Abstract
Scope
Broccoli sprouts are a rich source of glucosinolates, a group of phytochemicals that when hydrolyzed, are associated with cancer prevention. Our objectives were to investigate the metabolism, distribution, and interconversion of isothiocyanates (ITCs) in mice fed thermally processed broccoli sprout powders (BSPs) or the purified ITC sulforaphane.
Methods and results
For 1 wk, mice were fed a control diet (n = 20) or one of four treatment diets (n = 10 each) containing nonheated BSP, 60°C mildly heated BSP, 5-min steamed BSP, or 3 mmol purified sulforaphane. Sulforaphane and erucin metabolite concentrations in skin, liver, kidney, bladder, lung, and plasma were quantified using HPLC-MS/MS. Thermal intensity of BSP processing had disparate effects on ITC metabolite concentrations upon consumption. Mild heating generally resulted in the greatest ITC metabolite concentrations in vivo, followed by the nonheated and steamed BSP diets. We observed interconversion between sulforaphane and erucin species or metabolites, and report that erucin is the favored form in liver, kidney, and bladder, even when only sulforaphane is consumed.
Conclusion
ITC metabolites were distributed to all tissues analyzed, suggesting the potential for systemic benefits. We report for the first time tissue-dependent ratio of sulforaphane and erucin, though further investigation is warranted to assess biological activity of individual forms.
Keywords: Broccoli sprouts, Erucin, Interconversion, Isothiocyanates, Sulforaphane
1 Introduction
Epidemiological evidence demonstrates a particularly strong link between consumption of cruciferous vegetables, such as broccoli, and decreased risk of cancer at several organ sites [1], including the bladder [2]. Cell culture and animal studies suggest that isothiocyanates (ITCs), phytochemicals unique to cruciferous vegetables, are the source of this chemoprevention. Several anticarcinogenic mechanisms are associated with ITCs, including modulation of phase I and II enzyme systems [3,4], anti-inflammatory processes [5], and induction of apoptosis [6].
ITCs are derived from naturally occurring, biologically inactive precursors called glucosinolates, via an enzymatic hydrolysis. The endogenous plant enzyme myrosinase, physically segregated from glucosinolates in the plant cell, is released upon chopping or chewing, thus hydrolyzing glucosinolates to a variety of products, including ITCs and nitriles [7]. Alternatively, if myrosinase has been thermally destroyed during food processing or preparation (e.g. from blanching, steaming, or microwaving), thioglucosidases in animal and human gut microflora may also hydrolyze consumed glucosinolates to ITCs. However, microflora hydrolysis has been reported to be inefficient and highly variable in humans [8–11].
In some broccoli cultivars, the presence of the epithiospecifier protein (ESP) drives formation of glucosinolates to nitrile products, which unlike ITCs, have no biological activity [7, 12]. Matusheski et al. determined that in broccoli with active ESP, hydrolysis of the predominant glucosinolate, glucoraphanin, produced nine times more inactive nitrile product than ITC [13]. Interestingly, due to the different thermal labilities of ESP and myrosinase, mild heating (60–70°C) has been demonstrated to selectively inactivate ESP while retaining myrosinase activity. As a result, nitrile production is minimized with a concomitant increase in ITC formation [13]. These findings have significant implications for consumers, as they suggest mild heating may maximize the dose of ITCs delivered upon consumption, potentially increasing the health benefits of broccoli consumption [14]. However, to our knowledge, this hypothesis has not been studied in vivo.
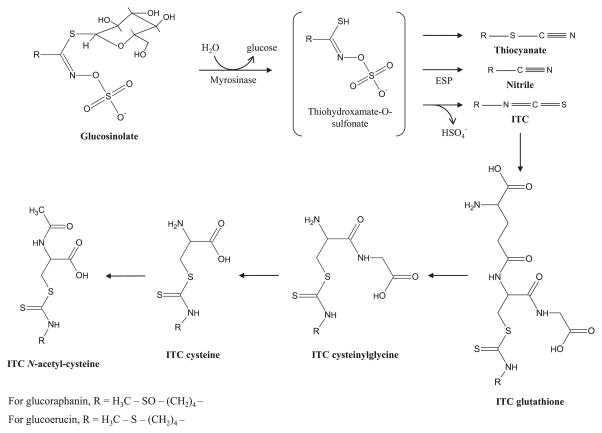
After conversion from glucosinolates, consumed ITCs are metabolized via the mercapturic acid pathway. An ITC is first conjugated to glutathione (GSH), followed by sequential conversion to the conjugates ITC-cysteinylglycine (CG), ITC-cysteine (Cys), and ITC-N-acetylcysteine (NAC) (Fig. 1). A recent study in mice measured the concentration of the ITC sulforaphane, along with its metabolic conjugates, in multiple tissues and observed the highest concentrations in the small intestine, prostate, and kidney [15]. However, this study likely underestimated true ITC metabolite concentrations in the examined tissues because sulforaphane, a sulfoxide, is reduced in part to its thioether analog, the ITC erucin, in vivo [16,17].
Figure 1.
Metabolism of isothiocyanates through the mercapturic acid pathway. Glucosinolates are hydrolyzed to thiocyanates, nitriles, or ITCs by the enzyme myrosinase. The presence or absence of ESP can dictate if nitriles or ITCs are favored. ITCs are conjugated to GSH, followed by sequential hydrolysis to CG, Cys, and NAC.
In this study, we sought to compare consumption of non-heated, steamed, and mildly heated broccoli sprout powders (BSPs) to determine the impact of these processing treatments on ITC metabolite concentrations in vivo. To further investigate how the metabolites of sulforaphane and erucin are metabolized, distributed, and interconverted, an additional group of mice consumed a diet supplemented with purified sulforaphane and no BSP.
