Abstract
The association between leptin and reproduction originated with the leptin-mediated correction of sterility in ob/ob mice and initiation of reproductive function in normal female mice. The uncovering of a central leptin pathway regulating food intake prompted the dissection of neuroendocrine mechanisms involving leptin in the metabolic control of reproduction. The absence of leptin receptors on GnRH neurons incited a search for intermediary neurons situated between leptin responsive and GnRH neurons. This review addresses the most significant findings that have furthered our understanding of recent progress in this new field. The role of leptin in puberty was impacted by the discovery of neurons that co-express kisspeptin, neurokinin B and dynorphin and that could act as leptin intermediates. Furthermore, the identification of first-order leptin-responsive neurons in the premammilary ventral nucleus and other brain regions opens new avenues to explore their relationship to GnRH neurons. Central to these advances is the unveiling that AgRP/NPY neurons project onto GnRH and kisspeptin neurons, allowing a crosstalk between food intake and reproduction. Finally, whereas puberty is a state of leptin sensitivity, mid-gestation represents a state of leptin resistance aimed at building energy stores to sustain pregnancy and lactation. Mechanisms underlying leptin resistance in pregnancy have lagged, however the establishment of this natural state is significant. Reproduction and energy balance are tightly controlled and backed up by redundant mechanisms that are critical for the survival of our species. It will be the goal of the next decade to shed new light on these complex and essential pathways.
The survival of any species depends on its ability to reproduce otherwise it will become extinct. Reproduction is an energy demanding process and its physiological costs vary widely in the animal kingdom, but there is no doubt that the burden of reproduction lies with females. The ability to reproduce starts at different ages in humans but invariably involves a period of sexual maturation that culminates with the first menstrual cycle. In most inbred strains of mice, the first ovulation occurs between 6–8 weeks of age, however in humans, puberty is attained in females at about 12–13 years of age. While the onset of puberty hallmarks the reproductive lifespan of a female, pregnancy, parturition and lactation are the crux of reproductive biology and remain the most physiologically complex and energy demanding life processes.
The adipose mass of an organism represents an energy storage reservoir. In organisms that undergo periods of hibernation or torpor, buildup of the adipose mass sustains the low metabolic rates and is essential for reproductive function, as best exemplified in ground squirrels (Forger, et al. 1986). Except in marsupials, torpor in mammals is not a requirement for survival, but the ability to fatten up at critical periods is an essential component of normal physiology. In this review, we will address the leptin-mediated effects and associated mechanisms that pertain to the accumulation of adipose mass at critical times during the reproductive events of a female, namely at puberty and pregnancy.
The seminal discovery that leptin is secreted from adipocytes (Zhang, et al. 1994) heightened an interest in the mechanisms of food intake and adipose tissue accumulation and their impact on the obesity epidemic. However, the link between adipose tissue and reproduction predate obesity, which often confounds and aggravates studies of leptin and the reproductive axis. The inspirational papers of Gordon Kennedy pioneered the link between energy intake and reproduction by placing these two pathways within the hypothalamic network (Kennedy and Mitra 1963a, b, c). Later, Rose Frisch unveiled her critical weight hypothesis (Frisch and McArthur 1974) and demonstrated that perturbations in the adipose mass negatively impact on female fertility (Frisch, et al. 1981; Frisch, et al. 1980; Vigersky, et al. 1977). While much of important physiology was unveiled in these early days, it is unfortunate that the groundbreaking parabiosis experiments of the late Doug Coleman between db/db and ob/ob mice (Coleman 1973) did not involve investigation of the reproductive axis during the months long response of ob/ob mice to the db/db-originated leptin. Rose Frisch studies were critical in providing a basis for the role of leptin in reproduction and are analogous to Coleman’s groundbreaking parabiosis experiments that provided a framework for the roles of leptin and leptin receptor. It was not until leptin rescued the sterility of ob/ob mice (Chehab, et al. 1996; Mounzih, et al. 1997) and advanced the onset of puberty in normal mice (Ahima, et al. 1997; Chehab, et al. 1997; Yura, et al. 2000) that Kennedy and Frisch’s works on the link between the adipose tissue and reproduction was revived and a tying metabolic factor identified in leptin. As a result, the reproductive field has widened and leptin, acknowledged as a permissive puberty factor (Cheung, et al. 1997), could activate a torrent orchestrated by multiple factors to culminate in the pulsatile secretion of gonadotropin releasing hormone (GnRH). Another key observation that built on the link between leptin and reproduction is the secretion of leptin from human placenta (Masuzaki, et al. 1997), further establishing an association between leptin and pregnancy, another state of high energy demands. The demonstration that mid-pregnancy in mice is a state of leptin resistance (Mounzih, et al. 1998) added further mechanistic insights into pathways responsible for increasing energy storage during pregnancy. The interplay of leptin, mediating the regulation of orexigenic and anorexigenic pathways (Boston, et al. 1997; Cowley, et al. 2001) and those inducing a state of leptin resistance during pregnancy, indicates symbiotic and likely overlapping associations between these two pathways.
Overall, initial studies have highlighted two essential physiological functions of leptin pertaining to its role in reproduction, namely in puberty and pregnancy. While leptin impacts both processes, their mechanisms are distinct in that the former is a state of leptin sensitivity highlighted by a leptin surge in rodents and primates (Ahima, et al. 1998; Suter, et al. 2000), while the latter imparts on increasing food intake via a leptin resistance state (Mounzih et al. 1998). Thus, this review will focus on progress aimed mostly at increasing our understanding of a leptin sensitive state in puberty and to a lesser extent a leptin resistant state during pregnancy.
I- Leptin in Puberty
Our knowledge of the mechanisms and cues that control the onset of puberty have considerably increased in the 20th century with much of the work originating from rodent and primate animal models. There is no doubt that the onset of puberty is gated by multiple metabolic factors, which altogether inform the reproductive system about the extent of energy stores in an organism in order to activate the GnRH pulse generator. In fact, increases of adipose tissue mass advance the onset of menarche (Kimm, et al. 2001; Wattigney, et al. 1999) whereas fat depletion delays it (Frisch et al. 1980). Paradoxically, leptin deficient individuals are obese but due to the lack of leptin fail to advance to puberty unless treated with exogenous leptin (Farooqi, et al. 1999; Montague, et al. 1997). These studies highlight the critical role of leptin in the onset of human reproductive function. There is also little doubt that any serious puberty-triggering factor must be able to influence directly or indirectly the secretion or regulation of GnRH. A requirement for such a factor is that mutations in its gene, whether in human and/or mouse models, must result in infertility or significant reproductive dysfunction. Leptin fulfills both of these requirements as shown by the infertility of leptin-deficient mice and humans and their leptin mediated rescue (Chehab et al. 1996; Farooqi et al. 1999; Gibson, et al. 2004) as well as its indirect stimulation of GnRH (Nagatani, et al. 1998; Reynoso, et al. 2003) and the potentiation of LH release from pituitary gonadotropes in the presence of GnRH (Avelino-Cruz, et al. 2009). Leptin also appears to exert GnRH independent effects on the reproductive system as shown by its ability to induce ovulation in GnRH deficient mice (Barkan, et al. 2005), an intriguing but interesting finding. Overall, leptin is a metabolic factor that bridges the regulation of the fat mass with reproduction.
