The worldwide epidemic of excess body weight, including overweight and obesity, is associated with increased prevalence of cardiovascular risks comprising hypertension – a condition that promote stroke, heart disease and end-stage organ damage which are major causes of death and disability (1;2). The close relationship between excess adipose mass and hypertension is well documented, with population-based studies showing excess adiposity as the strongest known risk factor for hypertension in male and female subjects of different ages and races (1–5). In particular, patients with visceral or abdominal obesity are at the greatest risk of developing hypertension and other cardiovascular risks (5;6). Moreover, obesity is a major risk factor for resistant hypertension, i.e. patients who have uncontrolled blood pressure despite being on three or more antihypertensive medications (7;8).
Clinical and animal studies have documented that weight gain can raise arterial pressure whereas weight loss has a beneficial effect on blood pressure control (9–11). However, there are many factors that influence the blood pressure response to weight change leading to substantial interindividual variations. In this regard, accumulating evidence suggests that genetic variants may impact the sensitivity to weight loss as well as blood pressure response to weight change. This is based on the observation that patients carrying certain polymorphisms related to obesity and hypertension seems more likely to benefit from dietary interventions for weight loss and blood pressure control (12;13).
Lifestyle modifications that promote weight loss are generally recommended as the first line of treatment for the increasing problem of obesity-associated hypertension (14). At first glance, this recommendation seems compelling, but evidence suggests that weight loss through lifestyle modifications for obesity-associated hypertension is neither simple nor sustained and is also not consistently effective as antihypertensive therapy (15). Given that excessive sympathetic activity is common in obesity, β-blockers appear as an obvious pharmacologic therapy to treat the hypertension associated with this condition, but treatment with β-blockers leads to metabolic complications including weight gain (16–18). Thus, novel approaches are needed for better management of obesity-associated hypertension. However, designing better therapies will require enhancing our understanding of the pathophysiological processes and molecular pathways that account for the blood pressure elevation in obesity. Here, I will review the recent progress in deciphering the mechanisms linking obesity and hypertension.
Excessive sympathetic nerve activity
Activation of the sympathetic nervous system which is a common feature of obesity is widely recognized as a major contributor to the development and maintenance of hypertension. Interestingly, sympathetic overdrive was also found to be closely related to subclinical cardiovascular and renal alterations that develop in obese subjects even in the absence of hypertension (19).
The recent development of radiotelemetric measurement of sympathetic nerve traffic allowed a close assessment of the temporal relationship between obesity, sympathetic overdrive and hypertension. Rabbits instrumented for telemetric recording of renal sympathetic activity and hemodynamic measurements were found to develop a rapid elevation in renal sympathetic tone, blood pressure and heart rate and impairment in baroreflex function when fed a high fat diet (20). Indeed, these changes appeared as soon a 1 week after starting the high fat diet and sustained after 3 weeks (20). Rats fed high fat diet also exhibited a rapid increase in radiotelemetric lumbar sympathetic activity which progresses with time, in parallel with hemodynamic and metabolic changes (21). Further analysis revealed that high fat feeding in rats increased lumbar sympathetic discharge during the entire 24-hour period and this increase was primarily caused by a rise in burst amplitude and not through changes in burst frequency (21), indicating an increasing number of recruited nerve fibers.
Additional evidence demonstrating the critical role of the sympathetic nerves subserving the kidneys derives from studies using renal denervation in animal models of obesity as well as obese patients. Selective ablation of the renal nerves abolished the hypertension in obese dogs independently from changes in body weight (22). Interestingly, renal denervation did not lower the elevated heart rate or the plasma norepinephrine levels in the obese dogs (22). The mechanisms by which renal denervation normalizes blood pressure in obesity are not well understood. It also remains to be determined whether disruption of sympathetic vasomotor pathways to beds other than the kidneys has beneficial effects on blood pressure and other cardiovascular alterations in obesity.
As a result of the significant technological advances achieved in recent years novel devices targeting the sympathetic nervous system have been developed. An implantable device for bilateral electric baroreceptor stimulation that decreases sympathetic tone alleviates obesity-associated hypertension in experimental and clinical settings (22–24). Another interventional strategy developed recently consists in the ablation of the renal sympathetic nerves with a radiofrequency-emitting catheter inserted percutaneously into the femoral artery in patients. Several open-label trials have documented the efficacy of renal denervation in subjects with resistant hypertension many of whom are obese (24–28). This new strategy offered patients with resistant hypertension another therapeutic option that is relatively safe as it can be performed under local anesthesia. However, it should be noted that there are some inconsistencies in the efficacy of this approach between studies (29). Moreover, in a recent blinded, sham-controlled study (SYMPLICITY HTN-3), after 6 months, the blood pressure lowering effect of renal denervation was not significantly different from that observed in the sham group (30). There are many factors that could account for these inconsistencies in the efficacy of renal denervation including the effectiveness of the denervation and possible interindividual variation in the response. Nonetheless, the disappointing outcomes of SYMPLICITY HTN-3 represent a major setback for renal denervation strategy which may compromise its future development and use for blood pressure management in patients with resistant hypertension.
