Abstract
In humans, infection with Coxiella burnetii, the causative agent of Q fever, leads to acute or chronic infection, both associated with specific clinical symptoms. In contrast, no symptoms are observed in goats during C. burnetii infection, although infection of the placenta eventually leads to premature delivery, stillbirth and abortion. It is unknown whether these differences in clinical outcome are due to the early immune responses of the goats. Therefore, peripheral blood mononuclear cells (PBMCs) were isolated from pregnant goats. In total, 17 goats were included in the study. Six goats remained naive, while eleven goats were infected with C. burnetii. Toll-like receptor (TLR) and cytokine mRNA expression were measured after in vitro stimulation with heat-killed C. burnetii at different time points (prior infection, day 7, 35 and 56 after infection). In naive goats an increased expression of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-10 and interferon (IFN)-γ mRNA upon C. burnetii stimulation was detected. In addition, TLR2 expression was strongly up-regulated. In goats infected with C. burnetii, PBMCs re-stimulated in vitro with C. burnetii, expressed significantly more TNF-α mRNA and IFN-γ mRNA compared to naive goats. In contrast, IL-10 mRNA production capacity was down-regulated during C. burnetii infection. Interestingly, at day 7 after inoculation a decreased IFN-γ protein level was observed in stimulated leukocytes in whole blood from infected goats, whereas at other time-points increased production of IFN-γ protein was seen. Our study shows that goats initiate a robust pro-inflammatory immune response against C. burnetii in vitro. Furthermore, PBMCs from C. burnetii infected goats show augmented pro-inflammatory cytokine responses compared to PBMCs from non-infected goats. However, despite this pro-inflammatory response, goats are not capable of clearing the C. burnetii infection.
Introduction
Q fever is a zoonosis caused by the intracellular Gram-negative bacterium Coxiella burnetii. In humans, infection may remain unnoticed in approximately half of the infected individuals and causes disease, a short flu-like acute febrile disease, pneumonia or hepatitis, in the other half [1]. After infection, either unrecognized or symptomatic, 1 to 5% may develop chronic Q fever months to years later, which presents primarily as endocarditis or vascular infection and may be lethal when left untreated [1]. Infected goats, sheep and cattle are the main reservoir for human infections, these animals can excrete high numbers of C. burnetii into the environment during parturition [2], [3].
In contrast to human infections, infection in goats by C. burnetii does not lead to recognizable systemic disease in non-pregnant animals, while in pregnant animals infection will lead only to abortion, stillbirth or premature delivery explained by the high bacterial numbers in the placenta and severe local inflammation [4]. Although clinical symptoms are absent in goats, two weeks after infection a clear specific humoral immune response occurs with the appearance of IgM and IgG anti-Phase 2 C. burnetii antibodies [3]. So far little is known about the early immune response in goats and the differences in the immune response to C. burnetii in man and goat.
Toll-like receptors (TLRs) are assumed to be the principal receptors involved in the early recognition and signalling of C. burnetii by innate immune cells. Upon recognition, peripheral blood mononuclear cells (PBMCs) derived from healthy humans produce various cytokines including interleukin-1beta (IL-1β), tumour necrosis factor-alpha (TNF-α) and interleukin-10 (IL-10), but no interferon gamma (IFN-γ) [5]–[7]. After infection, stimulation of human PBMCs with C. burnetii also leads to up-regulation of IL-1β [6], [8]–[10]. In patients with chronic Q-fever, the anti-inflammatory cytokine IL-10 is up-regulated [7], [11].
It is generally assumed that the type and degree of the early innate immune response relates to the severity of early disease manifestations and determines the early outgrowth of the infection [12]. Therefore it may be hypothesized that this response is different in goats which do not develop disease after infection as compared to humans. To test this hypothesis we evaluated the C. burnetii induced TLR and cytokine responses of PBMCs derived from naive and from infected pregnant goats in the last months of pregnancy. Furthermore, Roest et al. recently examined the circulating cytokine levels in naive and C. burnetii infected goats. They observed an absence of a systemic cytokine mRNA response and hypothesized that PBMCs might not have been in contact with C. burnetii during infection. To clarify this we focus on PBMCs and their ability to induce an immune response against heat-killed C. burnetii.
Material and Methods
Animals, Infection and Experimental Design
Seventeen pregnant healthy Alpine yearling goats were included in the study. The experimental setup has been published in detail by Roest et al. [3]. In short, on day 76 of the pregnancy eleven goats were intranasally inoculated with 1 ml of 1×10∧6/ml C. burnetii X09003262-001 (C. burnetii 3262) in culture medium. As a negative control,six goats were inoculated with 1 ml of culture medium only. Serum samples were taken weekly to measure antibody titers against C. burnetii (LSIVET RUMINANT milk/serum Q-fever ELISA kit, LSI, France). EDTA and heparin blood samples for stimulation experiments were taken from each goat; at day 0 (before inoculation) and at day 7, 35 and 56. To prove successful C. burnetii infection of the infected goats, the placenta was sampled after parturition from each goat for immunohistochemistry (IHC) and vaginal mucus was sampled for detection of C. burnetii DNA by PCR.
Ethics Statement
All animal experiments were approved by the Animal Experiment Commission of the Central Veterinary Institute, part of Wageningen UR, in accordance with the Dutch regulations on animal experimentation (registration number 2011111.c). Everything possible was done to minimise animal suffering. Humane endpoints were defined in advance. Whenever these endpoints were reached, animals were euthanized.
