proteins with 16S- and 23S-like rRNA molecules may form the apiRibosome of B. microti. No 5S ribosomal RNA-encoding rff gene could be found in the B. microti apicoplast genome. This finding suggests that the apicoplast ribosomes of B. microti are independent of 5S rRNA or that the apicoplast can either import 5S RNA from the cytoplasm, as was previously shown for mammalian mitochondria [25], [26], or expresses a gene with a sequence highly divergent from known rff genes. Noteworthy, whereas the chloroplast genome of Chromera expresses an rff gene, no rff genes were found in the apicoplast genomes of all apicomplexan parasites sequenced to date.
Annotation of the apicoplast genome of B. microti revealed 5 hypothetical coding sequences (hypA–E). The encoded proteins do not share significant homology with any protein in available databases and do not contain any recognizable functional domains. Examination of the apicoplast genomes of other apicomplexan parasites shows the presence of unknown but dissimilar proteins in the same genomic regions. Whether these CDSs are expressed or are an artifact of annotation remains to be determined.
B. microti carries the minimal apicoplast genome of apicomplexa
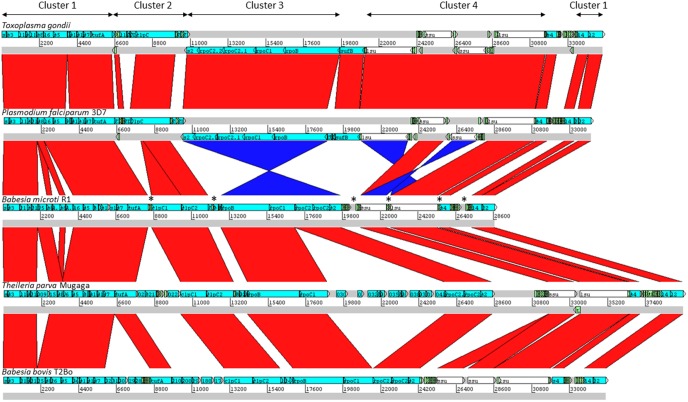
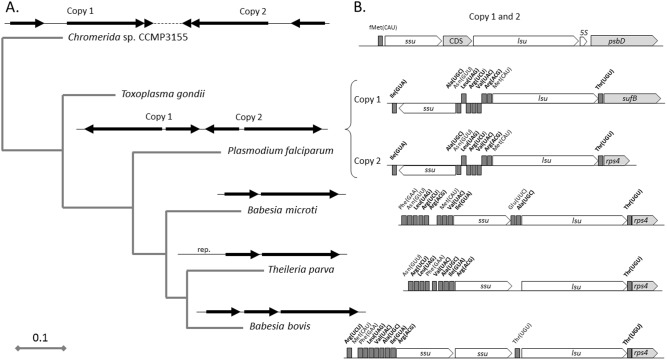
Four gene clusters in the B. microti apicoplast genome were found to be in a synteny with those found in other apicomplexan parasites (Fig. 2 and Figures S1 and S2 in File S1) as well as the chloroplast genomes of Chromera algae [27]. Cluster 1 includes genes encoding ribosomal proteins and the EF-Tu elongation factor (Fig. 3). Similar to the gene organization found in Theileria parva, Toxoplasma gondii and Babesia bovis, Cluster 1 of the apicoplast genome of B. microti lacks the rpl23 gene. This gene is present in Chromera and Plasmodium species between rpl2 and rpl4 genes but was lost during the evolution of the apicoplast in most apicomplexa. Two copies of the rps8-hypA genes encoding S8 ribosomal protein and a hypothetic protein (Hyp-A) are found in the Cluster, whereas no rps13-like gene could be found in this genome. In Chromera sp., rps13 is located between rps5 and rpl36, whereas in most apicomplexan parasites, this region contains a gene with unknown function or lacks a CDS as in the case in T. gondii (Fig. 3).
Figure 2. Schematic representation of gene clusters in the apicoplast genomes of various apicomplexan parasites.