2 Materials and methods
2.1 Chemicals
Optima-grade solvents were used for all analyses involving MS and HPLC-grade solvents were used for all other analyses (Fisher Scientific; Pittsburgh, PA, USA). 2-mercaptoethanol, TFA, and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Triethylamine was from EMD Chemical (Gibbstown, NJ, USA). D,L-Sulforaphane used for external standard calibration was from Toronto Research Chemicals (Toronto, Ontario, Canada) and D,L-Sulforaphane used for the mouse diet was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Glucoraphanin and glucoerucin standards were purchased from the Royal Veterinary and Agricultural University (Copenhagen, Denmark). ITC metabolite standards were synthesized in lab, as described below, with the initial ITC standards purchased from LKT Laboratories (St. Paul, MN, USA).
2.2 Processing of fresh broccoli sprouts
Fresh, 6-day old broccoli sprouts (Brassica oleracea) “Calabrese” were provided by a commercial supplier (Sun Sprout Farms, Columbus, OH) and stored refrigerated until processing the same day. The sprouts received one of three processing treatments: freeze-dried raw without heating, mildly heated at 60°C and freeze-dried, or steamed and freeze-dried. The 60°C mild heat treatment was performed by placing raw broccoli sprouts in zipper storage bags, removing the air, and submerging in a 60°C water bath for 10 min. Sprouts were placed in the zipper storage bags to prevent leaching of glucosinolates into the water bath. To steam, raw sprouts were placed in a sieve, covered, and set over a boiling water bath for 5 min. To facilitate equal heating, the sprouts were tossed occasionally. Raw, 60°C-mildly heated, and steamed sprouts were flash-frozen in liquid nitrogen and freeze-dried. After lyophilization, all broccoli sprouts were ground to a powder with a mortar and pestle in a low humidity environment to prevent uptake of water.
2.3 Glucosinolate extraction and analysis
The glucosinolates glucoraphanin and glucoerucin were quantified in the three freeze-dried BSPs, using a previously reported method [10]. BSP (40 mg) was added to a glass vial, followed by addition of 3 mL boiling water to denature endogenous myrosinase and preserve the glucosinolates. The loosely capped vial was immediately placed in a boiling water bath for 5 min, after which it was alternately vortexed and sonicated for 5 min. The sample was centrifuged for 5 min at 600 × g and the aqueous extract was decanted and set aside. Twice more, the pellet was resuspended in 3 mL of room temperature water and vortexed/sonicated for 10 min, followed by centrifugation and decanting. The three supernatants were combined, yielding approximately 9 mL aqueous extract. This extract was brought up to 10 mL with water for analysis.
Glucoraphanin and glucoerucin were separated on a Zorbax SB-CN RP column (4.6 × 250 mm; 5 μm; Agilent Technologies, Santa Clara, CA, USA) with an Agilent 1100 Series HPLC. A binary gradient, consisting of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), was used at a flow rate of 1.5 mL/min. Initial conditions consisted of 0% B for 3 min, followed by linear increases to 10% B by 4 min, 50% B by 8 min, and 95% B by 9 min, after which the column was equilibrated at 0% B for 3 min. An AB Sciex QTrap 5500 mass spectrometer (Concord, Ontario, Canada), operated in electrospray negative-ion mode, was used for quantitation. Selected reaction monitoring was based on the common liberation of HSO43 anion from the glucosinolate. Thus, the transitions for glucoraphanin (436 > 97) and glucoerucin (420 > 97) were used, with dwell times of 140 ms. Other relevant MS parameters include a desolvation gas temperature of 550°C, declustering potential of 70 V, entrance potential was 10 V, collision cell exit potential was 11 V, collision energy of 30 eV, ion spray potential of 4.5 kV, gas 1 at 60 psi, gas 2 at 55 psi, and the curtain gas pressure at 30 psi. An external standard curve of authentic standards (glucoraphanin, glucoerucin) was used to quantify glucosinolates.
2.4 Glucosinolate hydrolysis, ITC extraction, and analysis
Sulforaphane formation in the three BSPs was measured using a method described by Vermeulen et al. [18], with minor modifications. Approximately, 25 mg of the freeze-dried powder was combined with 5 mL water and incubated at 45°C for 2 h to allow glucosinolate hydrolysis. Next, 10 mL dichloromethane was added and the mixture was mechanically shaken for 20 min and centrifuged at 600 × g for 10 min, followed by removal of the dichloromethane layer. Once more, the sample was extracted in dichloromethane, and the dichloromethane layers were combined, creating ~20 mL extract, which was brought up to 25 mL with dichloromethane. A 3 mL aliquot was mixed with 1 mL of conjugating reagent (20 mM triethylamine and 200 mM 2-mercaptoethanol in dichloromethane) to stabilize and increase the molar extinction coefficient of the ITCs. This mixture was incubated at 30°C for 60 min and then dried under gaseous nitrogen.
Samples were reconstituted in 1:1 water:acetonitrile, then passed through 0.45 μm nylon filters into HPLC vials. Separation and quantification of the 2-mercaptoethanol conjugate of sulforaphane was achieved using a Waters 2695 separations module coupled to a Waters 996 photo diode array detector (Milford, MA, USA), with 10 μL injected into a Waters Symmetry C18 column (3.5 μm, 4.6 × 75 mm). A linear gradient, consisting of solution A (0.1% formic acid in water) and solution B (0.1% formic acid in acetonitrile) was employed. Initial conditions of 0% B increased to 50% B over 15 min. The 2-mercaptoethanol conjugate of sulforaphane was quantified by creating an external standard curve. It was assumed that sulforaphane conjugated with equal efficiency in the extract and standard solution.
