Abstract
Background and objectives
Immunotherapy for drug addiction is being investigated in several laboratories but most studies are conducted in animals of one sex. Yet, women show heightened immune responses and are more likely to develop autoimmune diseases than men. The purpose of this study was to compare the effects of an active anti-cocaine vaccine, succinyl-norcocaine conjugated to keyhole limpet hemocyanin, for its ability to elicit antibodies and alter cocaine-induced ambulatory activity in male versus female mice.
Methods
Male and female BALB/c mice were vaccinated (n=44) or served as non-vaccinated controls (n=34). Three weeks after initial vaccination, a booster was given. Ambulatory activity induced by cocaine (20 mg/kg) was assessed at 7-wk and plasma obtained at 8-wk to assess antibody levels.
Results
High antibody titers were produced in mice of both sexes. The vaccine reduced ambulatory activity cocaine-induced but this effect was greater in female compared to male mice.
Discussion and conclusions
The efficacy of this anti-cocaine vaccine is demonstrated in mice of both sexes but its functional consequences are greater in females than males.
Scientific significance
Results point to the importance of testing animals of both sexes in studies of immunotherapies for addiction.
Keywords: sex differences, vaccine, addiction, cocaine antibodies
Introduction
Cocaine addiction is a serious problem in the United States but there are no approved pharmacotherapies (1). A common approach to developing medications for addiction is to test if an agent alters the behavioral effects of the abused drug (2, 3). Agents are often chosen because they target neuropharmacological mechanisms affected by the abused drug. Rather than selecting agents because they block or mimic effects of the abused drug in brain, we, and others are developing immunotherapies for addiction (4–6). An effective addiction vaccine should produce antibodies that sequester the drug in circulation, if ingested, to slow drug entry into the brain. Slower drug entry would reduce the drug’s reinforcing effects (7–9) and presumably help the recovering addict maintain abstinence. We previously provided clinical support for the efficacy of a cocaine vaccine by showing it reduced cocaine-positive urines, particularly in addicts who produced high antibody levels (10, 11).
There was a great deal of variability in antibody responses among the subjects in the Martell studies and some participants produced very low levels of antibodies. We believe that this vaccine, made from succinyl norcocaine (SNC) and conjugated to cholera toxin B (12), could be improved by altering the carrier protein to which the SNC hapten is attached (4). Our recent work testing vaccines for methamphetamine (13) and morphine (14) utilized keyhole limpet hemocyanin (KLH) as the carrier protein and these vaccines blocked conditioned effects of the targeted drugs in rodents. KLH is likely to improve upon the immunogenicity of cholera toxin B. It can cause activation and maturation of monocyte derived dendritic cells for antigen presentation via signaling through the mannose receptor and eliciting high interleukin 12 production (15, 16). Thus, we constructed a new cocaine conjugate vaccine, SNC-KLH, and tested it in mice.
A second purpose of this study is to include assessments of the cocaine vaccine in mice of both sexes. This is important for vaccine treatment development in general because, relative to men, women show greater antiviral, inflammatory, and cellular immune responses, and are more likely to develop autoimmune diseases (17). For example, a candidate herpes simplex virus (HSV) vaccine is more efficacious in women than men (18). Sex hormones can affect vaccine responses; ovariecotomized female mice exhibit less protection against HSV infection than those given progesterone and 17β-estradiol (19). Further, gender should be considered in the design and application of anti-drug vaccines because male and female cocaine addicts differ in the course of addiction (20, 21) and in response to traditional treatments (22). Although there are more adult male cocaine users than females, this gender gap is closing among adolescent cocaine users (23) suggesting a possible increased prevalence of cocaine addiction among women in the future. A vaccine treatment may work well in females, but not males, but could be abandoned from further tests if only male subjects are evaluated. Or, women may show a more efficacious response (17) suggesting a lower amount be used to avoid potential adverse side-effects. Thus, we tested the ability of active immunization with the anti-cocaine vaccine, SNC-KLH, to alter the acute locomotor activating effects of cocaine in male and female BALB/c mice.
Methods
Animals and housing
Male and female BALB/c mice were housed in groups of five by sex in standard, shoebox cages under temperature- and humidity-controlled conditions. Food and water were available ad libitum in a colony maintained on a 12:12 light/dark cycle with lights on at 0600. All procedures were approved by the Institutional Animal Care and Use Committee in accordance with regulations of the Animal Welfare Act.