Comparison was performed using Mauve and BLAST analyses. The red and blue bars between chromosomal DNA sequences represent highly conserved regions in the forward and reverse directions respectively. Only highly conserved and syntenic regions were included in the present analysis. tRNA genes are marked by *.
Figure 3. Gene organization of cluster 1 in the apicoplast genomes of B. microti (R1), B. bovis (T2Bo), P. falciparum (3D7), T. gondii and T. parva (Mugaga) and the chloroplast genome of Chromera sp. (CCMP3155).
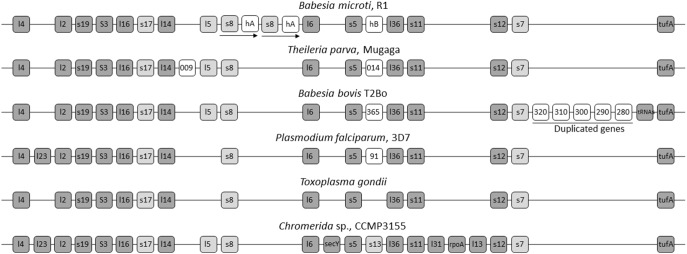
Light grey boxes represent highly divergent genes. White boxes corresponds to genes restricted to one species.
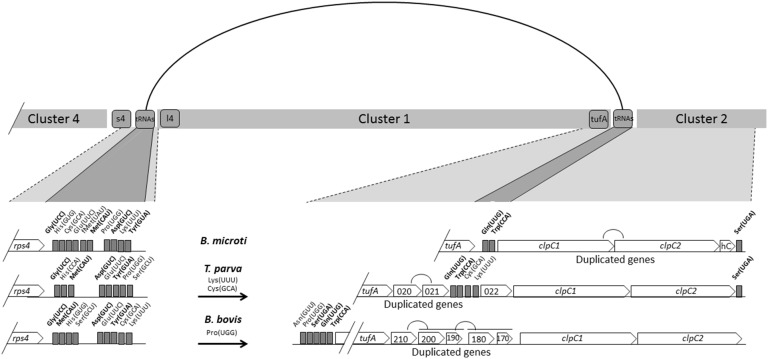
Cluster 1 of the B. microti apicoplast genome is surrounded by 10 tRNA genes on the side adjacent to Cluster 4 and 2 tRNA genes for Gln(UUG) and Trp(CCA) on the side adjacent to Cluster 2 (Fig. 4). Interestingly, in T. parva the junction between Cluster 1 and Cluster 2 contains two more tRNA genes for Lys(UUU) and Cys(GCA), whereas in B. bovis this region lacks tRNA regions. Unlike B. microti, the junction between Cluster 1 and Cluster 2 in T. parva and B. bovis contains several putative CDSs of unknown function some of which are identical copies of the same CDS (Tp020 and Tp021 in T. parva) and (Bb210 and Bb200, and cluster Bb200–Bb190 and Bb180–Bb170). The duplicated CDSs found in T. parva do not share homology with those found in B. bovis (Fig. 4). Altogether, these data suggest that the region between Clusters 1 and 2 might be a hot spot of recombination, and that major recombination events involving of regions adjacent to Cluster 1 may have taken place during the evolution of piroplasmida (Fig. 4). Furthermore, the lack of duplicated genes in B microti may account for the differences we see in size between these organisms.
Figure 4. Schematic representation of the DNA regions surrounding cluster 1 in B. microti R1, B. bovis T2Bo and T. parva Mugaga.
A line connecting the two ends of Cluster 1 indicate possible recombination events accounting for differences found in the gene organization and size between apicoplast genomes of piroplasmida.