2.5 Creation of broccoli sprout and sulforaphane diets
Five diets were produced for this study: a control diet, diets containing either the nonheated, 60°C-mildly heated, or 5-min steamed BSPs, and a diet containing purified sulforaphane. AIN-93G powder mouse diet was used as the control diet, and a modified AIN-93G diet, equalized for total calories and macronutrient content, was used as the base for the broccoli sprout and sulforaphane diets (Harlen-Teklad, Madison, WI, USA). The freeze-dried BSPs were mixed by hand until homogeneous, and added to their respective diets at 4% (w/w). For the sulforaphane diet, the diet manufacturer dispersed sulforaphane in soybean oil prior to blending with the other ingredients, creating a diet with a final concentration of 3 mmol sulforaphane/kg diet. This concentration was chosen to approximate the molar concentration of glucosinolates in the BSP diets. Analysis of the sulforaphane diet confirmed that sulforaphane was the only ITC present (data not shown). All diets were stored refrigerated until use.
2.6 Animals and housing
The Ohio State University Institutional Animal Care and Use Committee approved all animal procedures (protocol 2010A00000083). Female SKH-1 hairless mice, 8 wk of age, were purchased from Charles Rivers Laboratories (Wilmington, MA, USA). SKH-1 hairless mice were chosen because a secondary objective of this study was to evaluate dermatologic outcomes (data not presented here). Mice were divided into five groups, with 20 mice consuming the control diet, and 10 mice each consuming the nonheated, 60°C-mildly heated, steamed, and sulforaphane diets. Mice were housed five per cage on a 12 h light/dark cycle in a room maintained at 21–23°C. Prior to beginning the study, all procedures were approved by the Ohio State Institutional Animal Care and Use Committee. Mice were acclimated to a powder AIN-93G control diet for 6 days, followed by being fed experimental diets for 1 wk. Fresh food was provided daily and water was provided ad libitum. The powdered food was provided in jars to prevent mice from scattering it throughout the cage and to reduce contact of the food with water and urine. Body weights were measured at several points during the experiment.
2.7 Synthesis of metabolite standards
ITC metabolite standards were synthesized based on the method of Vermeulen [19]. This method exploits the spontaneous reaction between an ITC and GSH, CG, Cys, or NAC. After synthesis, metabolites were purified using semipreparative RP chromatography on a 10 × 250 mm, 5 μm C18 Bondpak column (Waters) with a water and acetonitrile mobile phase. Acetonitrile was removed through rotary evaporation, and the remaining aqueous portion was freeze-dried, resulting in a powder appearing pure by HPLC-photodiode array detection (PDA) and MS analysis. The powder was weighed, and extinction coefficients were determined in triplicate. In methanol at 270 nm, these molar extinction coefficients (M−1 cm−1) were determined to be 7334 sulforaphane-GSH, 4796 sulforaphane-CG, 7551 sulforaphane-CYS, 6832 sulforaphane-NAC, 1653 erucin-GSH, 2302 erucin-CG, 1609 erucin-CYS, and 3391 erucin-NAC [10].
2.8 Mouse plasma and tissue analysis
Mice were sacrificed and skin, liver, kidney, bladder, lung, and blood were collected. A 10 mm skin punch was taken for ITC and metabolite analysis. Blood was collected into EDTA-coated tubes, placed on ice, fractionated within 2 h of collection, and stored at −80°C until analysis. All tissue samples were frozen in liquid nitrogen immediately after collection and stored at −80°C until analysis.
Plasma samples were prepared for analysis, as has previously been described [20], by thawing on ice, then acidifying to 10% with ice-cold TFA. The sample was vortexed, centrifuged for 5 min at 21 000 × g, and the supernatant was removed. Tissue extraction was based on a previously published method [17]. With the exception of bladders, all solid tissue samples were prepared by freezing in liquid nitrogen and grinding to a powder with a tissue grinder. Tissue powder (100 mg) was transferred to a microcentrifuge tube and 400 μL of 1% formic acid was added. The sample was vortexed, and then probe sonicated for 3 s. Ice-cold TFA (50 μL) was added to precipitate proteins and the sample was vortexed again, followed by centrifugation at 21 000 × g for 5 min. The supernatant was removed and kept on ice. Next, the pellet was resuspended in 450 μL of a 10% TFA, 1% formic acid solution, and probe sonicated for 3 s. The sample was centrifuged and the supernatants were pooled. For kidney only, the pellet was extracted a third time, as the third fraction contained >10% of the total ITC metabolite content based on recovery experiments. For bladder analysis, the bladders were weighed and the above procedure was used, except the bladders were not ground to a powder, as probe sonication was sufficient to break up the whole bladder for chemical extraction, as described above.
Sulforaphane, its metabolic conjugates, and the erucin conjugates (but not free erucin) were separated with a Waters Acuity Ultra Performance LC system interfaced, splitless, with a triple quadruple mass spectrometer (Quattro Ultima, Micromass, Manchester, UK). The supernatant (5 μL) was injected directly onto an Acquity BEH Ultra Performance LC column (Waters, 100 × 2.1 mm, 1.7 μm). The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). Initial conditions of 0% B increased linearly to 10% B at 1 min, 33.3% B at 2.5 min, 72% B at 4 min, and equilibrated back to 0% B by 6 min (curve = 1). Selected reaction monitoring of the collision induced dissociation transitions for nine analytes with nonoverlapping time windows where possible were as follows: sulforaphane (178 > 114), sulforaphane-GSH (485 > 136), sulforaphane-CG (356 > 114), sulforaphane-Cys (299 > 136), sulforaphane-NAC (341 > 114), erucin-GSH (469 > 179), erucin-CG (340 > 103), erucin-Cys (283 > 103), and erucin-NAC (325 > 164). Dwell times ranged from 80 to 150 ms. The desolvation temperature was 450°C, cone voltage was 35 V, RF1 was 12.5 V, and collision energy was 10–17 eV. The CID argon pressure was 3 × 10−3 mbar. Mixed external standard curves were prepared for quantification of the analytes. Use of stable isotope or other internal standards was not necessary as we confirmed the method functions without matrix suppression. This was evident from analyte signals scaling with increasing injection volumes up to 5 μL.