The central question is the nature of the critical metabolic factor(s) that signal to GnRH neurons the appropriate physiological readiness to initiate GnRH release and activate the puberty cascade. Nutritional and metabolic factors have long been suspected to participate in the process and could elicit a direct or indirect release of GnRH. While mouse and human studies convincingly demonstrate an essential role of leptin in puberty (Ahima et al. 1997; Chehab et al. 1996; Chehab et al. 1997; Cheung et al. 1997; Farooqi et al. 1999), the absence of signaling competent-leptin receptor expression on GnRH neurons strongly suggests that an intemediary factor or pathway mediates the essential effects of leptin on the activation of GnRH neurons. Potential intermediate candidates are insulin, insulin growth factor 1 (IGF1), growth hormone (GH), ghrelin, neuropeptide Y (NPY), orexin, melanin concentrating hormone (MCH), adiponectin, kisspeptin and possibly gut peptides. Which of these factors is the single, if any, stimulus and what are its backup or co-factors? From an evolutionary perspective, it makes sense that reproduction, a fundamental component of a species survival, has developed redundant systems. To put this concept into perspective of critical metabolic and nutritional factors, we can turn to knockout mouse models to determine the impact on reproduction of a specific gene encoding a metabolic factor.
Insulin/IGF1
The insulin/IGF-1/GH axis is the hallmark of anabolic hormones that impact GnRH neurons at puberty. This is best demonstrated by the infertility of neuron specific insulin receptor knockout female mice (NIRKO), which exhibit a 90% reduction in LH but remain responsive to a GnRH agonist (Bruning, et al. 2000). Thus, neuronal expression of the insulin receptor appears to be critical for reproduction. In a milieu of elevated leptin levels at puberty, it is conceivable that leptin and insulin together influence GnRH secretion. The critical role of IGF1 is also shown by the postnatal lethality of IGF1 null mice (Liu, et al. 1993; Powell-Braxton, et al. 1993). However, a mixed genetic background of IGF-1 null mice rescues their lethal phenotype and these mice exhibit growth retardation and a failure to attain puberty (Liu and LeRoith 1999). In human, homozygous deletion of the IGF-1 gene resulted, as in genetically heterogeneous mice, in postnatal survival and retarded growth but normal, if not early puberty (Woods, et al. 2000; Woods, et al. 1996). Thus, unlike in NIRKO mice, IGF-1 does not appear to be critical and necessary for the onset of puberty but remains an important factor as it correlates with growth. An elegant experiment (Divall, et al. 2010) targeted the deletion of the IR or IGF1R to GnRH neurons and determined puberty and fertility outcomes in knockout mice. Mice with a targeted deletion of the IR on GnRH neurons displayed normal puberty and fertility. However, male and female mice with a deletion of the IGF1R on GnRH neurons showed delayed puberty but normal fertility. Interestingly, IGF1 administration advanced puberty in normal but not IGF1R knockout mice. Although IGF1R knockout mice showed delayed but not a block in puberty, IGF1R signaling on GnRH neurons appears to be an important contributor to the timing of puberty.
Orexigenic and Anorexigenic Factors
Orexigenic factors such as ghrelin, neuropeptide Y (NPY), melanin concentrating hormone (MCH), agouti-related protein (AgRP) and orexin constitute a food intake-regulatory circuit that could influence the hypothalamic-pituitary-gonadal (HPG) axis. Ghrelin, an endogenous ligand for the growth hormone secretagogue receptor (Howard, et al. 1996) is similar to leptin in that they are both secreted from the periphery, the former from stomach and the latter from adipose tissue, and exert their major effects on the arcuate nucleus (Cowley et al. 2001; Hewson, et al. 2002), including the HPG axis (Kluge, et al. 2007; Steiner, et al. 2003). The fact that leptin and ghrelin are antagonistic in their effects on food intake, their respective anorexigenic and orexigenic roles make them ideal candidates to regulate the reproductive system in times of nutritional variation. Ghrelin exerts a negative effect on GnRH (Fernandez-Fernandez, et al. 2005) whereas leptin stimulates it or facilitates its secretion (Nagatani et al. 1998; Reynoso et al. 2003). However, unlike leptin-deficient mice, ghrelin knockout mice are neither obese nor reproductively impaired (De Smet, et al. 2006). Similalry, orexin knockout mice suffer no energy or reproductive peculiarities (Chemelli, et al. 1999). Interestingly, knockout mice for neuropeptide Y are fertile and lean (Erickson, et al. 1996a). However, ob/ob mice deficient in NPY or in the NPY-Y4 receptor show an attenuation of obesity and improved fertility (Erickson, et al. 1996b; Sainsbury, et al. 2002), suggesting that a lack of NPY or its Y4 receptor alleviates the restraint placed by a deficiency of leptin on the reproductive axis and most importantly, demonstrating a central role for NPY in reproduction. Although NPY has long been known to stimulate GnRH secretion (Khorram, et al. 1988; Sutton, et al. 1988), it was also found to have both inhibitory and stimulatory effects on GnRH, via the NPY Y1 and NPY Y4 receptors, respectively (Roa and Herbison 2012). Thus, it would be revealing to determine the physiological states and mechanisms under which NPY exerts this variable effect on GnRH neurons. Another observation that links NPY to GnRH is their migration origin. Both GnRH and NPY neurons originate from the olfactory placode and migrate into the central nervous system during embryogenesis (Hilal, et al. 1996), implying that their mutual relocation to the hypothalamus could be somewhat evolutionary and functionally linked.
AgRP is expressed in leptin responsive neurons of the arcuate nucleus and exerts significant effects on energy intake when overexpressed in transgenic mice (Ollmann, et al. 1997), but has no obvious effects on adiposity or fertility in AgRP knockout and double AgRP/NPY knockout mice (Qian, et al. 2002; Xu, et al. 2005). However, ablation of AgRP-expressing neurons in leptin deficient ob/ob and leptin receptor deficient db/db mice, remarkably, restores fertility (Israel, et al. 2012; Sheffer-Babila, et al. 2013; Wu, et al. 2012). Thus, the findings that NPY and AgRP play critical roles in reducing sterility in leptin or leptin-signaling deficient states (Erickson et al. 1996b; Israel et al. 2012; Wu et al. 2012) uncover an essential link between energy balance and reproductive pathways. While it is reasonable to assume that AgRP/NPY projections onto GnRH neurons might serve as the long sought leptin intermediate, a more plausible hypothesis is that they represent a secondary alternate reproductive pathway, namely because single or double AgRP/NPY knockout mice without leptin or leptin receptor mutations fail to exhibit any reproductive defect. However, AgRP stimulates GnRH release (Stanley, et al. 1999) and thus this secondary pathway is fundamental and may underlie yet unexplained mechanisms of fertility in ob/ob mice such as when food restricted or bred on mixed genetic backgrounds (Ewart-Toland, et al. 1999; Lane and Dickie 1954; Qiu, et al. 2001). The facilitating and rescuing effects of this secondary pathway may also explain fertility in a female with a leptin receptor mutation (Nizard, et al. 2012).