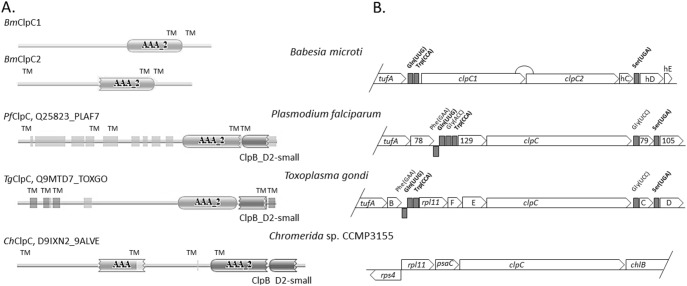
Cluster 2 of the B. microti apicoplast genome consists primarily of ClpC chaperones (Fig. 5). Similar to B. bovis and T. parva, the ClpC gene of B. microti is duplicated with both copies containing the AAA_2 ATPase domain (Fig. 4 and Fig. S3 in File S1). The region of Cluster 2 adjacent to Cluster 3 contains a Ser(UGA) tRNA and three hypothetical proteins (Hyp-C, Hyp-D and Hyp-E) (Fig. 1). The position of the Ser(UGA) tRNA is conserved in other apicomplexan parasites including T. parva (Fig. 4), P. falciparum and T. gondii (Fig. 5).
Figure 5. Domain structure and organization of Cluster 2 genes in B. microti and other apicomplexan parasites.
A. Comparison of ClpC domain structure between B. microti, P. falciparum, T. gondii and Chromera sp. B. microti apicoplast genome encodes two ClpC proteins that lack the N-terminus part as revealed by Pfam and TMpred predictions. Other apicomplexan ClpC structures have been obtained from Pfam database using UNIPROT accession numbers. Two PfamA domains are found in ClpC proteins of apicomplexa: AAA_2 (ATPase catalytic function) and ClpB_D2-small (conserved C-terminal domain). Light grey boxes indicate regions of low complexity. Transmembrane domains were predicted by Pfam only for T. gondii ClpC proteins (TM). B. Gene organization of cluster 2. The tRNA genes of cluster 2, which are conserved in all three apicoplast genomes are in bold. Three putative genes, C, D and E present at 3′ end of B. microti cluster 2 have no significant homologies with each other and lack homologs in other parasites.
Similar to B. bovis, P. falciparum and T. parva no rpl11 ribosomal gene was found in the B. microti apicoplast genome (Fig. 2 & 5). This finding suggests that either the ribosomes of these parasites do not require the L11 protein or that protein translation in the apicoplast of these parasites involves an rpl11-like gene radically divergent from that found in T. gondii, and prokaryotes and located on a different site in the apicoplast genome or possibly encoded by the nuclear genome. Because of the conserved gene order rpl11-clpC in Chromera, and T. gondii, the loss of the rpl11 gene in the apicoplast genomes of parasites within the Class Aconoidasida (which includes Haemosporida and Piroplasmida) might be linked to the rearrangement of the tRNA region adjacent to the clpC gene.
Cluster 3 of the B. microti apicoplast genome includes the “RNApol cluster” and contains in addition to the RNA polymerase genes (rpoB, rpoC1, rpoC2.1 and rpoC2.2), the gene encoding the S2 ribosomal protein, rps2 (Fig. 1 and 2). In B. microti as well as other apicomplexa, the alpha subunit of RNA polymerase (rpoA) gene is encoded by the nuclear genome, whereas in algae chloroplast genomes the gene encoding is present on cluster 1 (Fig. 3). Orientation of Cluster 3 genes in B. microti, T. parva and B. bovis is opposite to that found in P. falciparum and T. gondii, suggesting an inversion event that took place early during the evolution of piroplasmida (Fig. 2). Such an event might be responsible for the loss of the sufB gene in piroplasmida.
Cluster 4 of the apicoplast genome of B. microti includes rDNA genes. This region consists of a single set of ssu and lsu genes, which are transcribed in the same orientation (Fig. 6). In Chromera sp., T. gondi and P. falciparum apicoplast genomes, this cluster consists of two sets of ssu and lsu genes in opposite orientation (Fig. 6). Gene content and gene order in this cluster differ between species. In Chromera, the ssu and lsu genes are in the same orientation and separated by a CDS; in Toxoplasma and Plasmodium the ssu and lsu genes are in opposite orientation; and in B. bovis, 2 ssu genes are located upstream of the lsu gene and all three genes are transcribed in the same orientation. Unlike B. microti and T. parva, a second Thr(UGU) tRNA exists between the ssu and lsu genes in B. bovis (Fig. 6). This gene organization is likely the result of duplication events that occurred in the rDNA region during the evolution of B. bovis.