Coxiella burnetii strains
C. burnetii 3262 was used as infectious organism. This strain was isolated from the placenta from a goat that aborted on a farm during the Q fever outbreak in the Netherlands [13]. The strain was genotyped as CbNL01, which was the main C. burnetii genotype during the Dutch Q fever outbreak [13]. Ex vivo stimulation experiments were performed with the heat-killed C. burnetii Nine Mile (NM) reference strain. Both C. burnetii NM and C. burnetii 3262 were cultured at the Central Veterinary Institute as described previously [3]. The strains represent LPS phase I. LPS phase determination was performed by SDS-PAGE and silver staining, using purified phase I (RSA493) and phase II (RSA439) C. burnetii NM LPS (kindly provided by R. Toman) as controls [14], [15]. The number of Coxiella DNA copies was determined using Taqman (Quanta BioSciences, USA) real-time PCR as mentioned earlier [4]. MLVA typing was performed by using 12 of the 17 loci described by Arricau-Bouvery et al. [16]. A second series of stimulation experiments was simultaneously performed also with three heat-killed isolates, C. burnetii 14160-001, C. burnetii 3345937 and C. burnetii 3262. Details of all strains are mentioned in Table 1. Bacteria were killed by heating for 30 minutes at 99°C.
Table 1. List of C. burnetii isolates used in this study.
| Name | Country of origin | Source | LPS Phase | MLVA marker and number of repeats | |||||||||||
| 01 | 03 | 20 | 21 | 22 | 28 | 24 | 30 | 31 | 34 | 27 | 36 | ||||
| C. burnetii 3262 | The Netherlands | Goat placenta | I | 4 | 7 | 19 | 6 | 6 | 3 | 11 | 5 | 3 | 7 | 3 | 13 |
| C. burnetii 3345937 | The Netherlands | Human heart valve | I | 4 | 7 | 19 | 6 | 6 | 3 | 11 | 5 | 3 | 7 | 3 | 13 |
| C. burnetii 14160-001 | The Netherlands | Goat placenta | I | 3 | 6 | 15 | 6 | 6 | 7 | 13 | 6 | 3 | 9 | 2 | 4 |
| C. burnetii Nine Mile | USA | Tick | I | 4 | 7 | 15 | 6 | 6 | 6 | 27 | 6 | 5 | 5 | 4 | 4 |
Isolation and stimulation of peripheral blood mononuclear cells
The PBMC fraction was obtained by density centrifugation of EDTA blood using a Ficoll-Paque gradient (Pharmacia Biotech, USA) or Leucosep tubes prefilled with Ficoll-Paque (Greiner, the Netherlands). After washing, the cells were re-suspended in RPMI 1640 Dutch modified (Gibco Invitrogen, USA) supplemented with 50 mg/L gentamycin (Centrafarm, the Netherlands), 2 mM L-glutamine, and 1 mM pyruvate (Gibco Invitrogen, USA). A volume of 100 µl containing 5×105 mononuclear cells was added to round-bottom 96-well plates (Costar, Corning, The Netherlands) and incubated at 37°C and 5% CO2 for both 4 h and 24 h with either 50 µl RPMI (medium control), C. burnetii NM 1×107/ml, C. burnetii 3262 1×106/ml, C. burnetii 3345937 3.2×104/ml, C. burnetii 14160-001 1×107/ml and E. coli LPS 10 ng/ml in the presence of 50 µl 5% naive goat serum (in house). Following incubation, the supernatant was harvested and stored at −20°C. Subsequently Trizol (Invitrogen, USA) was added to cell pellet and stored at −80°C till further RNA isolation.
mRNA expression of Toll-like receptors and cytokines
RNA was isolated from the Trizol samples using the manufacturer's guidelines. In the end, the pellet was re-suspended in 25 µl RNAse free water (Sigma–Aldrich, USA) with 0.5 µl RNAseOUT Recombinant ribonuclease Inhibitor (Invitrogen, USA). Further purification of the samples was performed with the DNA-free kit (Ambion, USA) and the concentration of the RNA samples was determined using the Nanodrop (ND1000, NanoDrop Technologies, Inc, USA). Reverse transcription was performed using random primers and SuperScript III Reverse Transcriptase (Invitrogen, USA). PCR on the cytokines mRNA (TNF-α, IL-1β, IL-10 and IFN-γ) was performed using specific goat primers [3] and SYBR Green PCR Master Mix (Applied Biosystems, USA) in an ABI 7500 Real-time PCR system (PE Applied Biosystems, USA). Results were normalized according to the Succinate dehydrogenase complex subunit A (SDHA) gene of the same sample (Bos Taurus, Primerdesign Ltd, United Kingdom). Data was not corrected for the medium control, hereby the influences of the pregnancy on the cytokine mRNA expression remain visible. The primer sets for TLR1, TLR2, TLR4 and TLR6 were designed using PrimerExpress (Invitrogen, USA) (Table 2). The Livak method (2∧ΔΔCt) was used to represent the TLR expression data.
Table 2. Primer sequences for TLR1, TLR2, TLR4 and TLR6.
| Name | Primer sequence 5′-3′ Forward/reverse | Accession no. | Annealing Temperature |
| TLR1 | GGGTTGAGTGCCACACAGTTACCCCATAAGTATCTCCTAAGACCAATAAAA | HQ263209.1 | 60°C |
| TLR2 | ACTGGGTGGAGAACCTCATGGTCATTTGCCAGGGACGAAGTC | HQ263214.1 | 60°C |
| TLR4 | TCTGCCTTCACTACAGGGACTTTATGGCTCTTGTGGAAACCTTCCT | HM627213.2 | 60°C |
| TLR6 | TCTCAAGCATTTAGACCTCTCATTCACTGGGTCAAGTTGCCAAATTC | HQ263211.1 | 60°C |
Whole blood stimulation
Undiluted heparin blood (500 µl) was stimulated with either nothing or 25 µl heat-killed C. burnetii NM 1×107/ml. As control, samples were stimulated with concanavalin A (ConA) and/or phytohemagglutinine (PHA). After 24 h incubation at 37°C, the samples were centrifuged and the supernatant was stored at −20°C till further investigation. The production of IFN-γ protein by the leukocytes in the whole blood samples was analysed with the Bovigam IFN-γ test kit for cattle (Prionics, Schileren-Zurich, Switzerland) which was evaluated for goats. ELISA results were obtained as Optical Density determined at 450 nm (OD450) with a 635 nm reference filter (EL 808 Ultra micro plate reader, Bio-tek instruments, USA). Recombinant goat IFN-γ was purchased from Cusabio to invest to proportion between the measured OD values and the IFN-γ concentration. Hereto, a dilution series of IFN-γ was made with a maximum concentration of 25 ng/ml. The IFN-γ concentration and the corresponding OD values are proportional. Because the Bovigam IFN-γ test kit is able to measure goat IFN-γ in a dose-dependent manner, we decided to use this kit in our study.