Melanin concentrating hormone (MCH) is another orexigenic peptide expressed in the lateral hypothalamus, where leptin-responsive neurons are located. However, MCH knockout mice are lean, hypophagic and remain fertile (Shimada, et al. 1998), owing most likely to the presence of enough adipose tissue that secretes leptin. Furthermore, ob/ob mice lacking MCH remain infertile despite an attenuation of their obesity caused by an increase in energy expenditure, not decreased hyperphagia (Segal-Lieberman, et al. 2003).
Anorexigenic factors also impact the reproductive system. It is well known that perturbations in energy distribution and balance such as in lipodystrophy and anorexia nervosa cause interruptions of the menstrual cycles. Notable anorexigenic factors secreted by the gut in response to nutrient ingestion include protein tyrosine tyrosine (PYY), pancreatic polypeptide (PP), cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), oxyntomodulin (OXM), and apolipoprotein A-IV (apoA-IV). PYY knockout mice are obese and fertile (Batterham, et al. 2006). CCK knockout mice are normal and fertile (Lacourse, et al. 1999). GLP-1R knockout mice show impairments in behavioral and stress responses, minor reproductive disturbances, such as reduced gonadal weights in males and slight puberty delay in females, but overall normal fertility (MacLusky, et al. 2000). Similar to GLP-1, OXM results from the proglucagon gene, acts as an agonist at the GLP-1R and stimulates weight loss (Kosinski, et al. 2012), but has not yet been shown to exhibit any reproductive effect. Similarly, apoA-IV, which is secreted by the small intestine in response to fat absorption was suggested as a satiety factor but apoA-IV knockout mice showed only mild dyslipidemia and no reproductive defects (Weinstock, et al. 1997).
POMC neurons that activate the anorexigenic arm of the central leptin pathway are direct leptin targets in the arcuate nucleus. Mutations of the POMC gene in human and its ablation in mice result in obesity but no apparent effects on the reproductive axis (Krude, et al. 1998; Yaswen, et al. 1999). In addition, targeted deletion of the leptin receptor on POMC neurons resulted in no reproductive defects (Balthasar, et al. 2004; van de Wall, et al. 2008). However, deletions of both leptin and insulin receptors from POMC neurons caused insulin resistance and reduced fertility, a condition that resembled characteristics of the polycystic ovary syndrome (Hill, et al. 2010). Thus, POMC neurons also mediate normal function of the reproductive axis in females. α;-MSH is the POMC-derived ligand that binds to the MC4R located on second order leptin-responsive hypothalamic neurons, which stimulate the anorexigenic arm of the pathway. MC4R knockout mice are obese and subfertile (Huszar, et al. 1997) and interestingly, their reproductive dysfunction can be rescued with increasing exercise (Irani, et al. 2005). This observation is consistent with the ability of a melanocortin receptor antagonist to reverse reduced food intake in leptin treated ob/ob mice, without however affecting the activation of their reproductive axis, suggesting a dissociation of the melanocortin pathway from the reproductive system (Hohmann, et al. 2000). These observations are also consistent with normal fertility of adult obese individuals carrying heterozygous and homozygous deleterious mutations in the MC4R gene (Farooqi, et al. 2003). Thus, while melanocortin neurons could play a secondary role in reproduction, they are unlikely to serve as the critical leptin intermediates.
Overall, it appears that while the orexigenic and anorexigenic factors listed above could play single-handedly a critical role in fertility, it is more likely that altogether they would be the gatekeepers of reproduction. To gain further understanding into their individual role, a more informative approach would be to generate double and triple knockout alleles in a single mouse and then determine their combined effects on obesity and reproduction. While this strategy is quite laborious for traditional knockout strategies, the recent advent of genomic editing technologies such as the CRISPR/Cas9 system (Wang, et al. 2013), makes it likely to generate such informative experimental model systems.
Kisspeptins
The exciting discovery of the kisspeptin system offered a great candidate for a leptin intermediate acting between leptin responsive neurons and GnRH neurons. Kisspeptin, a product of the Kiss1 gene, is expressed in various mammalian species, predominantly in a large population of neurons in the arcuate nucleus (ARC) and to a lesser extent in the preoptic area (POA), rostral periventricular of the third ventricle as well as in scattered regions of the brain outside of the hypothalamus (Lehman, et al. 2010). Kisspeptin neurons connect to NPY and POMC neurons (Backholer, et al. 2010), which are first order leptin responsive neurons in the hypothalamus, heightening the coordination of the nutritional leptin axis to its reproductive counterpart.
Kisspeptin, encoded by the Kiss1 gene, emerged as a primary puberty candidate factor following the findings that individuals with hypogonadotropic hypogonadism carry mutations in the kisspeptin receptor GPR54 (de Roux, et al. 2003; Seminara, et al. 2003; Semple, et al. 2005). The first indication that kisspeptin could indeed be the long sought leptin intermediate was demonstrated in leptin deficient ob/ob mice that express low levels of Kiss1 mRNA, that are then stimulated to be increased with leptin treatment (Smith, et al. 2006). Targeted deletions in mice of Kiss1 or the Kiss1 receptor GPR54 gene, revealed infertility and abnormal sexual maturations (d'Anglemont de Tassigny, et al. 2007; Lapatto, et al. 2007), affirming a critical role for the kisspeptin system in the activation of the reproductive axis. Consistently, the finding that intracerebroventricular administration of kisspeptin stimulated maturation of the reproductive axis in hypoleptinemic states of prepubertal rats and hyperleptinemic fa/fa Zucker rats with a mutation in the leptin receptor (Navarro, et al. 2004), suggested that Kiss1 advances puberty when administered centrally and that its GnRH triggering function is upstream of GnRH neurons, which is what would be expected from a leptin intermediate. Most importantly, kisspeptins are potent stimulators of GnRH release (Irwig, et al. 2004; Messager, et al. 2005; Pielecka-Fortuna, et al. 2008; Thompson, et al. 2004). While these above findings were close to sealing the nature of the leptin intermediate, it was surprisingly found that deletion of the leptin receptor from Kiss1 neurons (Donato, et al. 2011) or ablation of >95% ablation of kisspeptin neurons prior to puberty resulted in normal puberty and fertility (Mayer and Boehm 2011). Furthermore, transgenic expression of the kisspeptin receptor in neurons of leptin receptor deficient mice failed to rescue their sterility (Cravo, et al. 2013). Thus, these studies dampened the excitement of a kisspeptin-leptin intermediate hypothesis, despite the fact that a small amount of kisspeptin might actually be sufficient to trigger puberty (Popa, et al. 2013). While kisspeptin expressing neurons are unlikely to be the leptin intermediate neurons, they nevertheless play a critical role in reproduction, perhaps in the maintenance of fertility after puberty. Thus, it would be interesting to tease out the effects of kisspeptin at puberty and subsequently for the maintenance of fertility. In addition to leptin, insulin receptors are located on kisspeptin neurons and could contribute to their effect on reproduction. Interestingly, deletion of the insulin receptor from kisspeptin neurons resulted in delayed puberty, but thereafter, normal fertility (Qiu, et al. 2013), adding insulin as a potential mediator of reproduction from kisspeptin neurons.