Figure 6. Organization and evolution of the rDNA region in the apicoplast genome of B. microti and other apicomplexa.
A. Phylogenetic analysis based on ssu and lsu genes. The tree was obtained using the maximum-likelihood method with (Bootstrap over 90%). Genomic organization of rDNA regions in the apicoplast or chloroplast genomes is given on top of each branch. B. Gene organization of the rDNA regions. The tRNA genes that are present in all apicomplexan genomes are shown in bold. Scale bar represents the number of substitutions per site.
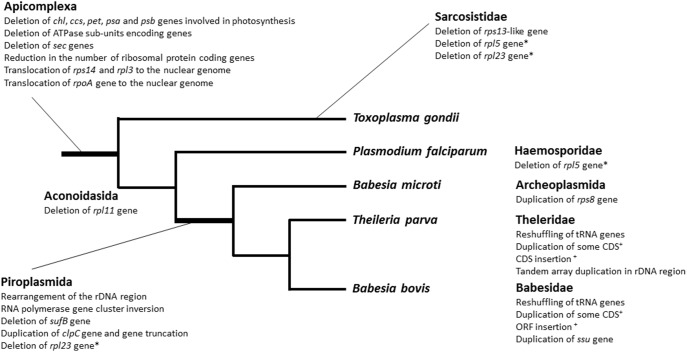
Comparison of different apicoplast genomes shows that major rearrangements took place during the various stages of apicoplast evolution (Fig. 7). While the loss of genes involved in photosynthesis represent a major early event in the evolution of the apicoplast, deletion of sufB, inversion of the RNApol region, reorganization of the rDNA region and duplication of the clpC gene represent important events that occurred during the early evolution of piroplasma. B. microti apicoplast genome carries these modifications but shows no DNA expansion (duplication of small regions) as is the case in B. bovis and T. parva. This suggests that B. microti may harbor the core apicoplast genome organization of apicomplexa.
Figure 7. Summary of the evolution of the organization of the apicoplast genome in apicomplexan parasites.
The unweighted tree was built using raw data from Figure S2 in File S1. The branch supporting the clade piroplasmida is associated with several major genomic rearrangements. *: events that occurred twice in the apicoplast evolution; +: rearrangement, duplication and insertion events observed in B bovis and T. parva involving distinct genes.
Conclusion
We have completed the sequencing and assembly of the apicoplast genome of B. microti. Our studies revealed that this 28.7 kb circular genome encodes a simple machinery devoted primarily to the transcription and translation events occurring within this organelle. The genome lacks genes associated with metabolic functions but harbors five genes encoding small hypothetical proteins whose function remains unknown. Our analysis of the apicoplast genome of B. microti complements our prior annotation of the nuclear genome of this pathogen, which identified several genes encoding structural and regulatory proteins and enzymes harboring an apicoplast targeting motif [11]. These nuclear encoded proteins are predicted to control important metabolic functions during the parasite life cycle in mammalian red blood cells and the tick vector. The potent activity of drugs such as azithromycin and clindamycin [28] against B. microti indicates that the apicoplast plays an essential role during the parasite intraerythrocytic life cycle. Targeting the apicoplast- and nuclear-encoded functions important for apicoplast maintenance and replication may help identify and design novel, potent and safer therapies for the treatment of human babesiosis.