Statistical analysis
Statistics regarding mRNA expression and cytokine production were performed using GraphPad Prism 5 software (Graphpad). Differences between experimental groups were tested using the Mann-Whitney U test, and differences with a p-value ≤0.05 were considered statistically significant. Samples with values lower or higher than two times the standard deviation were defined as outliers and excluded from the statistical analysis. These outliers are indicated in the figures as an open circle. In case the value of the outlier exceeded the y-axis of the graph, its value is mentioned. The PCR efficiency was determined for all TLRs and SDHA using a standard curve generated with different dilutions of a mixture of the cDNA of all samples.
Results
Experimental C. burnetii infection in goats
The experimental infection of the goats with C. burnetii 3262 was successful in all infected goats as shown by a positive IHC of the cotyledon with adjacent allantochorion of the placenta and by positive PCR of vaginal swabs. All naive goats remained negative in both tests. The IHC pictures of a C. burnetii positive and negative cotyledon can be found in the article of Roest et al.[4] in which the goats from experiment III resemble the goats in our study. Three infected goats gave birth to dead lambs, the lambs of the remaining six infected goats were alive. The offspring of the naive goats were all alive and healthy.
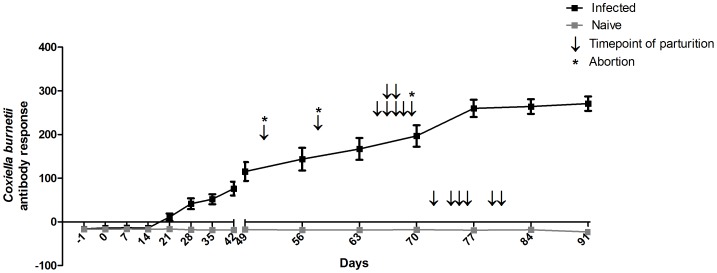
The weekly antibody response of all goats against C. burnetii during the infection period is shown in Figure 1. The antibody response in the infected goats increased strongly 21 days after infection, whereas the antibody response of the naive goats did not alter. The average pregnancy of the infected goats was 143 days (range 128–146 days), significantly shorter (p<0.001) than the 153 days (range 149–157 days) of the naive goats.
Figure 1. Successful experimental infection of goats with Coxiella burnetii 3262.
Eleven out of seventeen goats were intranasally infected with C. burnetii 3262. The remaining six were kept naive. During the infection, antibodies against C. burnetii were measured weekly in the serum of all goats using the LSIVET RUMINANT milk/serum Q-fever ELISA kit. Grey squares represent naive goats, black squares represent infected goats. Median values with SEM are shown. Parturition is indicated for each individual goat using an arrow. Abortion, defined as birth of dead lambs, is indicated with an asterisk. The final two remaining pregnant infected goats were culled for other purposes, and therefore not included in this graph.
TLR up-regulation and cytokine responses in C. burnetii stimulated PBMCs from all goats at day 0
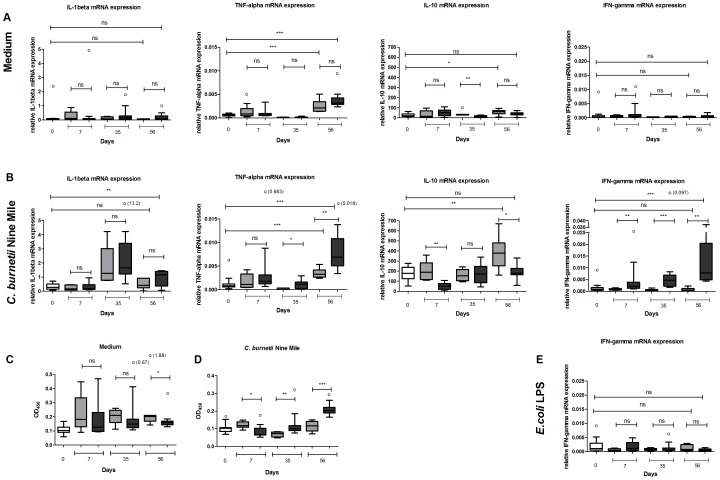
Stimulation of PBMCs from all pregnant goats at day 0 (prior to infection) with C. burnetii NM for 4 hours resulted in a slightly lower mRNA expression of TLR4, the mRNA expression of TLR1 and TLR6 was slightly up-regulated, and the expression of TLR2 mRNA was strongly up-regulated in comparison with unstimulated PBMCs (Figure 2A). In general, a fold induction of factor 2 indicates biological relevance. Despite the significantly increased TLR1, TLR2 and TLR6 mRNA expression after C. burnetii stimulation, the fold induction remained below factor 2. The mRNA expression of IL-1β and IFN-γ was up-regulated after stimulation of PBMCs from naive goats with the positive control E. coli LPS (data not shown), showing the reliability of the experimental approach to measure cytokine mRNA expression in goat PBMCs. Stimulation of goat PBMCs with C. burnetii NM resulted in an increased mRNA expression of IL-1β and the same trend was observed for TNF-α (Figure 2B). In PBMCs stimulated for 24 hours, the expression of the anti-inflammatory cytokine IL-10 strongly increased. Furthermore, the mRNA expression of the T-cell or NK-cell derived cytokine IFN-γ also showed an increase (Figure 2B), although this did not lead to increased IFN-γ protein concentrations in the supernatants of these cells (Figure 2C).
Figure 2. TLR up-regulation and cytokine responses of C. burnetii stimulated PBMCs from naive goats.