The involvement of kisspeptin in sexual maturation and reproduction extends to an exciting set of neurons in the ARC that co-express kisspeptin, neurokinin B and dynorphin. These neurons, termed KNDy, project onto GnRH neurons, stimulate GnRH release (Hrabovszky, et al. 2010; Ramaswamy, et al. 2010) and get activated during the LH surge (Merkley, et al. 2012). Furthermore, central administration of a neurokinin 3 receptor (NK3R) agonist in prepubertal females elicits LH secretion whereas infusion of an NK3R antagonist delays vaginal opening and LH pulse amplitude (Li, et al. 2014; Navarro, et al. 2012). The proportion of kisspeptin neurons that express the long form of the leptin receptor (LepRb) is controversial and ranges from 6% (Louis, et al. 2011) to 40% (Smith et al. 2006), implying that leptin action on KNDy neurons could be minimal or substantial. Analogous to the fact that a small amount of kisspeptin is required to trigger puberty (Popa et al. 2013), it is equally conceivable but remains to be determined, that a small number of KDNy neurons expressing LepRb could elicit GnRH release. The small proportion of neurons required to trigger reproductive function is reminiscent of the fact that implantation of preoptic slices containing only 3–140 GnRH neurons are enough to correct hypogonadism in GnRH deficient mice (Silverman, et al. 1985). Whereas STAT3 signaling is not required for reproduction (Bates, et al. 2003). only 15% of KNDy neurons in the ARC phosphorylate STAT3 upon leptin exposure, (Cravo, et al. 2011). Thus, KDNy neurons that bind leptin, express LepRb but suppress STAT3 phosphorylation will be critical for unraveling a leptin-kisspeptin STAT3-independent pathway for initiating reproductive function.
Premammilary Ventral Nucleus
An illuminating finding that throws new light on the search for a leptin intermediate was the identification that the premammilary ventral nucleus (PMV), a site in the hypothalamus that exhibits broad expression of receptors involved in energy metabolism, encompasses leptin responsive neurons (Donato, et al. 2010). Lesions in the PMV resulted in decreased activation of GnRH and Kiss1 neurons, causing low estrogen secretion and deficient feedback of GnRH on proestrus (Donato, et al. 2013), demonstrating that the PMV is a critical area that mediates the effects of leptin on reproduction. Another tantalizing finding is the uncovering that neurons directly responsive to leptin, located in various brain sites including the PMV, POA, ARC and dorsomedial hypothalamus (DMH) release the neurotransmitter nitric oxide (Donato et al. 2010). While it was previously known that targeted inactivation of the mouse nitrous oxide synthase (NOS) gene caused infertility in the presence of normal leptin levels (Gyurko, et al. 2002), recent studies showed that deletion of the neuronal NOS gene, or its pharmacological inhibition, blunted the ability of exogenous leptin to restore fertility to ob/ob mice (Bellefontaine, et al. 2014). In the same study, leptin was found to coordinate fertility by acting on neurons in the POA to induce NO synthesis via activation of neuronal NOS. Thus, leptin-responsive nitric oxide releasing neurons define a new class of PMV neurons that provide input to the neuroendocrine regulation of reproduction.
Overall it appears that the identification of a single critical leptin intermediate, which indirectly impacts GnRH secretion remains to be fully elucidated although the primary candidates are KNDy neurons and NO leptin responsive neurons. Consistent with the notion of redundancies in reproductive pathways, it is quite likely that a single leptin intermediate may not exist, but rather that a web of neuronal connections coordinate the firing of a complex system that is fundamental to species survival. The experimental dissection of these pathways will prove to be difficult but not impossible considering the increasing use of genome editing tools to knock-in and knock-out multiple genes in the same cell and generate corresponding mouse models (Wang et al. 2013). Another possibility, which remains to be fully explored and distinct from the stimulatory hypothesis of a leptin intermediate, is the removal of a restraint imposed on GnRH neurons, such as from epigenetic mechanisms. All these approaches should help us in the next decade to expand on novel findings and derive conclusive mechanisms for the reproductive side of leptin.
II- Leptin in maternal nutrition during pregnancy
The adipose tissue mass plays another role in reproduction, essentially for building up adequate energy reserves to sustain a pregnancy and for the subsequent energy demands of lactation. The first evidence of leptin playing a role in pregnancy originated from the report that pregnant women secrete elevated levels of leptin from the placenta into the maternal circulation (Masuzaki et al. 1997). The effects of leptin during pregnancy were subsequently assessed in leptin treated and mated male and female ob/ob mice, thus resulting in ob/ob pregnancies that were controlled with exogenous leptin (Mounzih et al. 1998). In this study, withdrawal of leptin treatment at 0.5, 6.5, 10.5 and 19.5 days p.c. of pregnant ob/ob mice did not affect implantation, gestation or parturition. However, the food intake of ob/ob females continuously treated with leptin during pregnancy, resulted as of day 10.5 p.c., in increased food intake compared to previous days, demonstrating an attenuation effect of the leptin treatment and the establishment of a leptin resistance state. Consistently, during the late pregnancy of rats, surges in food intake are associated with elevated plasma leptin levels and central administration of leptin during this period is less effective at reducing food intake compared to cyclic rats, again demonstrating a period of reduced leptin sensitivity (Johnstone and Higuchi 2001). The onset of leptin resistance in pregnancy was appropriately associated with decreased STAT3 phosphorylation in the VMH (Ladyman and Grattan 2004, 2005) and by the dysregulation of AgRP/NPY and melanocortin neurons (Ladyman, et al. 2009). Thus, mid-gestation in the mouse represents the beginning of a leptin resistance state that could conceivably be derived from synaptic plasticity and reprograming of neuronal projections into the hypothalamus. These changes would be aimed at establishing a body weight setpoint that results in increased food intake and adipose mass accumulation. Maternal food intake, whether increased or decreased was found to program postnatal leptin expression, as demonstrated by elevated leptin expression in adipose tissue and plasma secretion in female pig offsprings, whose mothers were allowed higher food consumption during the second quarter of pregnancy (Eckert, et al. 2000). While decreased maternal nutrition during pregnancy can have devastating effects on fetal reprograming, one effect is that the postnatal leptin surge (Ahima et al. 1998) is severely attenuated by maternal under nutrition (Delahaye, et al. 2008), presumably resulting in delayed growth and puberty.