Supporting Information
Figure S1: Multiple alignment of apicoplast genomes from B. microti and other apicomplexan parasites using Mauve. Apicoplast genomes are laid out horizontally with colored blocks representing homologous regions. Gene synteny and BLAST analysis were used to define gene clusters. Figure S2: Major genomic rearrangements revealed by comparing apicoplast genomes of various apicomplexan parasites. Red and blue areas represent conserved syntenic regions in the forward and reverse directions, respectively. Figure S3: Domain structure of ClpC proteins encoded by the apicoplast genome of piroplasmida. The apicoplast genomes of the Babesia bovis T2Bo, Babesia microti R1 and Theileria parva Mugaga encode two ClpC proteins containing a conserved ATPase domain (PfamA AAA_2 domain) and several transmembrane domains predicted using TMPred (TM). The figure was generated using Pfam server either using UNIPROT accession number or by direct submission of the amino acid sequence. Regions of low complexity are represented in blue. PfamB domains are represented by horizontal lines. Table S1: Primers used to assemble and sequence the B. microti apicoplast genome. Coordinates are given according to the sequence available at accession number LK028575. Primer orientation: w for Watson strand and c for Crick strand.
(PDF)
Acknowledgments
We thank Drs Stephane Delbecq and Amina Dassouli for providing genomic DNA of the R1 isolate.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files. The complete sequence of the apicoplast genome was deposited in the European Nucleotide Archive with Accession Number LK028575 and will be automatically available in GenBank and DNA Data Bank of Japan.
Funding Statement
This work was partly supported by the National Institutes of Health [AI097218 to CBM], and the Bill and Melinda Gates Foundation (Awards OPP1069779 and OPP1086229 to CBM), the ANR program Investissement d’avenir (ANR-11-BINF-0002 to EC) and the French ministry of research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Vannier E, Krause PJ (2012) Human babesiosis. N Engl J Med 366: 2397–2407. [DOI] [PubMed] [Google Scholar]
- 2. Kjemtrup AM, Conrad PA (2000) Human babesiosis: an emerging tick-borne disease. International Journal for Parasitology 30: 1323–1337. [DOI] [PubMed] [Google Scholar]
- 3. Vannier E, Gewurz BE, Krause PJ (2008) Human babesiosis. Infectious Disease Clinics of North America 22: 469–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krause PJ, Spielman A, Telford SR III, Sikand VK, McKay K, et al. (1998) Persistent parasitemia after acute babesiosis. N Engl J Med 339: 160–165. [DOI] [PubMed] [Google Scholar]
- 5. Institute of Medicine of the National Academies (2011) Committee on lyme disease and other tick-borne diseases. Critical needs and gaps in understanding. Prevention, amelioration, and resolution of lyme and other tick-borne diseases. The Short-term and long-term outcomes. The National Academies Press. Washington, D. C: 2011 Available: http://www.nap.edu. [PubMed] [Google Scholar]
- 6.CDC (2011) Nationally Notifiable Infectious Conditions, United States 2011, Babesiosis.
- 7. Leiby DA (2011) Transfusion-transmitted Babesia spp: bull's-eye on Babesia microti. Clin Microbiol Rev 24: 14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Young C, Chawla A, Berardi V, Padbury J, Skowron G, et al. (2012) Preventing transfusion-transmitted babesiosis: preliminary experience of the first laboratory-based blood donor screening program. Transfusion 52: 1523–1529. [DOI] [PubMed] [Google Scholar]
- 9. Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, et al. (2011) Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 155: 509–519. [DOI] [PubMed] [Google Scholar]
- 10. Krause PJ, Lepore T, Sikand VK, Gadbaw J Jr, Burke G, et al. (2000) Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 343: 1454–1458. [DOI] [PubMed] [Google Scholar]
- 11. Cornillot E, Hadj-Kaddour K, Dassouli A, Noel B, Ranwez V, et al. (2012) Sequencing of the smallest Apicomplexan genome from the human pathogen Babesia microti. Nucleic Acids Res 40: 9102–9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cornillot E, Dassouli A, Garg A, Pachikara N, Randazzo S, et al. (2013) Whole genome mapping and re-organization of the nuclear and mitochondrial genomes of Babesia microti isolates. PLoS One 8: e72657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ralph SA, van Dooren GG, Waller RF, Crawford MJ, Fraunholz MJ, et al. (2004) Tropical infectious diseases: metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat Rev Microbiol 2: 203–216. [DOI] [PubMed] [Google Scholar]