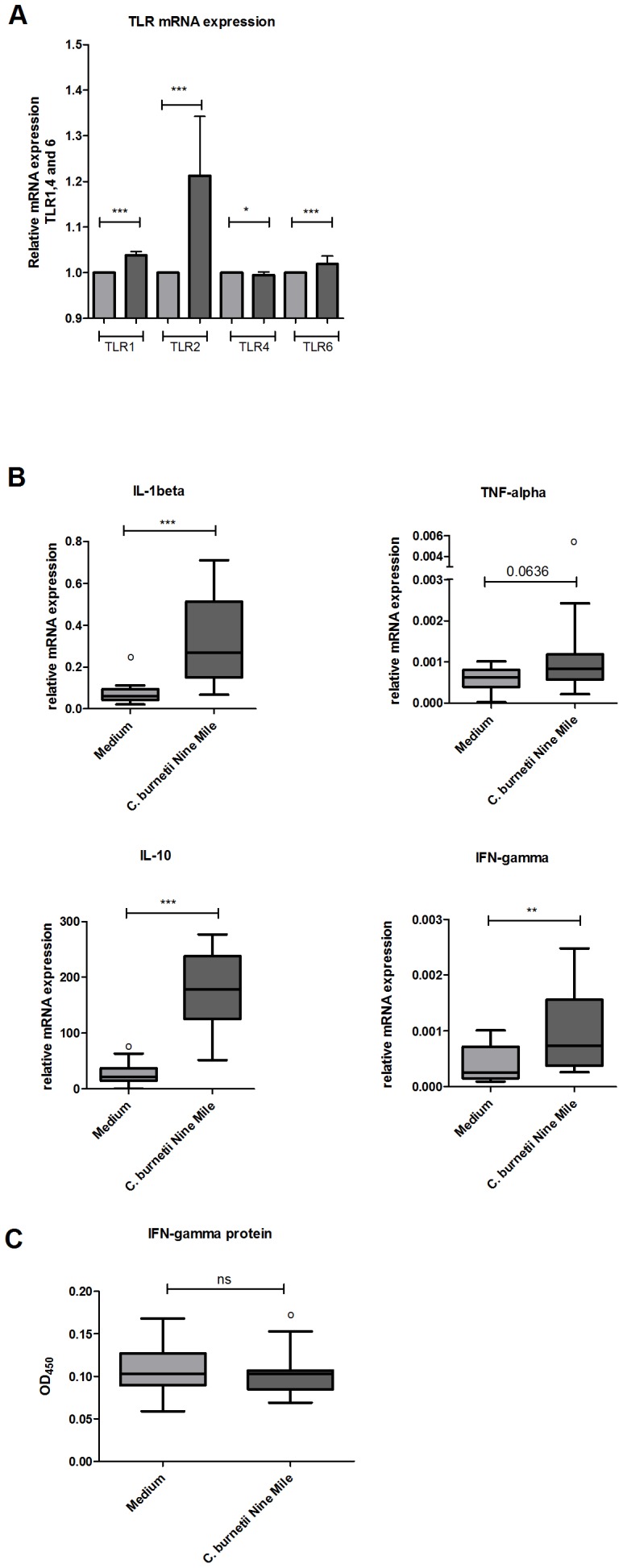
PBMCs were isolated from EDTA blood sampled from 17 goats prior to inoculation. 2A/B: PBMCs were incubated 4 h (TLRs, IL-1β, TNF-α) or 24 h (IL-10, IFN-γ) with either medium (grey bars) or heat killed C. burnetii NM 1×107/ml (dark grey bars). mRNA expression of TLR1, 2, 4, 6 and IL-1β, TNF-α, IL-10 and IFN-γ was determined using qPCR. 2C: IFN-γ protein was measured in the supernatant of PBMCs stimulated for 24 h with nil or C. burnetii NM 1×107/ml. IFN-γ was measured using the Bovigam Elisa kit. Box and Whisker plots are shown. Outliers are indicated as open circles. NS not significant; *p<0.05; **p<0.01; ***p<0.001, Mann-Whitney U-test.
PBMC stimulation experiments from naive and infected pregnant goats during the course of pregnancy
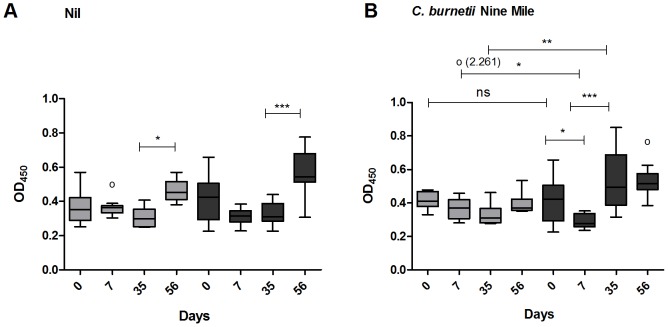
The course of the cytokine mRNA expression of PBMCs was investigated in unstimulated PBMCs, derived from naive and infected goats during the study period of 56 days (Figure 3A). IL-1β mRNA was not up-regulated in PBMCs from both naive and infected pregnant goats. At day 56 of the study TNF-α mRNA expression was increased in PBMCs derived from both naive and infected goats compared to TNF-α mRNA expression at the start of the study. In the infected goats this increase of TNF-α mRNA was significantly higher than in non-infected goats. In contrast, the IL-10 mRNA expression at day 56 was increased in the PBMCs from non-infected goats, while this increase was not observed in the PBMCs of infected goats. Both IFN-γ mRNA and IFN-γ protein levels were not up-regulated in respectively unstimulated PBMCs and unstimulated whole blood from both naive and infected pregnant goats during the study period (Figure 3A and 3C).
Figure 3. Cytokine profile of C. burnetii stimulated PBMCs from naive and C. burnetii infected goats.