Brain mechanisms
A wealth of information regarding the neuronal mechanisms that contribute to obesity-associated hypertension and sympathetic overdrive have been gathered in recent years (31). In particular, the brain melanocortinergic system has emerged as a crucial molecular pathway in the development of hypertension and other cardiovascular disorders in human obesity. The central melanocortin system consists of small peptides, derived from proopiomelanocortin (POMC) which is produced by a subset of neurons located mainly in the hypothalamus and brainstem. These peptides act on the melanocortin receptors (MCR), chiefly MC4R and MC3R, distributed throughout the central nervous system. The melanocortin system has a powerful influence on energy homeostasis and is critically involved in mediating the metabolic, sympathetic and cardiovascular effects of leptin and insulin (32–35).
Patients carrying loss-of-function mutations in MC4R were found to be protected from obesity-associated elevation in blood pressure, heart rate and sympathetic tone (36;37). Conversely, stimulation of the MC4R led to significant and dose-dependent increase in blood pressure in healthy overweight or obese subjects (36). These adverse cardiovascular effects of MC4R agonists detract from targeting this receptor for therapeutic use in the management of common human obesity despite its potent weight-reducing action. These findings also highlight the importance of assessing the cardiovascular effects when drugs are being considered for obesity management.
Animal studies have also provided several pieces of evidence implicating the melanocortin system as a key player in obesity-associated hypertension. Genetic studies have shown that interference with several signaling pathways in POMC neurons prevent the elevation of arterial pressure in obesity. For instance, blocking the proinflammatory pathway in POMC neurons by ablation of the gene encoding the activator IκB kinase-β prevented the development of hypertension caused by high fat diet in mice (38). Similarly, mice lacking leptin signaling in POMC neurons or in the arcuate nucleus of the hypothalamus, where most POMC neurons reside, through deletion of the leptin receptor itself (39;40) or components of its signaling pathway such as the signal transducer and activator of transcription-3 (41) are protected against obesity-associated hypertension. Together, these findings demonstrate that POMC neurons are critically involved in the development and maintenance of obesity hypertension.
Disruption of leptin receptor signaling in POMC neurons abolished the ability of exogenous leptin administration to increase blood pressure (39;41) which provides additional support to the notion that leptin is a critical hormone in coupling hypertension and obesity (Figure 1). In addition, these findings are consistent with the inability of leptin to increase renal sympathetic nerve traffic in mice bearing arcuate nucleus-specific deletion of the leptin receptor (40). Leptin receptor signaling in the subfornical organ has also been involved in mediating renal sympathetic nerve activity in a selective manner (42). Indeed, mice lacking the leptin receptor in the subfornical organ exhibited a blunted leptin-induced renal sympathetic excitation. However, these mice had unaltered body weight, food intake and brown adipose tissue sympathetic responses to leptin (42). The fact that several brain nuclei are implicated in the renal sympathetic nerve activation triggered by leptin is consistent with the idea that leptin action in the brain involves a distributed network. The ability of leptin to signal through various brain sites may also explain how this hormone can differentially alter baroreflex control of regional autonomic efferents (43). This was also touted as a potential mechanism underlying selective leptin resistance in obesity (44).
Figure 1.
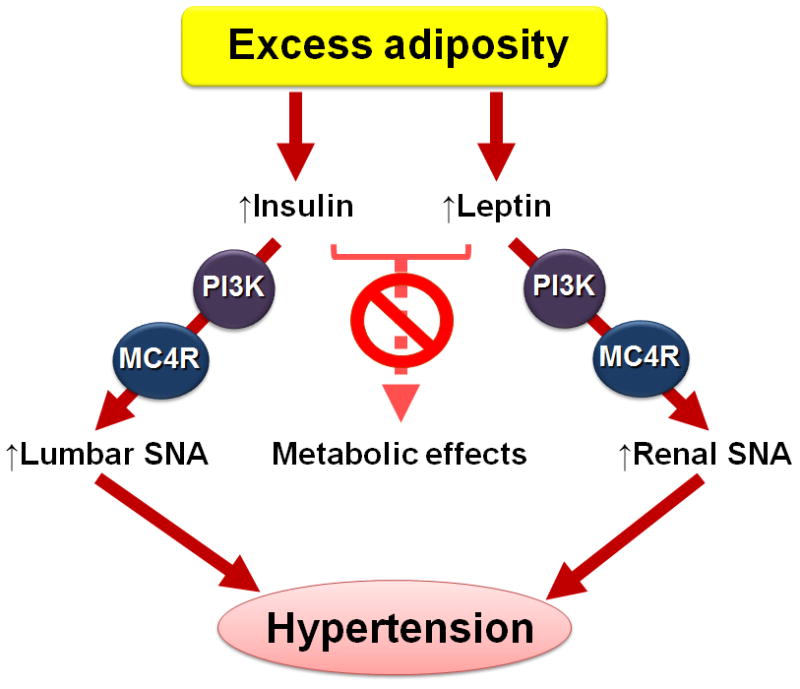
Schematic representation of the role of leptin and insulin in linking obesity and hypertension. Excess adiposity in obesity increases the circulating levels of leptin and insulin. This is associated with preserved ability of leptin and insulin to increase renal and lumbar sympathetic nerve activity (SNA), respectively. These sympathetic effects of leptin and insulin require PI3K and MC4R signaling in the brain. Actions of leptin and insulin promote excess sympathetic discharge leading to hypertension. On the other hand, the ability of leptin and insulin to regulate metabolic functions is diminished in obesity.