2.9 Statistics
In vitro glucosinolate and sulforaphane data are the result of three replicates and is presented as the average ± SD. All biological data are presented as the average, with SE of the mean as ±error bars. IBM SPSS Statistics 19 (Chicago, IL, USA) was used to determine the statistical significance of results. One-way ANOVA was employed, followed by Tukey’s post hoc test, and results were considered significant at the p < 0.05 level.
3 Results and discussion
3.1 Quantification of glucoraphanin and glucoerucin in BSPs
Broccoli sprouts have been used extensively in feeding studies, due to their significant concentrations of glucosinolates, in particular glucoraphanin, the precursor to sulforaphane [21]. Glucoraphanin and glucoerucin, the glucosinolate precursors to sulforaphane and erucin, respectively, were quantified in the freeze-dried BSPs. In many reports, glucoraphanin is the predominant glucosinolate in broccoli and broccoli sprouts [17,22–24]. Interestingly, we found the concentration of glucoerucin to be slightly greater than glucoraphanin in all broccoli sprout (“Calabrese”) samples (Table 1). Both our lab and others have previously reported comparable concentrations of glucoraphanin and glucoerucin in broccoli sprouts [24, 25]. In our study, the various processing techniques resulted in significantly different glucosinolate levels. Steamed broccoli sprouts had the greatest glucosinolate concentration, while the nonheated and 60°C-mildly heated BSPs had reduced levels. This disparity in glucosinolate content was likely a result of glucosinolates being converted to hydrolysis products during processing. We observed some loss in rigidity of the broccoli sprouts during the 60°C heat treatment, likely resulting in some decompartmentalization, consequent myrosinase release, and formation of hydrolysis products.
Table 1.
Quantification of glucoraphanin and glucoerucin in BSPs following processing, listed in order of increasing thermal intensity
| Processing treatment | Glucoraphanin (μmol/g DW) | Glucoerucin (μmol/g DW) |
|---|---|---|
| Freeze-dried raw (Nonheated) | 18.7 ± 0.37a | 22.5 ± 0.89d |
| 60°C + Freeze-dried | 14.2 ± 0.17b | 16.0 ± 0.40e |
| Steamed (5 min) + Freeze-dried | 22.5 ± 0.49c | 24.2 ± 1.15d |
DW, dry weight.
Values are the mean of three replicates ± SD. Different superscript letters (glucoraphanin and glucoerucin) represent significant differences between processing treatments (p < 0.05). Amounts of glucoraphanin and glucoerucin were not statistically compared.
3.2 In vitro glucoraphanin hydrolysis
Glucosinolates from the BSPs were hydrolyzed in vitro by adding water and allowing endogenous myrosinase to react with the glucosinolates (see Fig. 1), and the 2-mercaptoethanol conjugate of sulforaphane was quantified. The percent conversion from glucoraphanin to sulforaphane is reported in Table 2. As anticipated, steaming resulted in the lowest conversion to sulforaphane, approximately 4%. In the nonheated and 60°C-heated BSPs, approximately 23 and 98% conversion was observed, respectively. These vastly different conversion efficiencies likely indicate different levels of myrosinase and/or ESP activity. Our goal with the steaming treatment was to inactivate myrosinase—the extremely low sulforaphane yield (4%) following hydrolysis of the steamed BSP indicates this was accomplished. Conversely, in the non-heated BSP, our goal was to retain active myrosinase and ESP. The moderate conversion of glucoraphanin to sulforaphane suggests this was achieved. The observed 23% yield of sulforaphane from glucoraphanin in nonheated BSP is consistent with other reports [26, 27]. Lastly, our goal with the 60°C treatment was to inactivate ESP but retain myrosinase. The results strongly suggest that this was completed due to the nearly 100% conversion from glucoraphanin to sulforaphane, consistent with the selective inactivation of ESP [13]. The ability to modulate glucosinolate hydrolysis to ITCs can be used to optimize ITCs in future studies.
Table 2.
In vitro formation of sulforaphane and percent conversion from glucoraphanin, listed in order of increasing thermal intensity
| Processing treatment | Sulforaphane formed (μmol/g DW) | Percent conversion from glucoraphanin to sulforaphane |
|---|---|---|
| Freeze-dried raw (Nonheated) | 5.13 ± 0.050a | 22.8 |
| 60°C + Freeze-dried | 22.1 ± 0.3b | 97.9 |
| Steamed (5 min) + Freeze-dried | 0.94 ± 0.08c | 4.2 |
DW, dry weight.
To accurately reflect sulforaphane yields, the percent conversion was calculated by expressing the amount of sulforaphane formed as a percent of the total glucoraphanin content in the steamed broccoli sprouts. This value, 22.5 μmol/g DW, is considered the initial glucoraphanin concentration in all BSPs prior to any loss during processing.
Values are the mean of three replicates ± SD. Different superscript letters represent significant differences between processing treatments (p < 0.05).
3.3 Weight gain of mice consuming control and treatment diets
Mice consuming the control diet (no BSPs or sulforaphane) gained significantly more weight than mice consuming the four treatment diets (Supporting Information Table 1). As typical in mouse studies using powdered diets, food intake was not measured due to difficulties tracking intake of a powder diet, but the bitter flavor imparted by the BSPs/sulforaphane may have resulted in decreased consumption compared to the control diet. In the context of this study, differences in weight gain between the control and treatment diets are not of great consequence, as there were no significant weight gain differences within the four treatment diet groups. However, this dietary observation should be noted for future study.