3A/B: EDTA blood was sampled at day 7, 35 and 56 of the study from six naive goats (grey bars) and eleven infected goats (dark grey bars). The white bars show the mRNA expression at day zero. PBMCs were isolated and incubated for 4 h or 24 h with either medium (3A), heat killed C. burnetii NM 1×107/ml (3B) or E. coli LPS 10 ng/ml (3E). mRNA expression of TNF-α, IL-1β, IL-10 and IFN-γ was determined using qPCR. 3C/D: IFN-γ protein was measured in the supernatant of PBMCs stimulated for 24 h with nil (3C) or C. burnetii NM 1×107/ml (3D). IFN-γ was measured using the Bovigam Elisa kit. Grey bars represent six naive goats, dark grey bars represent eleven infected goats. Box and Whisker plots are shown. Outliers are indicated as open circles. NS; not significant *p<0.05; **p<0.01; ***p<0.001, Mann-Whitney U-test.
To investigate whether stimulation with C. burnetii resulted in different TLR up-regulation and cytokine responses of PBMCs derived from naive or infected goats we stimulated these cells in vitro with C. burnetii NM. The mRNA expression of TLR1, TLR2, TLR4 and TLR6 of C. burnetii NM stimulated PBMCs derived from naive and infected goats at day 35 equalled the expression as prior infection (data not shown). The expression of IL-1β and TNF-α mRNA of the infected goats was significantly higher at day 56 of the study than at the start of the study (Figure 3B). In contrast, C. burnetii stimulated PBMCs from naive goats did not show an increased IL-1β and IFN-γ mRNA expression. The expression of TNF-α mRNA at day 56 of the study was significantly higher in the infected goats compared to the naive goats. Compared to day zero, IL-10 mRNA expression increased within the 56 days of the study in the naive goats. In contrast, no significant difference in IL-10 mRNA expression between day zero and day 56 was observed in the infected goats. In addition, the expression of IL-10 mRNA was lower in the infected goats compared with the naive goats at day 7. The infected goats expressed more IFN-γ mRNA after PBMC stimulation starting from day 7 onwards (Figure 3B). Furthermore, at day 56 we found that in infected goats IFN-γ protein in the supernatants was higher compared to naive goats (Figure 3D) and unstimulated PBMCs (P<0.0017). At other time points, IFN-γ protein in the C. burnetii stimulated samples did not exceed that of the unstimulated samples, but the IFN-γ protein level was significantly lower in the PBMCs obtained of infected goats compared to PBMCs of naive goats at day 7 and higher at day 35. The increased IFN-γ mRNA expression found in the infected goats at day 35 and 56 after C. burnetii NM stimulation was not observed when PBMCs of these infected goats were stimulated with E. coli LPS (Figure 3E).
Whole blood stimulation experiments from naive and infected pregnant goats during the course of pregnancy
The role of whole blood components on the early immune response against C. burnetii was investigated by stimulation of whole blood from naive and infected goats with nil or C. burnetii NM. In non-stimulated blood from both naive and infected goats, IFN-γ production did not significantly alter at day 7 and day 35 of the study. However, at day 56, IFN-γ concentration increased in both naive as infected goats (Figure 4A). Figure 4B shows that in vitro stimulation of whole blood with C. burnetii NM does not lead to changes in the IFN-γ production in naive goats during the 56 days. In whole blood of the infected goats, the IFN-γ protein level is decreased at day 7 which was not observed in the naive goats. At day 35 of the study, the IFN-γ level is significantly higher in whole blood of infected goats compared to naive goats after stimulation with C. burnetii NM.
Figure 4. Increased Interferon-γ production in C. burnetii infected goats after stimulation with C. burnetii.
Stimulation of undiluted heparin blood with nil (4A) or heat killed C. burnetii NM 1×107/ml (4B). Grey bars represent six naive goats, dark grey bars represent eleven infected goats. Blood was sampled at day zero and at day 7, 35 and 56 of the study. IFN-γ was measured after 24 h incubation using the Bovigam IFN-γ ELISA kit. Box and Whisker plots are shown. Outliers are indicated as open circles. NS; not significant *p<0.05; **p<0.01; ***p<0.001, Mann-Whitney U-test.
In vitro stimulation of PBMCs from naive and infected pregnant goats with different C. burnetii strains
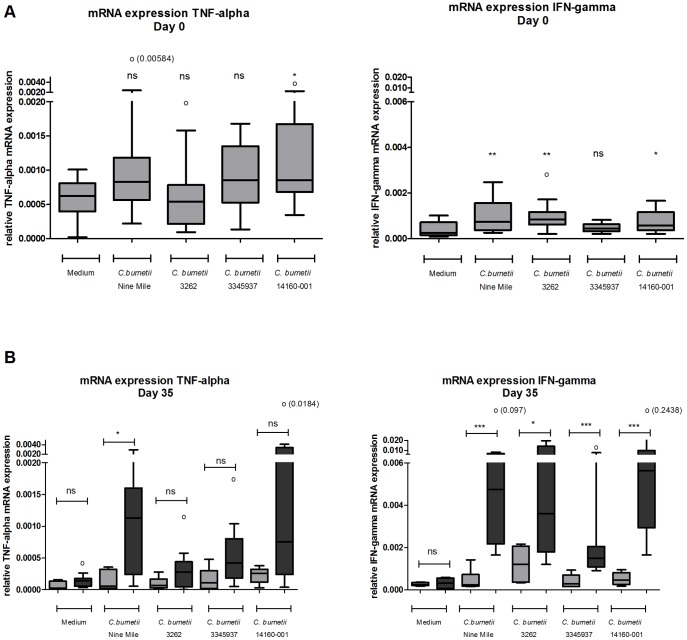
PBMCs from naive and infected goats were stimulated in vitro with four different C. burnetii strains to investigate whether all strains were able to induce up-regulation of cytokine mRNA levels. At day zero, only C. burnetii 14160-001 induced significantly more TNF-α mRNA compared to unstimulated PBMCs. The IFN-γ mRNA levels increased after PBMC stimulation with C. burnetii NM, C. burnetii 3262 and C. burnetii 14160-001. C. burnetii 3345937 stimulation did not induce either TNF-α and IFN-γ mRNA (Figure 5A). At day 35, PBMCs re-stimulated with all strains, except C. burnetii 3262, showed a trend towards an increased TNF-α mRNA expression in PBMCs of infected goats than PBMCs of naive goats. IFN-γ mRNA expression was significantly increased in PBMCs from infected goats after encounter with all four C. burnetii strains compared to naive goats (Figure 5B).