In a very recent study, Sayk and colleagues used microneurography to demonstrate that leptin causes sympathetic excitation in humans (45). They found that in young healthy man intravenous bolus administration of leptin caused a gradual increase in muscle sympathetic nerve activity that persisted for over 2 hours. These data combined with previous findings (46) suggest that leptin may be a major driver of cardiovascular sympathetic activation in human obesity just like in experimental obesity. It is worth noting that emerging evidences have extended the role of leptin and selective leptin resistance to other pathophysiological settings such as the fetal programming of obesity, hypertension and sympathetic overdrive (47–49).
The effectiveness of central administration of a leptin receptor antagonist in normalizing arterial pressure and renal sympathetic activity in obese rabbits provide direct evidence regarding the importance of leptin in driving hypertension and sympathetic overactivity in obesity (50). Notably, central administration of an insulin receptor antagonist to obese rabbits also decreased arterial pressure, but with no concomitant change in renal sympathetic traffic (50). This indicates that brain action of insulin is also involved in coupling obesity and hypertension (Figure 1). This appears to occur through sympathetic vasomotor pathways subserving beds other than the kidneys. Such possibility is consistent with the preserved ability of central action of insulin to increase lumbar, but not renal, sympathetic nerve traffic in obese mice (51). Strikingly, in obesity both phosphatidylinositol 3-kinase (PI3K) signaling and MC4R are required for the sympathetic control by leptin and insulin (Figure 1). Indeed, the PI3K-MC4R axis mediates the preserved renal and lumbar sympathoexcitatory effects of leptin (52;53) and insulin (51), respectively. In future studies it will be important to determine the molecular and cellular mechanisms that underlie the uncoupling of the metabolic and cardiovascular actions of both leptin and insulin in obesity.
Contribution of adipose tissue
Adipose tissue is now recognized as a major player in the regulation of physiological functions including cardiovascular regulation. Adipose tissue influences physiological and pathophysiological processes through various mechanisms including its ability to produce and release factors that act in a paracrine fashion to alter vascular reactivity with pathophysiological implications in obesity. For instance, adipocyte-derived aldosterone has emerged as a new paradigm in linking obesity with vascular disease (54). Adipocytes were found to possess the components necessary for aldosterone synthesis. Moreover, this study revealed that adipocyte-derived aldosterone regulates vascular function in a paracrine manner. Finally, the increased aldosterone production by adipocytes in obesity was shown to contribute to the vascular changes associated with this condition (54). These findings indicate that adipose tissue may be the source of the elevated circulating aldosterone levels that is commonly found in obese patients. These observations are also consistent with the beneficial effects of mineralocorticoid receptor blockade on blood pressure in obese subjects even in those displaying resistant hypertension (55).
Perivascular adipose tissue was known to influence vascular reactivity by releasing a transferable relaxing factor which produces relaxation by activating potassium channels in vascular smooth muscle cells in visceral arteries. The anti-contractile effects of perivascular adipose tissue has now been extended to peripheral skeletal muscle arteries and shown to involve the opening of a particular K+ channel (KCNQ) in smooth muscle (56). Moreover, targeting these K+ channels was found effective in reversing the decline in periadventitial regulation of arterial tone and peripheral resistance in hypertensive rats (56).