Mice were either administered the SNC-KLH vaccine as described below or served as non-vaccinated controls. The sample sizes per sex and vaccine group are as follows: 10 control females; 24 control males; 15 SNC-KLH females; and 29 SNC-KLH male mice.
Drug
Cocaine HCl (Research Triangle Institute, Research Triangle, NC) was dissolved in isotonic saline and prepared as salt base. A dose of 20 mg/kg was used because we find it increases ambulatory activity without inducing stereotypy.
SNC-KLH vaccine
Succinyl norcocaine (SNC) was prepared as described previously (12). The SNC-KLH vaccine was prepared according to the method we used previously to synthesize a 6-succinyl morphine-KLH conjugate vaccine (14).
Immunization and anti-cocaine determination
The vaccine (100 μg/mouse) plus 1.5 mg of alum (Sigma) was administered (IM) into the thigh of each hind leg (80–100 μl per site). A booster of the same amount was given at 3-wk. At 8-wks, blood was collected and titers of specific anti-cocaine IgG antibodies were determined using enzyme-linked immunosorbent assay (ELISA). Antibody titer levels were defined by the dilution of serum calculated to give an optical density of 1.0 (background in the ELISA was less than 0.2 for all samples al these dilutions). Sex differences in antibody titers were compared by t-test.
Locomotor activity
At 7-wks, mice were tested for effects of cocaine on ambulatory activity using an automated system of 15 apparatuses (Opto-M3; Columbus Instruments; Columbus, OH). Mice were placed individually into an acrylic test cage (17.5″ L X 17.5″ W X 8″ H) located between parallel bars of infrared sensors placed 1″ above the floor. Numbers of beam breaks were tabulated and relayed to a computer and data stored in 10-min time blocks. The first 60-min of the test session was used to habituate the mice to the apparatus with the last 10-min block utilized as baseline (non-drug). After habituation, mice were injected with cocaine (20 mg/kg; IP) and placed back into the apparatus for another 60-min. This session length was chosen because IP cocaine administration in mice leads to peak brain values within 5-min and has a half-life of 16-min in plasma and brain (24).
Assessments of cocaine-induced locomotor activity were determined by tabulating ambulatory counts per 10-min time period across the session. The activity levels from the final 10-min time block of the habituation session were used as co-variate in the analysis of the cocaine-induced activity levels. That is, the data were analyzed with 2 X 2 X 6 ANOCOVA representing between group factors of Sex and Vaccine with repeated measures on Time. Significance level was set at P<0.05. Data are presented as mean (± S.E.M.).
Results
Anti-Cocaine antibody levels
Antibody titers in serum did not differ between female (117.8 × 10−3 ± 21.4 × 10−3) and male (143.7 × 10−3 ± 18.3 × 10−3) mice at 8-wk after the initial immunization, P>0.10.
Locomotor activity
Ambulatory activity counts during the 60-min habituation period did not differ by Sex or Vaccine group nor was the interaction significant, P’s>0.10. There was a significant Time effect, F(5,370)=14.78; P<0.0001. Activity levels decreased across this period (data not shown). The ambulatory activity counts during the final 10-min segment of the habituation period are shown on the left side of Fig. 1 and were used as a co-variate in the analysis of the cocaine-induced activity level data.
FIGURE 1.
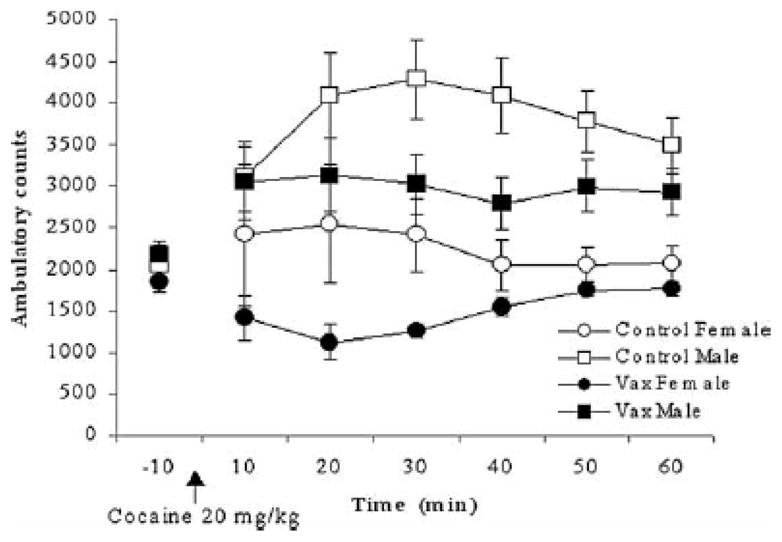
Mean (±SEM) ambulatory counts for vaccinated (Vax; closed symbols) and non-vaccinated (control; open symbols), female (circles) and male (squares) mice are shown by 10-min intervals after a cocaine (20 mg/kg) injection. The −10 point is the mean ambulatory counts during the last 10-min of a 60-min habituation session. Cocaine-induced ambulatory activity levels are decreased by the SNC-KLH vaccine and are lower in female versus male mice. See text for statistics.