Figure 5. Stimulation of C. burnetii infected PBMCs results in an increased cytokine expression.
EDTA blood was sampled at day zero (5A) and day 35 (5B) of the study. Peripheral blood mononuclear cells were isolated and incubated for 4 h or 24 h with either medium, heat killed C. burnetii NM 1×107/ml, heat killed C. burnetii 3262 1×106/ml, heat killed C. burnetii 3345937 3.2×104/ml, heat killed C. burnetii 14160-001 1×107/ml. mRNA expression of TNF-α and IFN-γ was determined using qPCR. Grey bars represent six naive goats, dark grey bars represent eleven infected goats. Box and Whisker plots are shown. Outliers are indicated as open circles. NS not significant; *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001, Mann-Whitney U-test.
Discussion
PBMCs from pregnant goats have a clear cytokine response upon contact with C. burnetii. This response is observed both in goats without any previous contact with the bacterium as well as in goats who where recently infected. This latter observation is interesting because infections of pregnant goats do not lead to systemic disease of the animal except for stillbirth of the lamb. In PBMCs of healthy pregnant goats we observed that the mRNA expression of IL1-β, TNF-α, IL-10 and IFN-γ were up-regulated after C. burnetii stimulation. These findings correspond with other studies in which increased TNF-α protein levels were found in human monocyte-derived dendritic cells stimulated with the C. burnetii outer membrane protein 1, and THP-1 cells which showed increased TNF-α and IFN-γ expression after C. burnetii stimulation [17], [18]. Recently, Graham et al.[19] demonstrated that stimulation of human alveolar macrophages with C. burnetii skewed towards a Th1 response. Increased TNF-α and IFN-γ protein levels were also found in serum from mice challenged with C. burnetii, and TNF-α mRNA expression increased in mice of which bone marrow derived macrophages were stimulated with C. burnetii [20], [21]. Thus, our results show that PBMCs of goats do not differ from humans and mice in their augmented TNF-α and IFN-γ mRNA response after in vitro stimulation with C. burnetii. During C. burnetii infection increased IFN-γ protein was measured in C. burnetii stimulated whole blood of goats which corresponds with the higher IFN-γ protein levels found in infected mice and chronic Q fever patients [20], [22].
The expression of IL-1β, IL-10 and IFN-γ mRNA was not up-regulated in the PBMCs of pregnant goats who were infected with C. burnetii. The observed up-regulation of TNF-α mRNA expression in PBMCs derived from infected goats may be seen as a consequence of pregnancy because this pattern was also found in PBMCs from naive goats. A possible explanation for the absence of an increased cytokine mRNA expression is that the non re-stimulated PBMCs did not respond to a nasal infection of C. burnetii. One of the reasons can be that in goats the C. burnetii infection is localised specifically in the placenta, and therefore no persistent systemic mRNA up-regulation was observed in the PBMCs from infected goats. Ben Amara et al.[23] indeed found that C. burnetii replicates in vitro in placental derived trophoblasts and induces an up-regulation of cytokine mRNA. Therefore, they assumed that the specific inflammatory environment in the placenta, skewed towards tolerance and anti-inflammatory responses, permits persistence of C. burnetii. This specific compartmentalized infection is also observed in chronic Q fever patients, as in these persons C. burnetii is mainly localised in the heart valve and vessels [1].
At day 56 of our study TNF-α mRNA expression was increased in all goats compared to the initial level, but a significantly higher TNF-α mRNA expression was seen in the infected goats. This suggests that, besides the influence of the pregnancy, infection with C. burnetii also increases expression of TNF-α mRNA. We also showed a clear increase in IL-1β and IFN-γ mRNA expression in the C. burnetii NM stimulated PBMCs derived from infected goats from day zero till day 56 of the study. However, at day 56, only IFN-γ mRNA expression was significantly higher in the PBMCs from infected goats. The fold induction (C. burnetii stimulated PBMCs divided by unstimulated PBMCs) of naive and infected goats at day 56 was respectively 1.96 and 24.21, indicating the large effect on IFN-γ mRNA expression of C. burnetii stimulated PBMCs from infected goats.
The fold induction at day 56 in C. burnetii stimulated PBMCs from naive and goats was respectively 1.96 and 24.21. Besides the observed mRNA up-regulation, IFN-γ protein levels were increased in C. burnetii stimulated PBMCs and whole blood from infected goats compared to naive goats. Thus although no systemic response in goats could be observed, PBMCs of C. burnetii infected goats respond differentially to C. burnetii stimulation compared to PBMC of uninfected goats. Stimulation with E. coli LPS did not lead to increased up-regulation of IFN-γ mRNA expression in the C. burnetii infected goats, which demonstrates that the observed IFN-γ response is specific for C. burnetii. Even though we hypothesized that C. burnetii infected PBMCs are translocated to the placenta, we assume that remaining memory T-cells in the peripheral blood can be activated and play a role in this immune response and increased IFN-γ mRNA expression.
The importance of TNF-α and IFN-γ during C. burnetii infection has been shown by Andoh et al. [24]. These authors demonstrated that IFN-γ−/− and TNF-α−/− mice have respectively high and modest susceptibility to C. burnetii NM infection and that the disease progressed rapidly in these mice [24]. In humans, much research has been performed on the cytokine expression in chronic Q fever patients, a condition that in our opinion mostly resembles the C. burnetii re-stimulation of PBMCs from infected goats. TNF-α mRNA and TNF-α protein levels are increased in C. burnetii re-stimulated monocytes of chronic Q fever patients compared to healthy controls [9]. Similarly, blood cells of chronic Q fever patients respond to C. burnetii stimulation with higher IFN-γ production [10], [22].