The mechanisms underlying leptin resistance in pregnancy may be underlined by the same mechanisms as in obesity, however, the triggering factors in either case are likely to be distinct owing to the differences of both physiological states. The onset of leptin resistance in pregnancy is a natural process, which could be carried postpartum in subsets of women with obesity (Gunderson and Abrams 2000). The timing for the onset of leptin resistance in pregnancy is predictable and therefore could ease the uncovering of the triggering mechanisms and associated factors.
Conclusions and Future Perspectives
Puberty and mid-gestation are physiological states of leptin sensitivity and leptin resistance, respectively. Studies centering on understanding the mechanisms that underlie the onset of puberty and to a lesser extent those involved in leptin resistance during pregnancy, have been exhaustive in the past twenty years. Our knowledge of the triggering neurons has been substantial and potential leptin intermediates have emerged and enlightened the role of leptin in reproduction. First, the location of a previously unappreciated site for leptin action in the PMV opens new avenues to investigate neuronal projections, synaptic plasticity and neurotransmitters that signal the timing and firing of GnRH neurons to trigger the reproductive cascade. Second, the characterization of the kisspeptin system, specifically the KNDy neurons, which are upstream of GnRH and the potential role leptin plays on these neurons continues to be an exciting pathway to decipher and dissect. Third, the revealing role of AgRP/NPY neurons that influence GnRH neurons is a critical step that bridges the central nutritional pathway elicited by the binding of leptin to first order neurons to the leptin-responsive neurons in the reproductive axis. The essential criteria for neurons to qualify, as leptin intermediary neurons, is that they would have to respond to leptin, initiate leptin signaling, not necessarily via STAT3 phosphorylation and stimulate GnRH release most likely through KNDy neurons. In addition, site-specific deletion of the leptin receptor from these neurons would have to result in sterility, irrespective of the presence or absence of obesity. Furthermore, ob/ob and db/db mice should display a dysregulation of these intermediary neurons, stemming from their hypoactivation in leptin or leptin signaling deficiency.
While reproductive disturbances are largely dissociated from common obesity, nutritional factors and reproduction are closely connected, as exemplified in states of negative energy balance, when food intake corrects amenorrhea. For example, exogenous leptin induces menstruation in hypothalamic amenorrhea and is thus an appropriate fertility treatment for this disorder (Chou, et al. 2011; Welt, et al. 2004). Furthermore, in lipodystrophy, leptin treatment, withdrawal and reinstatement has effects on the progression, interruption and regain of puberty (Kamran, et al. 2012).
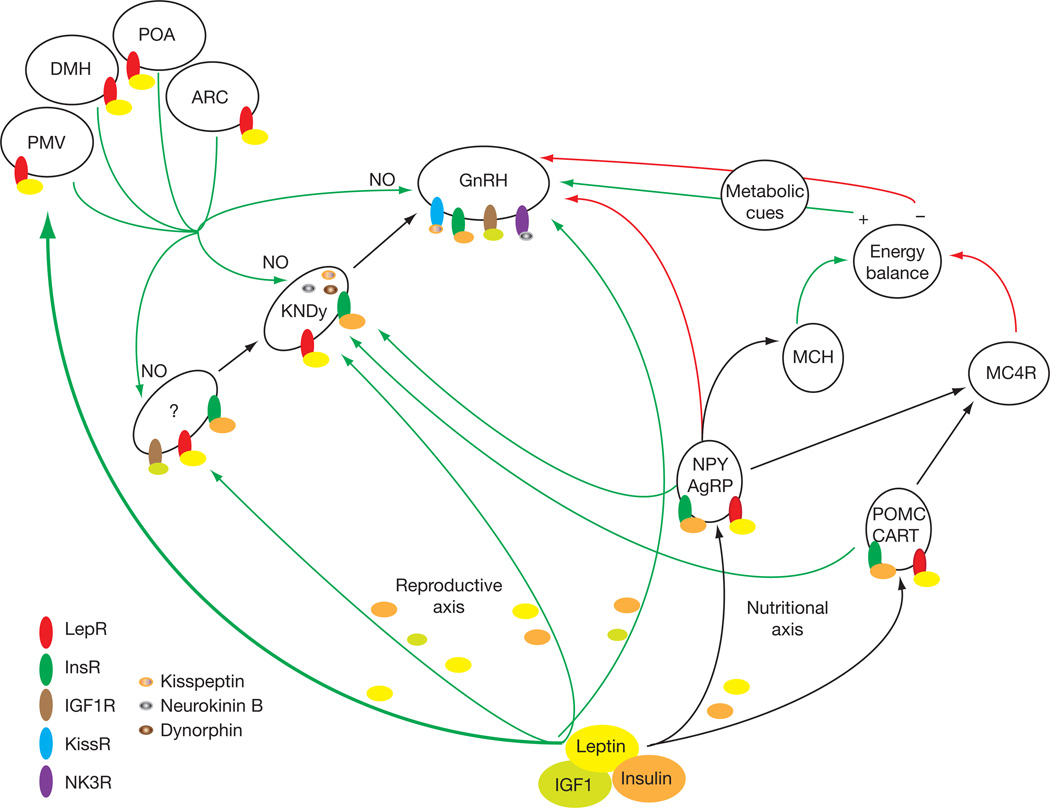
A proposed pathway that recapitulates the salient findings summarized in this review is shown in Fig. 1 and outlines the essential neuronal connections that involve leptin, insulin and IGF-1 or their surrogate neurons to converge onto GnRH neurons. As central pathways are critical for any leptin-mediated effect, the focus of future years will be on new first order sets of leptin-responsive neurons in the PMV and other brain regions that project onto GnRH neurons. These neurons will undoubtedly uncover mechanisms that will begin to unravel the complexities of a redundant system essential for species survival.
Fig. 1.
Schematic diagram and proposed model for the metabolic regulation and coordination of the leptin mediated reproductive and nutritional axes. Leptin, insulin and IGF-1 are shown as the most critical circulatory factors that act on hypothalamic and other brain networks to regulate food intake and GnRH secretion. The pathway, rich in redundancies, depicts the various direct and indirect inputs that converge on GnRH neurons. The crosstalks of the reproductive and nutritional axes are represented by the projections of the AgRP/NPY and POMC neurons to the KNDy neurons and by the AgRP/NPY neurons onto GnRH neurons. The first order leptin responsive neurons in the PMV and other hypothalamic areas are denoted with a thick green arrow to highlight their essential reproductive function and the release of the NO neurotransmitter. Ligands and receptors are shown on each set of neurons and green/red arrows represent stimulatory and inhibitory effects, respectively. The postulated elusive leptin intermediate neurons are denoted with a question mark. Acronyms in the figure are found in the text.