3.4 Effect of BSP diets on ITC metabolites in vivo
Several studies have reported low bioavailability of ITCs after consumption of broccoli in which endogenous myrosinase has been inactivated [8–11]. A recent investigation examined the excretion of ITCs in humans as a function of remaining myrosinase activity after broccoli processing. An 80% reduction in myrosinase activity was not associated with decreased excretion of sulforaphane or iberin conjugates in urine [28]. Additional studies have fed mildly heated, homogenized broccoli soups, resulting in hydrolysis of glucosinolates to sulforaphane and little or no sulforaphane nitrile formation [29,30]. However, to our knowledge, the effect of feeding broccoli or broccoli sprouts with intact glucosinolates and active myrosinase but inactive ESP has not been reported. While we did not directly measure myrosinase or ESP activity, a weakness of this study, our in vitro hydrolysis results (Table 2) support our goal of producing a broccoli sprout product with intact glucosinolates, active myrosinase, and inactive ESP in the 60°C-mildly heated BSP. While it is clear that mild heating enhances ITC formation in vitro, such as in a mildly heated, homogenized broccoli soup [29, 30], one cannot assume the same for in vivo consumption with intact glucosinolates. Conditions affecting in vitro hydrolysis, including pH, temperature, presence of iron, and incubation time, likely differ during in vivo digestion [13, 31, 32]. In addition, gut microflora play a significant role in glucosinolate hydrolysis in vivo [33]. Thus, we sought to determine differences in ITC metabolite concentrations in mice following consumption of BSPs which had been freeze-dried raw, mildly heated at 60°C and freeze-dried, or steamed for 5 min and freeze-dried.
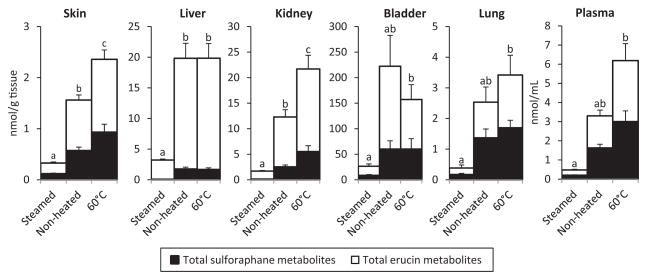
Mice were fed either a control diet, containing no BSPs, or one of the three BSP diets for 1 wk, after which they were sacrificed. Skin, liver, kidney, bladder, lung, and plasma samples were analyzed, and the sulforaphane and erucin metabolites were quantified in each sample. Data presented are the sum of the GSH, Cys, and NAC conjugates for either sulforaphane or erucin. Our method also measured free sulforaphane and the ITC-CG conjugates, but levels of these analytes were, at most, negligible, and thus excluded from calculations. ITC metabolites were detected in all plasma and tissue samples of mice, which consumed the broccoli sprout diets, indicating that ITC metabolites are readily distributed. No ITC metabolites were detected in mice consuming the control diet. In all tissues, differences between metabolite concentrations were observed as a result of the diet consumed, shown in Fig. 2. As expected from our in vitro hydrolysis study (Table 2), mice consuming the steamed BSP had the lowest average concentrations of ITC metabolites in all sample types analyzed. ITC metabolite concentrations in mice consuming the steamed BSP diet were significantly lower than those consuming the nonheated BSP in the skin, liver, and kidney by 79, 84, and 86%, respectively. Also consistent with the in vitro study, for all tissues and plasma, mice consuming the 60°C-mildly heated diet had significantly greater concentrations of ITC metabolites compared to mice which consumed the steamed BSP diet, ranging from approximately a sixfold increase in the bladder to 13-fold increase observed in plasma. Thus, these data affirm the importance of consuming glucosinolates with active myrosinase. Furthermore, in the skin and kidney, mice consuming the 60°C-mildly heated BSP diet had significantly higher average ITC metabolite concentrations than those consuming the nonheated diet, suggesting that a mild heat treatment may lead to increased ITC metabolite concentrations upon consumption.
Figure 2.
Concentration of ITC metabolites in mouse tissues and plasma following consumption of diets containing thermally processed BSPs. Total ITC concentrations were determined by calculating the sum of all sulforaphane (closed bars) and erucin metabolites (open bars). Different letters represent statistically significant differences between diets. Error bars represent the SEM for total sulforaphane or erucin metabolites.
3.5 Distribution of ITC metabolites to tissues
A pharmacokinetic study of ITC metabolism and distribution in mice following a single oral dose was recently published, which reported the ITC metabolite profile in plasma and various tissues [15]. In contrast, the present study provides a longer term observation of the distribution of ITC metabolites after consuming a BSP diet for 1 wk. A wide range of metabolite concentrations were observed in different tissues, varying from 50- to 100-fold in concentration (Fig. 2). Our data indicate that ITC metabolites are inclined to accumulate at specific sites, particularly the bladder, followed by the liver and kidney. Lower concentrations were detected in plasma, skin, and lung tissue. To our knowledge, this is the first report of detecting ITC metabolites in skin samples. In addition to the intestinal wall, the liver and kidneys are primary sites of ITC metabolism—ITCs are conjugated with GSH in the liver and then NAC in the kidney—while the bladder is the site of ITC metabolite excretion [34]. Therefore, it is not surprising that concentrations were higher in these tissues. Previously, Munday et al. [35] showed that dietary administration of a broccoli sprout extract significantly inhibited chemically induced bladder cancer in rats. They note that the bladder epithelium, the major site of bladder cancer development, comes into direct contact with ITC metabolites found in urine. Indeed, significant accumulation of sulforaphane in the bladder has been reported elsewhere [36]. Importantly, these results are corroborated by epidemiological evidence associating cruciferous vegetable consumption with decreased risk of bladder cancer [2].