The observed up-regulation of IFN-γ mRNA expression after C. burnetii NM stimulation was also found for the inoculum strain C. burnetii 3262 and the other C. burnetii strains which indicates that the different C. burnetii strains contain corresponding antigens on their surfaces which are recognized by goat PBMCs. Similar cross reactivity was demonstrated by Arricau-Bouvery et al. in a vaccination study in goats [25]. Based on these results we decided to perform our stimulation experiments with C. burnetii Nine Mile. The concentrations of the different C. burnetii strains used in this experiment were not identical, due to time restrictions and culture difficulties. Therefore, it is not possible to compare the strains and the cytokine mRNA expression they induce. Further studies have to be performed to investigate the possible divers immune response against C. burnetii strains by goats.
PBMCs derived from goats infected with C. burnetii expressed significantly less or equal amounts of IL-10 mRNA compared to the PBMCs of non-infected goats. We did not expect this, as in humans high IL-10 protein levels are related to chronic Q fever infection, and in acute Q fever patients a slight increase of IL-10 production was observed compared to healthy controls [7], [11]. The lower expression of IL-10 mRNA in the infected goats can be explained by the higher levels of IFN-γ mRNA and IFN-γ protein, as these two cytokines have an antagonistic effect on each other. Our results indicate that PBMCs from infected goats actually did interact with C. burnetii, leading to an augmented cytokine mRNA expression after a second C. burnetii encounter. The amount of IL-10 mRNA exceeded the mRNA levels of the housekeeping gene much more (up to 400 fold) than for IL-β and IFN-γ. However, the fold induction of IL-10 (C. burnetii stimulated PBMCs divided by unstimulated PBMCs) did not differ from the IL-1β fold induction or was even lower in the infected goats at day 35 and 56 when compared with IFN-γ. For example, at day 56, the fold induction of IL-1β, IL-10 and IFN-γ in the infected goats was respectively 5.89, 5.04 and 24.21.
We can speculate that regarding the pro-inflammatory cytokines, TNF-α and IFN-γ, PBMCs of infected goats re-stimulated with C. burnetii show the same cytokine profile as chronic Q fever patients, while the IL-10 mRNA expression in infected goats is not up-regulated as found in the chronic Q fever patients. These differences in IL-10 expression could be an explanation for the divergent clinical outcomes in humans and goats during C. burnetii infection. Yet, is should be taken in to account that, in contrast with the human study subjects, the infected goats are pregnant which can influence the cytokine responses in general and upon encounter of micro-organisms. Furthermore, possible differences in experimental approaches should be considered, i.e. our conclusions are based on mRNA expression levels, while other studies rely on protein levels in particular.
An interesting finding is the decreased IFN-γ protein level in C. burnetii stimulated PBMCs and whole blood from infected goats at day 7 of the study. This reduction was not observed in the PBMCs and whole blood of naive goats and is inconsistent with the increased IFN-γ protein levels observed in the PBMCs obtained from infected goats later in the infection. It can be hypothesized that the initial C. burnetii infection in the goats is capable to immune modulate PBMCs to produce less IFN-γ. Other microorganism infections, like Legionella pneumophila, Leishmania and Mycobacterium tuberculosis are also capable of modulating the Th1 response during early phases of infection [26]–[28]. Modulation of the IFN-γ response early during the infection, could create a favorable situation for C. burnetii to escape the early immune response of the goats, and might therefore be a strategy to pass over to the placenta without being eliminated. PBMCs obtained from infected goats later in the infection did not show the IFN-γ decrease after re-stimulation in vitro. This could mean that the immune modulation of PBMCs in vivo only occurred early in infection and once the Coxiella bacteria have reached the placenta, no systemic immune modulation is needed, or all systemic Coxiella bacteria are eliminated. Antigen specific lymphocyte sequestration can also be an explanation for the lower IFN-γ levels in infected goats up to 7 days after the infection. However, our results suggests that the effector T-cell population (Th1) remained in the PBMC fraction during Coxiella infection, as the mRNA expression of IFN-γ increased. However, based on the results of IL-10 mRNA expression, we can hypothesize that the regulatory T-cells (Th2) could be transferred to the lymph nodes during Coxiella infection.
During first contact with C. burnetii, PBMCs of healthy pregnant goats are capable of recognizing C. burnetii and activate their early immune response in vitro. Furthermore, PBMCs from infected goats showed an augmented pro-inflammatory cytokine mRNA expression compared to PBMCs of naive goats after C. burnetii stimulation, whereas the anti-inflammatory cytokine IL-10 was down-regulated. The finding that PBMCs from goats that have previously had contact with C. burnetii react differently than naive PBMCs can be useful in future vaccine strategies. Finally, although the PBMC response as found in infected goats is strongly geared towards a pro-inflammatory state, the infection is not cleared and the goats will still suffer abortions and stillbirths.
Acknowledgments
We thank dr. Rudolf Toman and dr. Gabriela Flores-Ramirez from the Institute of Virology, Slovak Academy of Sciences in Bratislava for their help with the LPS phase determination of the C. burnetii strains. We thank Frank Harders for designing the TLR primers. We thank the employees of the department of Animal Technology for taking care of the goats and the sampling during the experiment.