- 14. Fleige T, Limenitakis J, Soldati-Favre D (2010) Apicoplast: keep it or leave it. Microbes Infect 12: 253–262. [DOI] [PubMed] [Google Scholar]
- 15. van Dooren GG, Striepen B (2013) The algal past and parasite present of the apicoplast. Annu Rev Microbiol 67: 271–289. [DOI] [PubMed] [Google Scholar]
- 16. Hikosaka K, Tsuji N, Watanabe Y, Kishine H, Horii T, et al. (2012) Novel type of linear mitochondrial genomes with dual flip-flop inversion system in apicomplexan parasites, Babesia microti and Babesia rodhaini. BMC Genomics 13: 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, et al. (2000) Artemis: sequence visualization and annotation. Bioinformatics 16: 944–945. [DOI] [PubMed] [Google Scholar]
- 18. Schattner P, Brooks AN, Lowe TM (2005) The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res 33: W686–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stothard P, Wishart DS (2005) Circular genome visualization and exploration using CGView. Bioinformatics 21: 537–539. [DOI] [PubMed] [Google Scholar]
- 20. Darling AE, Mau B, Perna NT (2010) progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5: e11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carver TJ, Rutherford KM, Berriman M, Rajandream MA, Barrell BG, et al. (2005) ACT: the Artemis Comparison Tool. Bioinformatics 21: 3422–3423. [DOI] [PubMed] [Google Scholar]
- 22. Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, et al. (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36: W465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, et al. (2012) The Pfam protein families database. Nucleic Acids Res 40: D290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hofmann K, Stoffel W (1993) TMbase - A database of membrane spanning proteins segments. Biol Chem 374: 166. [Google Scholar]
- 25. Yoshionari S, Koike T, Yokogawa T, Nishikawa K, Ueda T, et al. (1994) Existence of nuclear-encoded 5S-rRNA in bovine mitochondria. FEBS Lett 338: 137–142. [DOI] [PubMed] [Google Scholar]
- 26. Magalhaes PJ, Andreu AL, Schon EA (1998) Evidence for the presence of 5S rRNA in mammalian mitochondria. Mol Biol Cell 9: 2375–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Janouskovec J, Horak A, Obornik M, Lukes J, Keeling PJ (2010) A common red algal origin of the apicomplexan, dinoflagellate, and heterokont plastids. Proc Natl Acad Sci U S A 107: 10949–10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aboulaila M, Munkhjargal T, Sivakumar T, Ueno A, Nakano Y, et al. (2012) Apicoplast-targeting antibacterials inhibit the growth of Babesia parasites. Antimicrob Agents Chemother 56: 3196–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Multiple alignment of apicoplast genomes from B. microti and other apicomplexan parasites using Mauve. Apicoplast genomes are laid out horizontally with colored blocks representing homologous regions. Gene synteny and BLAST analysis were used to define gene clusters. Figure S2: Major genomic rearrangements revealed by comparing apicoplast genomes of various apicomplexan parasites. Red and blue areas represent conserved syntenic regions in the forward and reverse directions, respectively. Figure S3: Domain structure of ClpC proteins encoded by the apicoplast genome of piroplasmida. The apicoplast genomes of the Babesia bovis T2Bo, Babesia microti R1 and Theileria parva Mugaga encode two ClpC proteins containing a conserved ATPase domain (PfamA AAA_2 domain) and several transmembrane domains predicted using TMPred (TM). The figure was generated using Pfam server either using UNIPROT accession number or by direct submission of the amino acid sequence. Regions of low complexity are represented in blue. PfamB domains are represented by horizontal lines. Table S1: Primers used to assemble and sequence the B. microti apicoplast genome. Coordinates are given according to the sequence available at accession number LK028575. Primer orientation: w for Watson strand and c for Crick strand.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files. The complete sequence of the apicoplast genome was deposited in the European Nucleotide Archive with Accession Number LK028575 and will be automatically available in GenBank and DNA Data Bank of Japan.