3.6 Interconversion of sulforaphane and erucin
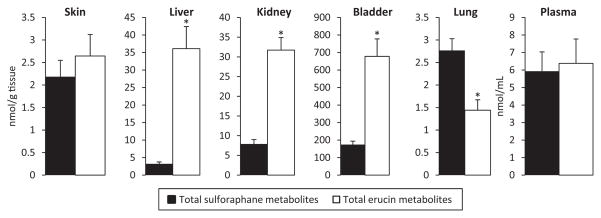
In addition to the BSP diets, one group of mice consumed an AIN-93G diet supplemented with purified sulforaphane and no BSP. The presence of both sulforaphane and erucin metabolites in the plasma and tissue of mice consuming this diet confirm that sulforaphane is reduced to erucin in vivo (Fig. 3). Previous studies have reported this phenomenon in both animals and humans [10, 16–18, 37], but were limited to plasma and urine analysis. By analyzing several types of mouse tissue, we found that the sulforaphane metabolite to erucin metabolite ratio is dependent on the tissue location. In plasma and skin, approximately equal quantities of the two ITC metabolites are present. However, in liver, bladder, and kidney, erucin is the predominant form. Notably, these three tissues were also found to have the highest ITC metabolite concentrations (Fig. 2). The concentration of erucin metabolites was over 11-fold higher than sulforaphane in the liver, despite erucin being absent from the sulforaphane diet. More research is needed to determine if the greater concentrations of erucin metabolites in these tissues is a result of tissue-specific interconversion, or if interconversion occurs outside the tissue and these tissues accumulate erucin metabolites differentially. Only in the lung was sulforaphane, the oxidized form of erucin, present in statistically significantly greater concentrations. Surprisingly, in the liver, kidney, and bladder, similar ratios of sulforaphane to erucin were observed between mice consuming the BSP and sulforaphane diets (data not shown), even though the BSP diets contained both glucoraphanin and glucoerucin, while the sulforaphane diet contained only purified sulforaphane. This is supported by a study in humans, which shows a consistent sulforaphane-to-erucin ratio in plasma and urine within subjects, with different sources of glucosinolates, and over time (1 month) [10, 38]. These findings suggest that within certain tissues, equilibrium between the two ITC species may be reached, independent of single or multiple ITCs/glucosinolates being consumed.
Figure 3.
Concentration and inter-conversion of sulforaphane and erucin metabolites in mice fed a diet containing purified sulforaphane. An asterisk denotes statistically significant differences between total sulforaphane metabolites and total erucin metabolites at each site. Error bars represent the SEM for total sulforaphane or erucin metabolites
At this time, it is unknown what drives the interconversion between sulforaphane and erucin. While reduction of sulforaphane to erucin by human colonic bacteria is possible [39], human data suggest that the interconversion between sulforaphane and erucin likely takes place postabsorption [10], but further verification is necessary. It remains to be seen if tissue-dependent interconversion or accumulation occurs in humans, and if interconversion is relevant to the biological activity of ITCs and their metabolites. These observations have several implications for future work with cruciferous vegetables and ITCs. While sulforaphane has previously been considered the most potent inducer of phase II enzymes among ITCs [3], the large extent converted to erucin brings into question whether the consumed form is important. In addition to phase II enzyme induction, erucin but not sulforaphane has the added ability to act as a direct, preventative antioxidant, interfering with initiation of peroxidation reactions [40], though this reaction has not been observed in vivo. During this oxidation reaction, the thioether group of erucin is oxidized to a sulfoxide, creating sulforaphane. We have observed interindividual variability of this ratio in human subjects [10, 38], though it has not yet been determined if this ratio has biological significance. It would also be of interest to feed mice a diet containing purified erucin to determine if the ratio of sulforaphane to erucin in various tissues is consistent with results from the present study.
3.7 Relative abundance of individual ITC metabolites
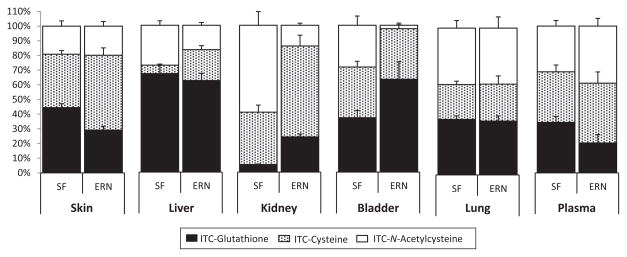
The relative abundances of individual ITC metabolites were comparable between mice consuming the BSP diets and mice fed the sulforaphane diet; thus, only data from mice consuming the sulforaphane diet are shown here (Fig. 4). Details on the ITC metabolites formed in biological samples following consumption of the three BSP diets are provided in Supporting Information Figs. 1–3. In the skin, liver, lung, and plasma the relative proportions of the GSH, Cys, and NAC-sulforaphane metabolites appeared similar compared to their matching erucin counterparts. However, the trend for sulforaphane and erucin metabolite profiles in kidney and bladder differs. In the kidney, the relative abundance of sulforaphane-GSH, Cys, and NAC conjugates was approximately 5, 35, and 60%, respectively, while the GSH, Cys, and NAC conjugates for erucin were 25, 60, and 15% of the total, respectively. In the bladder, approximately equal proportions (i.e. ~33%) of the three sulforaphane conjugates were observed. However, for erucin, the NAC metabolite was almost nonexistent, and the GSH conjugate accounted for over 60% of the total. This is surprising, considering the NAC conjugate is the predominant form shown to be excreted in the urine [20,41]. As discussed above, the kidney and bladder were also the tissues with higher levels of total ITC metabolites and a lower sulforaphane to erucin ratio. To our knowledge, this is the first time relative sulforaphane and erucin metabolite levels have been compared in tissues.