Adipose tissue produces hormones that act in an endocrine manner including leptin, discussed above, and peptides such as angiotensinogen, the only known precursor of Ang II - the main effector of the renin-angiotensin system (RAS). The contribution of adipocyte-derived angiotensinogen to the circulating pool of this protein is well established, but its role in metabolic and blood pressure regulation was not clear. Yiannikouris et al. (57) have now directly assessed the relevance of the adipose tissue angiotensinogen to the development of obesity-associated hypertension by studying mice that lacks the angiotensinogen gene only in adipose tissue. Selective ablation of adipose tissue angiotensinogen did not alter the weight gain and other metabolic changes caused by high fat diet, but it protected mice from hypertension (57). Perhaps the most intriguing observation in this study relate to the demonstration that adipose tissue modulate blood pressure through its profound effect on Ang II synthesis, but the mechanisms involved remain to be defined (58).
The significant impact of adipocyte-derived angiotensinogen on blood pressure has triggered great interest in understanding the genetic determinants of its regulation. Analysis of human adipose tissues obtained during surgery revealed a great variability in the total level of angiotensinogen gene expression among subjects (59). This analysis also showed enhanced levels in the subcutaneous adipose tissue of the mRNA derived from a particular allele of the angiotensinogen gene bearing the −20C variant in its promoter. This was associated with enriched binding to the −20C allele of a transcription factor, the upstream stimulatory factor 2, which is essential for angiotensinogen transcriptional regulation (59). Future studies are needed to examine the relevance of these genetic determinants in angiotensinogen gene to obesity and hypertension.
In addition to its paracrine and endocrine influence on cardiovascular regulation, a recent study implicated afferent reflex originating from white adipose tissue in the control of blood pressure and sympathetic nerve activity and in linking obesity to hypertension and sympathetic activation (60). Capsaicin-mediated stimulation of afferent reflex originating from inguinal fat depot was found to increase efferent renal sympathetic discharge and blood pressure in rats. Notably, these sympathetic and pressor responses elicited by adipose afferent reflex activation were enhanced in obese animals. Conversely, disruption of the white adipose tissue sensory afferents caused a rapid and long-lasting decrease in renal sympathetic tone and arterial pressure in obese rats, but not in controls. Moreover, obese rats given a leptin receptor antagonist into the inguinal and retroperitoneal fat depots exhibited a very rapid decrease in renal sympathetic activity and arterial pressure that lasted for about 15 minutes (60). These intriguing results indicate that leptin-mediated enhancement in white adipose tissue sensory afferent activity as a major pathophysiological mechanism of hypertension and vasomotor sympathetic overdrive in obesity.
Renal mechanisms
Volume expansion and sodium retention are major features of obesity. The importance of altered renal excretory capacity in the etiology of experimental and human hypertension is well established. Longitudinal studies have shown that alterations in renal excretory function occur prior to the onset of obesity hypertension in animal models and humans (61;62) indicating that renal dysfunction may drive arterial pressure elevation in obesity. The sodium retention associated with experimental obesity appears due to an increase in tubular reabsorption of sodium as indicated by the increased glomerular filtration rate and decreased fractional sodium excretion (22).
Obesity is associated with several other changes in the kidney that promote the development of hypertension as well as chronic kidney disease and end-stage renal disease. These changes include fat accumulation, histological lesions, matrix expansion, inflammation, oxidant stress, fibrosis, and the presence of proteins in the urine (63–66). Interestingly, sex appears to influence the development of renal changes in obesity. This is based on the observation that female sheep are protected from adverse renal effects caused by obesity despite exhibiting similar metabolic and cardiovascular phenotypes as the male counterparts (67).
Excessive sympathetic activity is a major cause of the abnormal sodium retention in obesity. This is supported by the finding that systemic sympathetic inhibition eliminated the renal abnormalities in obese dogs (22). However, despite its efficacy to control blood pressure selective ablation of the renal nerves failed to correct the glomerular hyperfiltration observed in obesity (22). In fact, renal denervation in obese dogs led to a further increase in glomerular filtration rate whereas fractional sodium excretion remained suppressed (22). Thus, the mechanisms by which renal denervation correct obesity-associated hypertension remain unclear. It should be noted, however, that ablating the renal nerves in obesity reduced plasma renin activity presumably through inhibition of renal renin production and/or release (22). This finding points to the RAS as a potential mechanism by which renal denervation improves blood pressure control in obesity.
Several other mechanisms have been implicated in the renal changes that occur in obesity. For instance, improving insulin sensitivity in obese mice through Protein Tyrosine Phosphatase 1B deletion corrected the reduced albumin excretion, but not Na+ and K+ excretion nor the structural changes in the kidney (64). This finding point to a pathological role of insulin resistance in the albuminuria associated with obesity although it is possible that such correction may reflect the normalization of arterial pressure. The type 2 scavenger receptor, CD36, and Na/K-ATPase have also emerged as key molecules in mediating the renal inflammation triggered by obesity in mice (68).