Finally, a provocative question is whether the primary function of leptin pertains to its metabolic regulation of the reproductive axis. Then conceivably, dysregulation in segments of this pathway, perhaps those that involve the leptin intermediate(s), could cause overweight disorders. While innumerable and challenging arguments would certainly be raised on either side of this hypothesis, it remains worthy of consideration. We still have many more years of intriguing questions to ask and additional lessons to be learned from the role of leptin in the metabolic control of neuroendocrine reproductive biology.
Acknowledgements
I wish to thank Jun Zhu for his help with this review and the members of my laboratory, Khalid Mounzih, Jun Qiu, Amanda Ewart-Toland, Scott Ogus, Mary Lim and Roghua Lu, who helped to elucidate much of the early work in establishing a role for leptin in reproduction. Their efforts would not have been fruitful without NIH grants HD35142 and T32 DK07636.
REFERENCES
- Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest. 1997;99:391–395. doi: 10.1172/JCI119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101:1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avelino-Cruz JE, Flores A, Cebada J, Mellon PL, Felix R, Monjaraz E. Leptin increases L-type Ca2+ channel expression and GnRH-stimulated LH release in LbetaT2 gonadotropes. Mol Cell Endocrinol. 2009;298:57–65. doi: 10.1016/j.mce.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, Li Q, Clarke IJ. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010;151:2233–2243. doi: 10.1210/en.2009-1190. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Barkan D, Hurgin V, Dekel N, Amsterdam A, Rubinstein M. Leptin induces ovulation in GnRH-deficient mice. FASEB J. 2005;19:133–135. doi: 10.1096/fj.04-2271fje. [DOI] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Bellefontaine N, Chachlaki K, Parkash J, Vanacker C, Colledge W, d'Anglemont de Tassigny X, Garthwaite J, Bouret SG, Prevot V. Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction. J Clin Invest. 2014 doi: 10.1172/JCI65928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boston BA, Blaydon KM, Varnerin J, Cone RD. Independent and additive effects of central POMC and leptin pathways on murine obesity. Science. 1997;278:1641–1644. doi: 10.1126/science.278.5343.1641. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- Chehab FF, Mounzih K, Lu R, Lim ME. Early onset of reproductive function in normal female mice treated with leptin. Science. 1997;275:88–90. doi: 10.1126/science.275.5296.88. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997;138:855–858. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]
- Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, Brinkoetter MT, Gong H, Arampatzi K, Mantzoros CS. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A. 2011;108:6585–6590. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9:294–298. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Cravo RM, Frazao R, Perello M, Osborne-Lawrence S, Williams KW, Zigman JM, Vianna C, Elias CF. Leptin signaling in Kiss1 neurons arises after pubertal development. PLoS One. 2013;8:e58698. doi: 10.1371/journal.pone.0058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazao R, Lee CE, Gautron L, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet B, Depoortere I, Moechars D, Swennen Q, Moreaux B, Cryns K, Tack J, Buyse J, Coulie B, Peeters TL. Energy homeostasis and gastric emptying in ghrelin knockout mice. J Pharmacol Exp Ther. 2006;316:431–439. doi: 10.1124/jpet.105.091504. [DOI] [PubMed] [Google Scholar]
- Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, Lesage J, Vieau D. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149:470–475. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- Divall SA, Williams TR, Carver SE, Koch L, Bruning JC, Kahn CR, Wondisford F, Radovick S, Wolfe A. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest. 2010;120:2900–2909. doi: 10.1172/JCI41069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J, Jr, Cravo RM, Frazao R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, et al. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355–368. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J, Jr, Frazao R, Fukuda M, Vianna CR, Elias CF. Leptin induces phosphorylation of neuronal nitric oxide synthase in defined hypothalamic neurons. Endocrinology. 2010;151:5415–5427. doi: 10.1210/en.2010-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J, Jr, Lee C, Ratra DV, Franci CR, Canteras NS, Elias CF. Lesions of the ventral premammillary nucleus disrupt the dynamic changes in Kiss1 and GnRH expression characteristic of the proestrus-estrus transition. Neuroscience. 2013;241:67–79. doi: 10.1016/j.neuroscience.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert JE, Gatford KL, Luxford BG, Campbell RG, Owens PC. Leptin expression in offspring is programmed by nutrition in pregnancy. J Endocrinol. 2000;165:R1–R6. doi: 10.1677/joe.0.165r001. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996a;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996b;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- Ewart-Toland A, Mounzih K, Qiu J, Chehab FF. Effect of the genetic background on the reproduction of leptin-deficient obese mice. Endocrinology. 1999;140:732–738. doi: 10.1210/endo.140.2.6470. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez R, Tena-Sempere M, Navarro VM, Barreiro ML, Castellano JM, Aguilar E, Pinilla L. Effects of ghrelin upon gonadotropin-releasing hormone and gonadotropin secretion in adult female rats: in vivo and in vitro studies. Neuroendocrinology. 2005;82:245–255. doi: 10.1159/000092753. [DOI] [PubMed] [Google Scholar]
- Forger NG, Dark J, Barnes BM, Zucker I. Fat ablation and food restriction influence reproductive development and hibernation in ground squirrels. Biol Reprod. 1986;34:831–840. doi: 10.1095/biolreprod34.5.831. [DOI] [PubMed] [Google Scholar]
- Frisch RE, Gotz-Welbergen AV, McArthur JW, Albright T, Witschi J, Bullen B, Birnholz J, Reed RB, Hermann H. Delayed menarche and amenorrhea of college athletes in relation to age of onset of training. JAMA. 1981;246:1559–1563. [PubMed] [Google Scholar]
- Frisch RE, McArthur JW. Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science. 1974;185:949–951. doi: 10.1126/science.185.4155.949. [DOI] [PubMed] [Google Scholar]
- Frisch RE, Wyshak G, Vincent L. Delayed menarche and amenorrhea in ballet dancers. N Engl J Med. 1980;303:17–19. doi: 10.1056/NEJM198007033030105. [DOI] [PubMed] [Google Scholar]
- Gibson WT, Farooqi IS, Moreau M, DePaoli AM, Lawrence E, O'Rahilly S, Trussell RA. Congenital leptin deficiency due to homozygosity for the Delta133G mutation: report of another case and evaluation of response to four years of leptin therapy. J Clin Endocrinol Metab. 2004;89:4821–4826. doi: 10.1210/jc.2004-0376. [DOI] [PubMed] [Google Scholar]
- Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev. 2000;22:261–274. doi: 10.1093/oxfordjournals.epirev.a018038. [DOI] [PubMed] [Google Scholar]
- Gyurko R, Leupen S, Huang PL. Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology. 2002;143:2767–2774. doi: 10.1210/endo.143.7.8921. [DOI] [PubMed] [Google Scholar]
- Hewson AK, Tung LY, Connell DW, Tookman L, Dickson SL. The rat arcuate nucleus integrates peripheral signals provided by leptin, insulin, and a ghrelin mimetic. Diabetes. 2002;51:3412–3419. doi: 10.2337/diabetes.51.12.3412. [DOI] [PubMed] [Google Scholar]
- Hilal EM, Chen JH, Silverman AJ. Joint migration of gonadotropin-releasing hormone (GnRH) and neuropeptide Y (NPY) neurons from olfactory placode to central nervous system. J Neurobiol. 1996;31:487–502. doi: 10.1002/(SICI)1097-4695(199612)31:4<487::AID-NEU8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann JG, Teal TH, Clifton DK, Davis J, Hruby VJ, Han G, Steiner RA. Differential role of melanocortins in mediating leptin's central effects on feeding and reproduction. Am J Physiol Regul Integr Comp Physiol. 2000;278:R50–R59. doi: 10.1152/ajpregu.2000.278.1.R50. [DOI] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31:1984–1998. doi: 10.1111/j.1460-9568.2010.07239.x. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Irani BG, Xiang Z, Moore MC, Mandel RJ, Haskell-Luevano C. Voluntary exercise delays monogenetic obesity and overcomes reproductive dysfunction of the melanocortin-4 receptor knockout mouse. Biochem Biophys Res Commun. 2005;326:638–644. doi: 10.1016/j.bbrc.2004.11.084. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Israel DD, Sheffer-Babila S, de Luca C, Jo YH, Liu SM, Xia Q, Spergel DJ, Dun SL, Dun NJ, Chua SC., Jr Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology. 2012;153:2408–2419. doi: 10.1210/en.2011-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone LE, Higuchi T. Food intake and leptin during pregnancy and lactation. Prog Brain Res. 2001;133:215–227. doi: 10.1016/s0079-6123(01)33016-9. [DOI] [PubMed] [Google Scholar]
- Kamran F, Rother KI, Cochran E, Safar Zadeh E, Gorden P, Brown RJ. Consequences of stopping and restarting leptin in an adolescent with lipodystrophy. Horm Res Paediatr. 2012;78:320–325. doi: 10.1159/000341398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. J Physiol. 1963a;166:408–418. doi: 10.1113/jphysiol.1963.sp007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy GC, Mitra J. Hypothalamic control of energy balance and the reproductive cycle in the rat. J Physiol. 1963b;166:395–407. doi: 10.1113/jphysiol.1963.sp007111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy GC, Mitra J. Spontaneous pseudopregnancy and obesity in the rat. J Physiol. 1963c;166:419–424. doi: 10.1113/jphysiol.1963.sp007113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorram O, Pau KY, Spies HG. Release of hypothalamic neuropeptide Y and effects of exogenous NPY on the release of hypothalamic GnRH and pituitary gonadotropins in intact and ovariectomized does in vitro. Peptides. 1988;9:411–417. doi: 10.1016/0196-9781(88)90277-x. [DOI] [PubMed] [Google Scholar]
- Kimm SY, Barton BA, Obarzanek E, McMahon RP, Sabry ZI, Waclawiw MA, Schreiber GB, Morrison JA, Similo S, Daniels SR. Racial divergence in adiposity during adolescence: The NHLBI Growth and Health Study. Pediatrics. 2001;107:E34. doi: 10.1542/peds.107.3.e34. [DOI] [PubMed] [Google Scholar]
- Kluge M, Schussler P, Uhr M, Yassouridis A, Steiger A. Ghrelin suppresses secretion of luteinizing hormone in humans. J Clin Endocrinol Metab. 2007;92:3202–3205. doi: 10.1210/jc.2007-0593. [DOI] [PubMed] [Google Scholar]
- Kosinski JR, Hubert J, Carrington PE, Chicchi GG, Mu J, Miller C, Cao J, Bianchi E, Pessi A, Sinharoy R, et al. The glucagon receptor is involved in mediating the body weight-lowering effects of oxyntomodulin. Obesity (Silver Spring) 2012;20:1566–1571. doi: 10.1038/oby.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- Lacourse KA, Swanberg LJ, Gillespie PJ, Rehfeld JF, Saunders TL, Samuelson LC. Pancreatic function in CCK-deficient mice: adaptation to dietary protein does not require CCK. Am J Physiol. 1999;276:G1302–G1309. doi: 10.1152/ajpgi.1999.276.5.G1302. [DOI] [PubMed] [Google Scholar]
- Ladyman SR, Grattan DR. Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology. 2004;145:3704–3711. doi: 10.1210/en.2004-0338. [DOI] [PubMed] [Google Scholar]
- Ladyman SR, Grattan DR. Suppression of leptin receptor messenger ribonucleic acid and leptin responsiveness in the ventromedial nucleus of the hypothalamus during pregnancy in the rat. Endocrinology. 2005;146:3868–3874. doi: 10.1210/en.2005-0194. [DOI] [PubMed] [Google Scholar]
- Ladyman SR, Tups A, Augustine RA, Swahn-Azavedo A, Kokay IC, Grattan DR. Loss of hypothalamic response to leptin during pregnancy associated with development of melanocortin resistance. J Neuroendocrinol. 2009;21:449–456. doi: 10.1111/j.1365-2826.2009.01862.x. [DOI] [PubMed] [Google Scholar]
- Lane PW, Dickie MM. Fertile, Obese Male Mice. Relative Sterility in Obese Males Corrected by Dietary Restriction. J Hered. 1954;45:56–58. [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Res. 2010;1364:90–102. doi: 10.1016/j.brainres.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, Li XF, Hu MH, Shao B, Poston L, Lightman SL, O'Byrne KT. Neurokinin B receptor antagonism decreases luteinising hormone pulse frequency and amplitude and delays puberty onset in the female rat. J Neuroendocrinol. 2014 doi: 10.1111/jne.12167. [DOI] [PubMed] [Google Scholar]
- Liu JL, LeRoith D. Insulin-like growth factor I is essential for postnatal growth in response to growth hormone. Endocrinology. 1999;140:5178–5184. doi: 10.1210/endo.140.11.7151. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Louis GW, Greenwald-Yarnell M, Phillips R, Coolen LM, Lehman MN, Myers MG., Jr Molecular mapping of the neural pathways linking leptin to the neuroendocrine reproductive axis. Endocrinology. 2011;152:2302–2310. doi: 10.1210/en.2011-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Cook S, Scrocchi L, Shin J, Kim J, Vaccarino F, Asa SL, Drucker DJ. Neuroendocrine function and response to stress in mice with complete disruption of glucagon-like peptide-1 receptor signaling. Endocrinology. 2000;141:752–762. doi: 10.1210/endo.141.2.7326. [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14:704–710. doi: 10.1038/nn.2818. [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997;138:1190–1193. doi: 10.1210/endo.138.3.5024. [DOI] [PubMed] [Google Scholar]
- Mounzih K, Qiu J, Ewart-Toland A, Chehab FF. Leptin is not necessary for gestation and parturition but regulates maternal nutrition via a leptin resistance state. Endocrinology. 1998;139:5259–5262. doi: 10.1210/endo.139.12.6523. [DOI] [PubMed] [Google Scholar]
- Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL. Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology. 1998;67:370–376. doi: 10.1159/000054335. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Ruiz-Pino F, Sanchez-Garrido MA, Garcia-Galiano D, Hobbs SJ, Manfredi-Lozano M, Leon S, Sangiao-Alvarellos S, Castellano JM, Clifton DK, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci. 2012;32:2388–2397. doi: 10.1523/JNEUROSCI.4288-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizard J, Dommergues M, Clement K. Pregnancy in a woman with a leptin-receptor mutation. N Engl J Med. 2012;366:1064–1065. doi: 10.1056/NEJMc1200116. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa SM, Moriyama RM, Caligioni CS, Yang JJ, Cho CM, Concepcion TL, Oakley AE, Lee IH, Sanz E, Amieux PS, et al. Redundancy in Kiss1 expression safeguards reproduction in the mouse. Endocrinology. 2013;154:2784–2794. doi: 10.1210/en.2013-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993;7:2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Ogus S, Mounzih K, Ewart-Toland A, Chehab FF. Leptin-deficient mice backcrossed to the BALB/cJ genetic background have reduced adiposity, enhanced fertility, normal body temperature, and severe diabetes. Endocrinology. 2001;142:3421–3425. doi: 10.1210/endo.142.8.8323. [DOI] [PubMed] [Google Scholar]
- Qiu X, Dowling AR, Marino JS, Faulkner LD, Bryant B, Bruning JC, Elias CF, Hill JW. Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from Kiss1 cells. Endocrinology. 2013;154:1337–1348. doi: 10.1210/en.2012-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494–4503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynoso R, Ponzo OJ, Szwarcfarb B, Rondina D, Carbone S, Rimoldi G, Scacchi P, Moguilevsky JA. Effect of leptin on hypothalamic release of GnRH and neurotransmitter amino acids during sexual maturation in female rats. Exp Clin Endocrinol Diabetes. 2003;111:274–277. doi: 10.1055/s-2003-41285. [DOI] [PubMed] [Google Scholar]
- Roa J, Herbison AE. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology. 2012;153:5587–5599. doi: 10.1210/en.2012-1470. [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Jenkins A, Oakes SR, Ormandy CJ, Herzog H. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev. 2002;16:1077–1088. doi: 10.1101/gad.979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal-Lieberman G, Bradley RL, Kokkotou E, Carlson M, Trombly DJ, Wang X, Bates S, Myers MG, Jr, Flier JS, Maratos-Flier E. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci U S A. 2003;100:10085–10090. doi: 10.1073/pnas.1633636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG, O'Rahilly S, Aparicio SA. Two novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:1849–1855. doi: 10.1210/jc.2004-1418. [DOI] [PubMed] [Google Scholar]
- Sheffer-Babila S, Sun Y, Israel DD, Liu SM, Neal-Perry G, Chua SC., Jr Agouti-related peptide plays a critical role in leptin's effects on female puberty and reproduction. Am J Physiol Endocrinol Metab. 2013;305:E1512–E1520. doi: 10.1152/ajpendo.00241.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Zimmerman EA, Gibson MJ, Perlow MJ, Charlton HM, Kokoris GJ, Krieger DT. Implantation of normal fetal preoptic area into hypogonadal mutant mice: temporal relationships of the growth of gonadotropin-releasing hormone neurons and the development of the pituitary/testicular axis. Neuroscience. 1985;16:69–84. doi: 10.1016/0306-4522(85)90048-x. [DOI] [PubMed] [Google Scholar]
- Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- Stanley SA, Small CJ, Kim MS, Heath MM, Seal LJ, Russell SH, Ghatei MA, Bloom SR. Agouti related peptide (Agrp) stimulates the hypothalamo pituitary gonadal axis in vivo & in vitro in male rats. Endocrinology. 1999;140:5459–5462. doi: 10.1210/endo.140.11.7248. [DOI] [PubMed] [Google Scholar]
- Steiner J, LaPaglia N, Kirsteins L, Emanuele M, Emanuele N. The response of the hypothalamic-pituitary-gonadal axis to fasting is modulated by leptin. Endocr Res. 2003;29:107–117. doi: 10.1081/erc-120022292. [DOI] [PubMed] [Google Scholar]
- Suter KJ, Pohl CR, Wilson ME. Circulating concentrations of nocturnal leptin, growth hormone, and insulin-like growth factor-I increase before the onset of puberty in agonadal male monkeys: potential signals for the initiation of puberty. J Clin Endocrinol Metab. 2000;85:808–814. doi: 10.1210/jcem.85.2.6371. [DOI] [PubMed] [Google Scholar]
- Sutton SW, Toyama TT, Otto S, Plotsky PM. Evidence that neuropeptide Y (NPY) released into the hypophysial-portal circulation participates in priming gonadotropes to the effects of gonadotropin releasing hormone (GnRH) Endocrinology. 1988;123:1208–1210. doi: 10.1210/endo-123-2-1208. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigersky RA, Andersen AE, Thompson RH, Loriaux DL. Hypothalamic dysfunction in secondary amenorrhea associated with simple weight loss. N Engl J Med. 1977;297:1141–1145. doi: 10.1056/NEJM197711242972103. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattigney WA, Srinivasan SR, Chen W, Greenlund KJ, Berenson GS. Secular trend of earlier onset of menarche with increasing obesity in black and white girls: the Bogalusa Heart Study. Ethn Dis. 1999;9:181–189. [PubMed] [Google Scholar]
- Weinstock PH, Bisgaier CL, Hayek T, Aalto-Setala K, Sehayek E, Wu L, Sheiffele P, Merkel M, Essenburg AD, Breslow JL. Decreased HDL cholesterol levels but normal lipid absorption, growth, and feeding behavior in apolipoprotein A-IV knockout mice. J Lipid Res. 1997;38:1782–1794. [PubMed] [Google Scholar]
- Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- Woods KA, Camacho-Hubner C, Bergman RN, Barter D, Clark AJ, Savage MO. Effects of insulin-like growth factor I (IGF-I) therapy on body composition and insulin resistance in IGF-I gene deletion. J Clin Endocrinol Metab. 2000;85:1407–1411. doi: 10.1210/jcem.85.4.6495. [DOI] [PubMed] [Google Scholar]
- Woods KA, Camacho-Hubner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- Wu Q, Whiddon BB, Palmiter RD. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc Natl Acad Sci U S A. 2012;109:3155–3160. doi: 10.1073/pnas.1120501109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Morton GJ, Ogimoto K, Stanhope K, Graham J, Baskin DG, Havel P, Schwartz MW, Barsh GS. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol. 2005;3:e415. doi: 10.1371/journal.pbio.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Yura S, Ogawa Y, Sagawa N, Masuzaki H, Itoh H, Ebihara K, Aizawa-Abe M, Fujii S, Nakao K. Accelerated puberty and late-onset hypothalamic hypogonadism in female transgenic skinny mice overexpressing leptin. J Clin Invest. 2000;105:749–755. doi: 10.1172/JCI8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]