Figure 4.
Relative abundance of sulforaphane and erucin metabolites in tissues and plasma of mice fed a diet supplemented with purified sulforaphane. Error bars represent SEM for each metabolite. SF, Sulforaphane; ERN, Erucin.
4 Concluding remarks
Our findings in mice reveal that consumption of steamed BSP results in lower concentrations of ITC metabolites in vivo compared to nonheated sprouts and mild heating at 60°C further increases ITC metabolites in most tissues, which corresponds with higher ITC metabolite production in vitro. ITC metabolites were distributed to all tissues analyzed, indicating the potential for systemic benefits of cruciferous vegetable consumption. We are the first to report that the in vivo ratio of sulforaphane and erucin is tissue-dependent, with erucin being the favored form in the liver, kidney, and bladder, even when feeding only sulforaphane. Overall, these results support the growing body of evidence suggesting health benefits from cruciferous vegetable consumption, and encourage further study on the biological relevance and mechanism behind the sulforaphane to erucin ratio in tissues.
Acknowledgments
We appreciate Sun Sprout Farms of Central Ohio for providing the broccoli sprouts used in this study. We also appreciate the use of the Nutrient and Phytochemical Analytic Shared Resource at Ohio State University.
Abbreviations
- BSP
broccoli sprout powder
- CG
cysteinylglycine
- Cys
cysteine
- ESP
epithiospecifier protein
- GSH
glutathione
- ITC
isothiocyanate
- NAC
N-acetylcysteine
Footnotes
The authors have declared no conflict of interest.
Additional supporting information may be found in the online version of this article at the publisher’s web-site
References
- 1.Traka MH, Mithen R. Glucosinolates, isothiocyanates and human health. Phytochem Rev. 2009;8:269–282. [Google Scholar]
- 2.Michaud DS, Spiegelman D, Clinton SK, Rimm EB, et al. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst. 1999;91:605–613. doi: 10.1093/jnci/91.7.605. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahéo K, Morel F, Langouët S, Ferrec EL. Inhibition of cytochromes P-450 and induction of glutathione S-transferases by sulforaphane in primary human and rat hepatocytes. Cancer Res. 1997;57:3649–3652. [PubMed] [Google Scholar]
- 5.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhäuser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 6.Gamet-Payrastre L, Li P, Lumeau S, Cassar G, et al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- 7.Matusheski NV, Jeffery EH. Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile. J Agric Food Chem. 2001;49:5743–5749. doi: 10.1021/jf010809a. [DOI] [PubMed] [Google Scholar]
- 8.Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, et al. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer. 2000;38:168–178. doi: 10.1207/S15327914NC382_5. [DOI] [PubMed] [Google Scholar]
- 9.Vermeulen M, Klöpping-Ketelaars I, van den Berg R, Vaes WHJ. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J Agric Food Chem. 2008;56:10505–10509. doi: 10.1021/jf801989e. [DOI] [PubMed] [Google Scholar]
- 10.Clarke JD, Hsu A, Riedl K, Bella D, et al. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol Res. 2011;64:456–463. doi: 10.1016/j.phrs.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egner PA, Chen JG, Wang JB, Wu Y, et al. Bioavailability of sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev Res. 2011;4:384–395. doi: 10.1158/1940-6207.CAPR-10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matusheski NV, Swarup R, Juvik JA, Mithen RF, et al. Epithiospecifier protein from broccoli (Brassica oleracea L. ssp. italica) inhibits formation of the anticancer agent sulforaphane. J Agric Food Chem. 2006;54:2069–2076. doi: 10.1021/jf0525277. [DOI] [PubMed] [Google Scholar]
- 13.Matusheski NV, Juvik JA, Jeffery EH. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry. 2004;65:1273–1281. doi: 10.1016/j.phytochem.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Wang GC, Farnham M, Jeffery EH. Impact of thermal processing on sulforaphane yield from Broccoli (Brassica oleracea L. ssp. italica) J Agric Food Chem. 2012;60:6743–6748. doi: 10.1021/jf2050284. [DOI] [PubMed] [Google Scholar]
- 15.Clarke JD, Hsu A, Williams DE, Dashwood RH, et al. Metabolism and tissue distribution of sulforaphane in Nrf2 knockout and wild-type mice. Pharm Res. 2011;28:3171–3179. doi: 10.1007/s11095-011-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassahun K, Davis M, Hu P, Martin B, Baillie T. Bio-transformation of the naturally occurring isothiocyanate sulforaphane in the rat: identification of phase I metabolites and glutathione conjugates. Chem Res Toxicol. 1997;10:1228–1233. doi: 10.1021/tx970080t. [DOI] [PubMed] [Google Scholar]
- 17.Abbaoui B, Riedl KM, Ralston RA, Thomas-Ahner JM, et al. Inhibition of bladder cancer by broccoli isothiocyanates sulforaphane and erucin: characterization, metabolism, and interconversion. Mol Nutr Food Res. 2012;56:1675–1687. doi: 10.1002/mnfr.201200276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermeulen M, van den Berg R, Freidig AP, van Bladeren PJ, Vaes WHJ. Association between consumption of cruciferous vegetables and condiments and excretion in urine of isothiocyanate mercapturic acids. J Agric Food Chem. 2006;54:5350–5358. doi: 10.1021/jf060723n. [DOI] [PubMed] [Google Scholar]