Vascular alterations
Alterations in the vasculature including structural changes, endothelial dysfunction, enhanced contractile response and altered stiffness are hallmarks of obesity. These vascular abnormalities are thought to contribute and even predict the development of hypertension and other cardiovascular complications (9;11;64;69–71). Consistent with the notion that in obesity vascular alterations precede hypertension, Weisbrod et al. (11) demonstrated that diet-induced obese mice develop arterial stiffness and endothelial dysfunction prior to the onset of hypertension. Importantly, obesity-associated vascular changes can be reversed by weight loss even after their establishment (11;69).
It should be noted, however, that vascular defects such as endothelial dysfunction are not universally associated with obesity. This is based on a number of studies showing the development of obesity without concomitant presence of vascular abnormalities (65;72). For instance, mice lacking the Bardet-Biedl syndrome 2 gene do not display endothelial dysfunction despite the presence of obesity (72). More striking is the finding that salt-sensitive (SS) rats fed high fat diet exhibit an improvement in endothelium-dependent vascular relaxation despite the significant weight gain and the presence of several cardiovascular risks including elevated blood pressure and reduced renal function (65). The suppressed RAS appears to account for the improvement in endothelial function in obese SS rats (65). The efficacy of chronic treatment with Ang 1–7, a peptide that negatively modulate RAS activity, in alleviating obesity-associated impairment in endothelium-dependent vasodilation supports further the pathophysiological role of the RAS in obesity-related endothelial dysfunction (73). Multiple other mechanisms including sympathetic overdrive, inflammatory mediators, excessive reactive oxygen species production, decreased NO bioactivity may contribute to the deleterious effects of obesity on the vasculature (70;71;74;75).
Conclusions and perspectives
The epidemic of obesity is associated with raising prevalence of cardiovascular risk factors including hypertension. In parallel, this epidemic triggered a great interest in understanding the mechanisms underlying the adverse cardiovascular effects of obesity which has led to significant progress in recent years. Defects in various biological processes ranging from genetic and humoral factors to basic cellular signaling pathways in different tissues have been involved in the pathogenesis of obesity-related hypertension. The diversity in the processes implicated is consistent with the polygenic and multifactorial nature of obesity and its comorbidities. The new knowledge gained in recent years should be taken into account when seeking novel diagnostic and therapeutic approaches for the cardiovascular disorders caused by excess adiposity. Given the evidence pointing to the significance of the neurogenic mechanisms in obesity-associated hypertension new strategies that disrupt these processes should be favored.
Despite the progress accomplished in recent years there are many important issues that remain unaddressed. For instance, studies to dissociate the processes that are involved in the control of energy homeostasis versus blood pressure and vasomotor sympathetic traffic should be pursued. This is necessary for the development of safe anti-obesity drugs that do not compromise cardiovascular health. This will also allow the identification of strategies that can be used to treat hypertension without interfering with metabolic regulation. Also, despite the wide range of available anti-hypertensive drugs there are still no specific guidelines for the treatment of hypertension in an obesity setting. Clinical trials are needed to address this issue. Finally, therapy for obesity-related hypertension should take into account progress in other research fields like pharmacogenomics, i.e. the genetic predisposition to drug response (76–78). Combining pharmacogenomics (and perhaps other “omics” such proteomics) with patient characteristics and co-morbidities will be useful to determine the most effective strategy to treat obesity-associated hypertension. This will allow hypertension care to move forward and enter the era of personalized medicine.
Acknowledgments
Sources of Funding
K. Rahmouni is supported by National Institutes of Health grant HL084207 and American Heart Association Established Investigator Award 14EIA18860041.
Footnotes
Disclosures
None.