Ambulatory activity levels after cocaine administration are shown in Fig. 1 by 10-min time blocks in control and vaccinated, female and male mice. Ambulatory activity induced by cocaine differed across the session as supported by the significant Time effect, F(5,370)=2.47; P<0.05. Activity levels were significantly less in female versus male mice as supported by the significant Sex effect, F(1,74)=18.17; P<0.0001. Mice vaccinated with SNC-KLH showed lower levels of ambulatory activity as supported by the significant Vaccine effect, F(1,74)=5.19; P<0.05. In addition, the effect of Time showed a significant two-way interaction with Vaccine, F(5,370)=4.63; P<0.00005, and a significant three-way interaction with Vaccine and Sex, F(5,370)=2.42; P<0.05. As seen in Fig 1, these interactions with Time may reflect that the greatest differences between vaccine groups of both sexes were seen earlier in the session (i.e., 20–40 min time blocks), particularly for females, compared to later in the session (50–60 min time blocks).
Inspection of the data in Fig. 1 shows that, relative to the habituation baseline, cocaine administration led to a decrease in activity levels in female mice that were vaccinated with SNC-KLH. In contrast, male mice exhibited increased ambulation in response to cocaine administration but this level was lower among male mice that were vaccinated with SNC-KLH. Because the main effect of Sex was significant, we explored the role of variable on the functional effect of the vaccine by performing analyses separately in male and female mice. The Vaccine effect was significant among female mice, F(1,23)=6.04; P<0.02, but not among male mice, P>0.10.
Discussion
The results of this study show that mice vaccinated with SNC-KLH produce antibodies against cocaine and exhibit attenuated cocaine-induced locomotor activity. Antibody titers do not differ between mice of both sexes. However, while the overall vaccine effect on locomotor activity was significant, separate analyses by Sex reveals that much of the significance is carried by female mice. This is seen despite the fact that there are almost twice as many male mice per Vaccine group than female mice. The interaction of Vaccine and Sex is not significant but this likely reflects that the vaccine decreased activity levels in both sexes. Overall, the results of this preliminary study point to the importance of testing potential vaccines for addiction in animals of both sexes.
While our prediction that female mice would show heightened immune responses relative to male mice did not hold (17), we did not anticipate that cocaine-induced locomotor activity would be lower in females compared to males. Much previous research conducted with rodents of both sexes shows the opposite – higher cocaine-induced activity levels in females versus males – most likely due to gonadal hormone effects (25–29). Indeed, female rats in estrus (29) or those that were ovarectomized and received hormone replacement with either estrogen alone or combined with progesterone, but not progesterone alone (27), exhibit enhanced cocaine-induced activity levels. Female rats in estrus also show enhanced responding for cocaine under a progressive ratio schedule of self-administration (30). In the present study, female mice were cycling freely and estrous stage was not assessed. It would be important to control for gonadal hormone status in future studies that assess immunization to cocaine.
Among most strains of mice and rats, females show greater activational effects of cocaine than males. Why the direction of this sex difference differs in some strains of mice, including the BALB/c strain (31, 32) that we employed in this study and in our prior work with vaccines (13), is not clear. However, the functional effect of the SNC-KLH vaccine was greater in female mice despite the fact that there was no sex difference in immune response. This suggests that females may be the preferred sex to use when examining potential vaccine treatments with this strain, a commonly employed strain among immunology researchers. It would be of interest to determine whether the SNC-KLH vaccine shows similar sex-dependent functional effects in other strains of rodents particularly those that show the more typical sex-dependent pattern of responsivity to cocaine.