- 19.Vermeulen M. Synthesis of isothiocyanate-derived mercapturic acids. Eur J Med Chem. 2003;38:729–737. doi: 10.1016/s0223-5234(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 20.Al Janobi AA, Mithen RF, Gasper AV, Shaw NP, et al. Quantitative measurement of sulforaphane, iberin and their mercapturic acid pathway metabolites in human plasma and urine using liquid chromatography-tandem electrospray ion-isation mass spectrometry. J Chromatogr B. 2006;844:223–234. doi: 10.1016/j.jchromb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kensler TW, Chen J, Egner PA, Fahey JW, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 23.Kushad M, Brown A, Kurilich A, Juvik J, et al. Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem. 1999;47:1541–1548. doi: 10.1021/jf980985s. [DOI] [PubMed] [Google Scholar]
- 24.Tian Q, Rosselot RA, Schwartz SJ. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal Biochem. 2005;343:93–99. doi: 10.1016/j.ab.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 25.Bellostas N, Kachlicki P, Sørensen JC, Sørensen H. Glucosinolate profiling of seeds and sprouts of B. oleracea varieties used for food. Sci Hortic. 2007;114:234–242. [Google Scholar]
- 26.Matusheski NV, Wallig MA, Juvik JA, Klein BP, et al. Preparative HPLC method for the purification of sulforaphane and sulforaphane nitrile from Brassica oleracea. J Agric Food Chem. 2001;49:1867–1872. doi: 10.1021/jf0013860. [DOI] [PubMed] [Google Scholar]
- 27.Mithen RF, Faulkner K, Magrath R, Rose P, et al. Development of isothiocyanate-enriched broccoli, and its enhanced ability to induce phase 2 detoxification enzymes in mammalian cells. Theor Appl Genet. 2003;106:727–734. doi: 10.1007/s00122-002-1123-x. [DOI] [PubMed] [Google Scholar]
- 28.Oliviero T, Verkerk R, Vermeulen M, Dekker M. In vivo formation and bioavailability of isothiocyanates from glucosinolates in broccoli as affected by processing conditions. Mol Nutr Food Res. 2014;58:1447–1456. doi: 10.1002/mnfr.201300894. [DOI] [PubMed] [Google Scholar]
- 29.Saha S, Hollands W, Teucher B, Needs PW, et al. Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared to fresh broccoli. Mol Nutr Food Res. 2012;56:1906–1916. doi: 10.1002/mnfr.201200225. [DOI] [PubMed] [Google Scholar]
- 30.Gasper AV, Al-Janobi A, Smith JA, Bacon JR, et al. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005;82:1283–1291. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 31.Vaughn SF, Berhow MA. Glucosinolate hydrolysis products from various plant sources: pH effects, isolation, and purification. Ind Crop Prod. 2005;21:193–202. [Google Scholar]
- 32.Williams DJ, Critchley C, Pun S, Chaliha M, O’Hare TJ. Key role of Fe(2+) in epithiospecifier protein activity. J Agric Food Chem. 2010;58:8512–8521. doi: 10.1021/jf904532n. [DOI] [PubMed] [Google Scholar]
- 33.Fahey JW, Wehage SL, Holtzclaw WD, Kensler TW, et al. Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res. 2012;5:603–611. doi: 10.1158/1940-6207.CAPR-11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verkerk R, Schreiner M, Krumbein A, Ciska E, et al. Glucosinolates in Brassica vegetables: the influence of the food supply chain on intake, bioavailability and human health. Mol Nutr Food Res. 2009;53:S219–S219. doi: 10.1002/mnfr.200800065. [DOI] [PubMed] [Google Scholar]
- 35.Munday R, Mhawech-Fauceglia P, Munday CM, Paonessa JD, et al. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008;68:1593–1600. doi: 10.1158/0008-5472.CAN-07-5009. [DOI] [PubMed] [Google Scholar]
- 36.Veeranki OL, Bhattacharya A, Marshall JR, Zhang Y. Organ-specific exposure and response to sulforaphane, a key chemopreventive ingredient in broccoli: implications for cancer prevention. Br J Nutr. 2013;109:25–32. doi: 10.1017/S0007114512000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bheemreddy RM, Jeffery EH. The metabolic fate of purified glucoraphanin in F344 rats. J Agric Food Chem. 2007;55:2861–2866. doi: 10.1021/jf0633544. [DOI] [PubMed] [Google Scholar]
- 38.Clarke JD, Riedl K, Bella D, Schwartz SJ, et al. Comparison of isothiocyanate metabolite levels and histone deacetylase activity in human subjects consuming broccoli sprouts or broccoli supplement. J Agric Food Chem. 2011;59:10955–10963. doi: 10.1021/jf202887c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luang-In V, Narbad A, Nueno-Palop C, Mithen R, et al. The metabolism of methylsulfinylalkyl- and methylthioalkyl-glucosinolates by a selection of human gut bacteria. Mol Nutr Food Res. 2013;58:875–883. doi: 10.1002/mnfr.201300377. [DOI] [PubMed] [Google Scholar]
- 40.Barillari J, Canistro D, Paolini M, Ferroni F, et al. Direct antioxidant activity of purified glucoerucin, the dietary secondary metabolite contained in rocket (Eruca sativa Mill.) seeds and sprouts. J Agric Food Chem. 2005;53:2475–2482. doi: 10.1021/jf047945a. [DOI] [PubMed] [Google Scholar]
- 41.Egner PA, Kensler TW, Chen J, Gange SJ, et al. Quantification of sulforaphane mercapturic acid pathway conjugates in human urine by high-performance liquid chromatography and isotope-dilution tandem mass spectrometry. Chem Res Toxicol. 2008;21:1991–1996. doi: 10.1021/tx800210k. [DOI] [PMC free article] [PubMed] [Google Scholar]