References
- 1.Jones A, Charakida M, Falaschetti E, Hingorani AD, Finer N, Masi S, Donald AE, Lawlor DA, Smith GD, Deanfield JE. Adipose and height growth through childhood and blood pressure status in a large prospective cohort study. Hypertension. 2012;59:919–925. doi: 10.1161/HYPERTENSIONAHA.111.187716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suglia SF, Clark CJ, Gary-Webb TL. Adolescent obesity, change in weight status, and hypertension: racial/ethnic variations. Hypertension. 2013;61:290–295. doi: 10.1161/HYPERTENSIONAHA.111.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tu W, Eckert GJ, DiMeglio LA, Yu Z, Jung J, Pratt JH. Intensified effect of adiposity on blood pressure in overweight and obese children. Hypertension. 2011;58:818–824. doi: 10.1161/HYPERTENSIONAHA.111.175695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selassie A, Wagner CS, Laken ML, Ferguson ML, Ferdinand KC, Egan BM. Progression is accelerated from prehypertension to hypertension in blacks. Hypertension. 2011;58:579–587. doi: 10.1161/HYPERTENSIONAHA.111.177410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pausova Z, Mahboubi A, Abrahamowicz M, Leonard GT, Perron M, Richer L, Veillette S, Gaudet D, Paus T. Sex differences in the contributions of visceral and total body fat to blood pressure in adolescence. Hypertension. 2012;59:572–579. doi: 10.1161/HYPERTENSIONAHA.111.180372. [DOI] [PubMed] [Google Scholar]
- 6.Bombelli M, Facchetti R, Sega R, Carugo S, Fodri D, Brambilla G, Giannattasio C, Grassi G, Mancia G. Impact of body mass index and waist circumference on the long-term risk of diabetes mellitus, hypertension, and cardiac organ damage. Hypertension. 2011;58:1029–1035. doi: 10.1161/HYPERTENSIONAHA.111.175125. [DOI] [PubMed] [Google Scholar]
- 7.Goodfriend TL, Calhoun DA. Resistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapy. Hypertension. 2004;43:518–524. doi: 10.1161/01.HYP.0000116223.97436.e5. [DOI] [PubMed] [Google Scholar]
- 8.Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 9.De CC, Porteri E, Rizzoni D, Corbellini C, La BE, Boari GE, Pilu A, Mittempergher F, Di BE, Casella C, Nascimbeni R, Rosei CA, Ruggeri G, Caimi L, Rosei EA. Effects of weight loss on structural and functional alterations of subcutaneous small arteries in obese patients. Hypertension. 2011;58:29–36. doi: 10.1161/HYPERTENSIONAHA.111.171082. [DOI] [PubMed] [Google Scholar]
- 10.Haufe S, Utz W, Engeli S, Kast P, Bohnke J, Pofahl M, Traber J, Haas V, Hermsdorf M, Mahler A, Busjahn A, Wiesner S, Otto C, Mehling H, Luft FC, Boschmann M, Schulz-Menger J, Jordan J. Left ventricular mass and function with reduced-fat or reduced-carbohydrate hypocaloric diets in overweight and obese subjects. Hypertension. 2012;59:70–75. doi: 10.1161/HYPERTENSIONAHA.111.178616. [DOI] [PubMed] [Google Scholar]
- 11.Weisbrod RM, Shiang T, Al SL, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62:1105–1110. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Qi Q, Liang J, Hu FB, Sacks FM, Qi L. Neuropeptide Y promoter polymorphism modifies effects of a weight-loss diet on 2-year changes of blood pressure: the preventing overweight using novel dietary strategies trial. Hypertension. 2012;60:1169–1175. doi: 10.1161/HYPERTENSIONAHA.112.197855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kostis WJ, Cabrera J, Hooper WC, Whelton PK, Espeland MA, Cosgrove NM, Cheng JQ, Deng Y, De SC, Pyle M, Maruthur N, Reyes I, Anderson CA, Liu J, Kostis JB. Relationships between selected gene polymorphisms and blood pressure sensitivity to weight loss in elderly persons with hypertension. Hypertension. 2013;61:857–863. doi: 10.1161/HYPERTENSIONAHA.111.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokubo Y. Prevention of Hypertension and Cardiovascular Diseases: A Comparison of Lifestyle Factors in Westerners and East Asians. Hypertension. 2014;63:655–660. doi: 10.1161/HYPERTENSIONAHA.113.00543. [DOI] [PubMed] [Google Scholar]
- 15.Mark AL. Dietary therapy for obesity: An emperor with no clothes. Hypertension. 2008;51:1426–1433. [Google Scholar]
- 16.Julius S, Valentini M, Palatini P. Overweight and hypertension - A 2-way street? Hypertension. 2000;35:807–813. doi: 10.1161/01.hyp.35.3.807. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Ma L, Habibi J, Whaley-Connell A, Hayden MR, Tilmon RD, Brown AN, Kim JA, Demarco VG, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension. 2010;55:880–888. doi: 10.1161/HYPERTENSIONAHA.109.145136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan J, Engeli S, Boschmann M, Weidinger G, Luft FC, Sharma AM, Kreuzberg U. Hemodynamic and metabolic responses to valsartan and atenolol in obese hypertensive patients. J Hypertens. 2005;23:2313–2318. doi: 10.1097/01.hjh.0000188734.98463.82. [DOI] [PubMed] [Google Scholar]