Funding Statement
This work was supported by The Netherlands Organisation for Health Research and Development (grant 205520004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Raoult D, Marrie TJ, Mege JL (2005) Natural history and pathophysiology of Q fever. Lancet Infectious Diseases 5: 219–226. [DOI] [PubMed] [Google Scholar]
- 2. Maurin M, Raoult D (1999) Q fever. Clin Microbiol Rev 12: 518–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roest HI, Post J, van Gelderen B, van Zijderveld FG, Rebel JM (2013) Q fever in pregnant goats: humoral and cellular immune responses. Vet Res 44: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roest HJ, van Gelderen B, Dinkla A, Frangoulidis D, van Zijderveld F, et al. (2012) Q fever in pregnant goats: pathogenesis and excretion of Coxiella burnetii. Plos One 7: e48949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghigo E, Capo C, Raoult D, Mege JL (2001) Interleukin-10 stimulates Coxiella burnetii replication in human monocytes through tumor necrosis factor down-modulation: Role in microbicidal defect of Q fever. Infection and Immunity 69: 2345–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoffelen T, Sprong T, Bleeker-Rovers CP, Wegdam-Blans MC, Ammerdorffer A, et al. (2013) A Combination of IFN-gamma and IL-2 Production by Coxiella burnetii Stimulated Circulating Cells Discriminates Between Chronic Q Fever and Past Q Fever. Clin Microbiol Infect. [DOI] [PubMed]
- 7. Honstettre AL, Imbert GV, Ghigo E, Gouriet FR, Capo C, et al. (2003) Dysregulation of cytokines in acute Q fever: Role of interleukin-10 and tumor necrosis factor in chronic evolution of Q fever. Journal of Infectious Diseases 187: 956–962. [DOI] [PubMed] [Google Scholar]
- 8. Capo C, Zugun F, Stein A, Tardei G, Lepidi H, et al. (1996) Upregulation of tumor necrosis factor alpha and interleukin-1 beta in Q fever endocarditis. Infect Immun 64: 1638–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dellacasagrande J, Ghigo E, Capo C, Raoult D, Mege JL (2000) Coxiella burnetii survives in monocytes from patients with Q fever endocarditis: involvement of tumor necrosis factor. Infect Immun 68: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Izzo AA, Marmion BP (1993) Variation in interferon-gamma responses to Coxiella burnetii antigens with lymphocytes from vaccinated or naturally infected subjects. Clin Exp Immunol 94: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Capo C, Zaffran Y, Zugun F, Houpikian P, Raoult D, et al. (1996) Production of interleukin-10 and transforming growth factor beta by peripheral blood mononuclear cells in Q fever endocarditis. Infection and Immunity 64: 4143–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Netea MG, van der Meer JW, van Deuren M, Kullberg BJ (2003) Proinflammatory cytokines and sepsis syndrome: not enough, or too much of a good thing? Trends Immunol 24: 254–258. [DOI] [PubMed] [Google Scholar]
- 13. Roest HIJ, Ruuls RC, Tilburg J, Nabuurs-Franssen MH, Klaassen CHW, et al. (2011) Molecular Epidemiology of Coxiella burnetii from Ruminants in Q Fever Outbreak, the Netherlands. Emerging Infectious Diseases 17: 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hitchcock PJ, Brown TM (1983) Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol 154: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schramek S, Galanos C (1981) Lipid A component of lipopolysaccharides from Coxiella burnetii. Acta Virol 25: 230–234. [PubMed] [Google Scholar]
- 16. Arricau-Bouvery N, Hauck Y, Bejaoui A, Frangoulidis D, Bodier CC, et al. (2006) Molecular characterization of Coxiella burnetii isolates by infrequent restriction site-PCR and MLVA typing. BMC Microbiol 6: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Xiong X, Wu D, Wang X, Wen B (2011) Efficient activation of T cells by human monocyte-derived dendritic cells (HMDCs) pulsed with Coxiella burnetii outer membrane protein Com1 but not by HspB-pulsed HMDCs. BMC Immunol 12: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Zhang G, Hendrix LR, Tesh VL, Samuel JE (2012) Coxiella burnetii induces apoptosis during early stage infection via a caspase-independent pathway in human monocytic THP-1 cells. Plos One 7: e30841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Graham JG, MacDonald LJ, Hussain SK, Sharma UM, Kurten RC, et al. (2013) Virulent Coxiella burnetii pathotypes productively infect primary human alveolar macrophages. Cell Microbiol 15: 1012–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meghari S, Bechah Y, Capo C, Lepidi H, Raoult D, et al. (2008) Persistent Coxiella burnetii infection in mice overexpressing IL-10: an efficient model for chronic Q fever pathogenesis. PLoS Pathog 4: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang G, Russell-Lodrigue KE, Andoh M, Zhang Y, Hendrix LR, et al. (2007) Mechanisms of vaccine-induced protective immunity against Coxiella burnetii infection in BALB/c mice. J Immunol 179: 8372–8380. [DOI] [PubMed] [Google Scholar]
- 22. Schoffelen T, Joosten LA, Herremans T, de Haan AF, Ammerdorffer A, et al. (2013) Specific Interferon gamma Detection for the Diagnosis of Previous Q Fever. Clin Infect Dis 56: 1742–1751. [DOI] [PubMed] [Google Scholar]
- 23. Ben Amara A, Ghigo E, Le Priol Y, Lepolard C, Salcedo SP, et al. (2010) Coxiella burnetii, the Agent of Q Fever, Replicates within Trophoblasts and Induces a Unique Transcriptional Response. Plos One 5: e15315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andoh M, Zhang G, Russell-Lodrigue KE, Shive HR, Weeks BR, et al. (2007) T cells are essential for bacterial clearance, and gamma interferon, tumor necrosis factor alpha, and B cells are crucial for disease development in Coxiella burnetii infection in mice. Infection and Immunity 75: 3245–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arricau-Bouvery N, Souriau A, Bodier C, Dufour P, Rousset E, et al. (2005) Effect of vaccination with phase I and phase II Coxiella burnetii vaccines in pregnant goats. Vaccine 23: 4392–4402. [DOI] [PubMed] [Google Scholar]
- 26. Segal G, Shuman HA (1999) Possible origin of the Legionella pneumophila virulence genes and their relation to Coxiella burnetii. Mol Microbiol 33: 669–670. [DOI] [PubMed] [Google Scholar]
- 27. Rocha PN, Almeida RP, Bacellar O, de Jesus AR, Filho DC, et al. (1999) Down-regulation of Th1 type of response in early human American cutaneous leishmaniasis. J Infect Dis 180: 1731–1734. [DOI] [PubMed] [Google Scholar]
- 28. Wang X, Barnes PF, Dobos-Elder KM, Townsend JC, Chung YT, et al. (2009) ESAT-6 inhibits production of IFN-gamma by Mycobacterium tuberculosis-responsive human T cells. J Immunol 182: 3668–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]