A number of researchers have tested effects of passive or active immunization against cocaine in rodents but each study was conducted with rodents of one sex. Antibodies from active immunization with anti-cocaine vaccines reduced the conditioned rewarding, analgesic, and discriminative stimulus effects of cocaine in female rats (33, 34) and decreased the locomotor effects of cocaine in male rats (35, 36). Active and passive immunizations against cocaine in male rats also attenuated cocaine self-administration and blocked the reinstatement of this behavior in rats (12, 37). The results of the present study show the utility of testing potential anti-cocaine vaccines in animals of both sexes. Future studies should test the ability of cocaine (or other anti-drug) vaccines on a number of behavioral procedures using a wider range of doses in both males and females as well as investigate the contribution of gonadal hormones.
Acknowledgments
This material is the result of work supported with resources and use of facilities at the Michael E. DeBakey Veterans Affairs Medical Center, Houston, TX and supported by grants DP1DA033502 and U01023898 from The National Institute on Drug Abuse (NIDA).
The authors thank Y. Wu and B. Mao for their excellent technical assistance.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.SAMHSA. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-46, HHS Publication No (SMA) 13-4795 2013 [cited; Available from: http://www.samhsa.gov/data/NSDUH/2012SummNatFindDetTables/NationalFindings/NSDUHresults2012.pdf.
- 2.Koob GF, Lloyd GK, Mason BJ. Development of pharmacotherapies for drug addiction: a Rosetta Stone approach. Nature Reviews Drug Discovery. 2009;8:500–15. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haile CN, Kosten TR, Kosten TA. Pharmacogenetic treatments for drug addiction: cocaine, amphetamine and methamphetamine. American Journal of Drug and Alcohol Abuse. 2009;35:161–77. doi: 10.1080/00952990902825447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinsey BM, Kosten TR, Orson FM. Anti-cocaine vaccine development. Expert Reviews Vaccines. 2010;9:1109–14. doi: 10.1586/erv.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens SM, Atchley WT, Hambuchen MD, Peterson EC, Gentry WB. Monoclonal antibodies as pharmacokinetic antagonists for the treatment of (+)-methamphetamine addiction. CNS and Neurological Disorders Drug Targets. 2011;10:892–8. doi: 10.2174/187152711799219370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janda KD, Trewwek JB. Vaccines targeting drugs of abuse: Is the glass half-empty or half-full? Nature Reviews Immunology. 2012;12:67–72. doi: 10.1038/nri3130. [DOI] [PubMed] [Google Scholar]
- 7.deWit H, Bodker B, Amber J. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology. 1992;107:352–8. doi: 10.1007/BF02245161. [DOI] [PubMed] [Google Scholar]
- 8.Marsch LA, Bickel WK, Badger GJ, et al. Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. Journal of Pharmacology and Experimental Therapeutics. 2001;299:1056–65. [PubMed] [Google Scholar]
- 9.Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. European Journal of Pharmacology. 2004;486:251–7. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Archives of General Psychiatry. 2009;66:1116–23. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biological Psychiatry. 2005;58:158–64. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Fox BS, Kantak KM, Edwards MA, et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nature Medicine. 1996;2:1129–32. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- 13.Shen XY, Kosten TA, Lopez AY, Kinsey BM, Kosten TR, Orson FM. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug and Alcohol Dependence. 2013;129:41–8. doi: 10.1016/j.drugalcdep.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosten TA, Shen XY, O’Malley PW, et al. A morphine conjugate vaccine attenuates the behavioral effects of morphine in rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2013;45:223–9. doi: 10.1016/j.pnpbp.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Presicce P, Taddeo A, Conti A, Villa ML, DellaBella S. Keyhole limpet hemocyanin induces the activation and maturation of human dendritic cells through the involvement of mannose receptor. Molecualr Immunology. 2008;45:1136–45. doi: 10.1016/j.molimm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Gloudemans AK, Plantinga M, Guilliams M, et al. The mucosal adjuvant cholera toxin B instructs non-mucosal dendritic cells to promote IgA production via retinoic acid and TGF-β. PLoS One. 2013;8:59822. doi: 10.1371/journal.pone.0059822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infectous Disease. 