- 19.Lambert E, Sari CI, Dawood T, Nguyen J, McGrane M, Eikelis N, Chopra R, Wong C, Chatzivlastou K, Head G, Straznicky N, Esler M, Schlaich M, Lambert G. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension. 2010;56:351–358. doi: 10.1161/HYPERTENSIONAHA.110.155663. [DOI] [PubMed] [Google Scholar]
- 20.Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, Head GA. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60:163–171. doi: 10.1161/HYPERTENSIONAHA.111.190413. [DOI] [PubMed] [Google Scholar]
- 21.Muntzel MS, Al-Naimi OA, Barclay A, Ajasin D. Cafeteria diet increases fat mass and chronically elevates lumbar sympathetic nerve activity in rats. Hypertension. 2012;60:1498–1502. doi: 10.1161/HYPERTENSIONAHA.112.194886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension. 2012;59:331–338. doi: 10.1161/HYPERTENSIONAHA.111.185074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, Peters T, Sweep FC, Haller H, Pichlmaier AM, Luft FC, Jordan J. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619–626. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- 24.Biaggioni I. Interventional approaches to reduce sympathetic activity in resistant hypertension: to ablate or stimulate? Hypertension. 2012;59:194–195. doi: 10.1161/HYPERTENSIONAHA.111.186809. [DOI] [PubMed] [Google Scholar]
- 25.Lambert GW, Hering D, Esler MD, Marusic P, Lambert EA, Tanamas SK, Shaw J, Krum H, Dixon JB, Barton DA, Schlaich MP. Health-related quality of life after renal denervation in patients with treatment-resistant hypertension. Hypertension. 2012;60:1479–1484. doi: 10.1161/HYPERTENSIONAHA.112.200865. [DOI] [PubMed] [Google Scholar]
- 26.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–464. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- 27.Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, Linz D, Schmieder R, Rump LC, Kindermann I, Sobotka PA, Krum H, Scheller B, Schlaich M, Laufs U, Bohm M. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension. 2012;60:419–424. doi: 10.1161/HYPERTENSIONAHA.112.193870. [DOI] [PubMed] [Google Scholar]
- 28.Ewen S, Mahfoud F, Linz D, Poss J, Cremers B, Kindermann I, Laufs U, Ukena C, Bohm M. Effects of Renal Sympathetic Denervation on Exercise Blood Pressure, Heart Rate, and Capacity in Patients With Resistant Hypertension. Hypertension. 2014;63:839–845. doi: 10.1161/HYPERTENSIONAHA.113.01985. [DOI] [PubMed] [Google Scholar]
- 29.Fadl Elmula FE, Hoffmann P, Fossum E, Brekke M, Gjonnaess E, Hjornholm U, Kjaer VN, Rostrup M, Kjeldsen SE, Os I, Stenehjem AE, Hoieggen A. Renal sympathetic denervation in patients with treatment-resistant hypertension after witnessed intake of medication before qualifying ambulatory blood pressure. Hypertension. 2013;62:526–532. doi: 10.1161/HYPERTENSIONAHA.113.01452. [DOI] [PubMed] [Google Scholar]
- 30.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL. A Controlled Trial of Renal Denervation for Resistant Hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 31.Hirooka Y, Kishi T, Ito K, Sunagawa K. Potential clinical application of recently discovered brain mechanisms involved in hypertension. Hypertension. 2013;62:995–1002. doi: 10.1161/HYPERTENSIONAHA.113.00801. [DOI] [PubMed] [Google Scholar]
- 32.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced Hypertension: Role of Sympathetic Nervous System, Leptin, and Melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harlan SM, Rahmouni K. Neuroanatomical determinants of the sympathetic nerve responses evoked by leptin. Clin Auton Res. 2013;23:1–7. doi: 10.1007/s10286-012-0168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlson SH, Wyss JM. Mechanisms underlying hypertension and obesity: a melanocortin linkage in the brain. Hypertension. 2011;57:375–376. doi: 10.1161/HYPERTENSIONAHA.110.161729. [DOI] [PubMed] [Google Scholar]
- 35.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension. 2011;57:435–441. doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O’Rahilly S, Farooqi IS. Modulation of Blood Pressure by Central Melanocortinergic Pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 37.Sayk F, Heutling D, Dodt C, Iwen KA, Wellhoner JP, Scherag S, Hinney A, Hebebrand J, Lehnert H. Sympathetic Function in Human Carriers of Melanocortin-4 Receptor Gene Mutations. J Clin Endocrinol Metab. 2010;95:1998–2002. doi: 10.1210/jc.2009-2297. [DOI] [PubMed] [Google Scholar]