2010;10:338–49. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Standberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. New England Journal of Medicine. 2002;247:1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 19.Gillgrass AE, Tang VA, Towarnicki KM, Rosenthal KL, Kaushic C. Protection against genital herpes infection in mice immunized under different hormonal conditions correlates with induction of vagina-associated lymphoid tissue. Journal of Virology. 2005;79:3117–26. doi: 10.1128/JVI.79.5.3117-3126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. Journal of Substance Abuse Treatment. 1993;10:63–6. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- 21.Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug and Alcohol Dependence. 1999;53:223–30. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- 22.Fattore L, Altea S, Fratta W. Sex differences in drug addiction: A review of animal and human studies. Women and Health. 2008;4:51–65. doi: 10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- 23.Cotto JH, Davis E, Dowling GJ, Elcano JC, Staton AB, Weiss SRB. Gender effects on drug use, abuse, and dependence: a special analysis of results from the National Survey on Drug Use and Health. Gender Medicine. 2010;7:402–12. doi: 10.1016/j.genm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Benuck M, Laitha A, Reith ME. Pharmacokinetics of systemically administered cocaine and locomotor stimulation in mice. Journal of Pharmacology and Experimental Therapeutics. 1987;243:144–9. [PubMed] [Google Scholar]
- 25.Thomsen M, Caine SB. Cocaine self-administration under fixed and progressive ratio schedules of reinforcement: comparison of C57BL/6J, 129X1/SvJ, and 129S6/SvEvTac inbred mice. Psychopharmacology. 2006;184:145–54. doi: 10.1007/s00213-005-0207-0. [DOI] [PubMed] [Google Scholar]
- 26.Schindler CW, Carmona GN. Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacology, Biochemistry & Behavior. 2002;72:857–63. doi: 10.1016/s0091-3057(02)00770-0. [DOI] [PubMed] [Google Scholar]
- 27.Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. Journal of Pharmacology and Experimental Therapeutics. 2000;293:879–86. [PubMed] [Google Scholar]
- 28.Sircar R, Kim D. Female gonadal hormones differentially modulate cocaine-induced behavioral sensitization in Fischer, Lewis, and Sprague-Dawley rats. Journal of Pharmacology & Experimental Therapeutics. 1999;289:54–65. [PubMed] [Google Scholar]
- 29.Quinones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behavioural Brain Research. 1999;101:15–20. doi: 10.1016/s0166-4328(98)00073-4. [DOI] [PubMed] [Google Scholar]
- 30.Roberts DCS, Bennett SAL, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–11. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- 31.Itzhak Y, Ali SF, Martin JL, Black MD, Huang PL. Reisistance of neuronal nitric oxide synthase-deficient mice to cocaine-induced locomotor sensitization. Psychopharmacology. 1998;140:378–86. doi: 10.1007/s002130050779. [DOI] [PubMed] [Google Scholar]
- 32.Thomsen M, Caine SB. Psychomotor stimulant effects of cocaine in rats and 15 mouse strains. Experimental and Clinical Psychopharmacology. 2011;19:321–41. doi: 10.1037/a0024798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ettinger RH, Ettinger WF, Harless WE. Active immunization with cocaine-protein conjugate attenuates cocaine effects. Pharmacology Biochemistry and Behavior. 1997;58:215–20. doi: 10.1016/s0091-3057(97)00005-1. [DOI] [PubMed] [Google Scholar]
- 34.Johnson MW, Ettinger RH. Active cocaine immunization attenuates the discriminative properties of cocaine. Experimental and Clinical Psychopharmacology. 2000;8:163–7. doi: 10.1037//1064-1297.8.2.163. [DOI] [PubMed] [Google Scholar]
- 35.Carrera MRA, Ashley JA, Wirsching P, Koob GF, Janda KD. A second-generation vaccine protects against the psychoactive effects of cocaine. Proceedings of the National Academy of Sciences. 2001;98:1988–92. doi: 10.1073/pnas.041610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrera MRA, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378:727–30. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- 37.Carrera MRA, Ashley JA, Zhou B, Wirsching P, Koob GF, Janda KD. Cocaine vaccines: Antibody protection against relapse in a rat model. Proceedings of the National Academy of Sciences. 2000;97:6202–6. doi: 10.1073/pnas.97.11.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]