- 38.Purkayastha S, Zhang G, Cai DS. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappa B. Nat Med. 2011;17:883–887. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.do Carmo JM, da Silva AA, Cai ZW, Lin SY, Dubinion JH, Hall JE. Control of Blood Pressure, Appetite, and Glucose by Leptin in Mice Lacking Leptin Receptors in Proopiomelanocortin Neurons. Hypertension. 2011;57:918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the Leptin Receptor in the Hypothalamic Arcuate Nucleus Abrogates Leptin-Induced Sympathetic Activation. Circ Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubinion JH, do Carmo JM, Adi A, Hamza S, da Silva AA, Hall JE. Role of proopiomelanocortin neuron Stat3 in regulating arterial pressure and mediating the chronic effects of leptin. Hypertension. 2013;61:1066–1074. doi: 10.1161/HYPERTENSIONAHA.111.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young CN, Morgan DA, Butler SD, Mark AL, Davisson RL. The brain subfornical organ mediates leptin-induced increases in renal sympathetic activity but not its metabolic effects. Hypertension. 2013;61:737–744. doi: 10.1161/HYPERTENSIONAHA.111.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li B, Shi Z, Cassaglia PA, Brooks VL. Leptin acts in the forebrain to differentially influence baroreflex control of lumbar, renal, and splanchnic sympathetic nerve activity and heart rate. Hypertension. 2013;61:812–819. doi: 10.1161/HYPERTENSIONAHA.111.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol. 2013;305:R566–R581. doi: 10.1152/ajpregu.00180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machleidt F, Simon P, Krapalis AF, Hallschmid M, Lehnert H, Sayk F. Experimental hyperleptinemia acutely increases vasoconstrictory sympathetic nerve activity in healthy humans. J Clin Endocrinol Metab. 2013;98:E491–E496. doi: 10.1210/jc.2012-3009. [DOI] [PubMed] [Google Scholar]
- 46.Eikelis N, Schlaich M, Aggarwal A, Kaye D, Esler M. Interactions between leptin and the human sympathetic nervous system. Hypertension. 2003;41:1072–1079. doi: 10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- 47.Samuelsson AM, Clark J, Rudyk O, Shattock MJ, Bae SE, South T, Pombo J, Redington K, Uppal E, Coen CW, Poston L, Taylor PD. Experimental hyperleptinemia in neonatal rats leads to selective leptin responsiveness, hypertension, and altered myocardial function. Hypertension. 2013;62(3):627–633. doi: 10.1161/HYPERTENSIONAHA.111.00691. [DOI] [PubMed] [Google Scholar]
- 48.Prior LJ, Davern PJ, Burke SL, Lim K, Armitage JA, Head GA. Exposure to a high-fat diet during development alters leptin and ghrelin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2014;63:338–345. doi: 10.1161/HYPERTENSIONAHA.113.02498. [DOI] [PubMed] [Google Scholar]
- 49.Rahmouni K. Sympathetic tone in the young: the mother weighs in. Hypertension. 2010;55:21–22. doi: 10.1161/HYPERTENSIONAHA.109.140764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension. 2013;61:628–634. doi: 10.1161/HYPERTENSIONAHA.111.00705. [DOI] [PubMed] [Google Scholar]
- 51.Morgan DA, Rahmouni K. Differential effects of insulin on sympathetic nerve activity in agouti obese mice. J Hypertens. 2010;28:1913–1919. doi: 10.1097/HJH.0b013e32833c2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgan DA, Thedens DR, Weiss R, Rahmouni K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1730–R1736. doi: 10.1152/ajpregu.90324.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harlan SM, Morgan DA, Dellsperger DJ, Myers MG, Jr, Mark AL, Rahmouni K. Cardiovascular and sympathetic effects of disrupting tyrosine 985 of the leptin receptor. Hypertension. 2011;57:627–632. doi: 10.1161/HYPERTENSIONAHA.110.166538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Briones AM, Nguyen Dinh CA, Callera GE, Yogi A, Burger D, He Y, Correa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, Touyz RM. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59:1069–1078. doi: 10.1161/HYPERTENSIONAHA.111.190223. [DOI] [PubMed] [Google Scholar]
- 55.de SF, Muxfeldt E, Fiszman R, Salles G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55:147–152. doi: 10.1161/HYPERTENSIONAHA.109.140988. [DOI] [PubMed] [Google Scholar]
- 56.Zavaritskaya O, Zhuravleva N, Schleifenbaum J, Gloe T, Devermann L, Kluge R, Mladenov M, Frey M, Gagov H, Fesus G, Gollasch M, Schubert R. Role of KCNQ channels in skeletal muscle arteries and periadventitial vascular dysfunction. Hypertension. 2013;61:151–159. doi: 10.1161/HYPERTENSIONAHA.112.197566. [DOI] [PubMed] [Google Scholar]
- 57.Yiannikouris F, Gupte M, Putnam K, Thatcher S, Charnigo R, Rateri DL, Daugherty A, Cassis LA. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension. 2012;60:1524–1530. doi: 10.1161/HYPERTENSIONAHA.112.192690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grobe JL, Rahmouni K. The adipose/circulating renin-angiotensin system cross-talk enters a new dimension. Hypertension. 2012;60:1389–1390. doi: 10.1161/HYPERTENSIONAHA.112.200543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park S, Lu KT, Liu X, Chatterjee TK, Rudich SM, Weintraub NL, Kwitek AE, Sigmund CD. Allele-specific expression of angiotensinogen in human subcutaneous adipose tissue. Hypertension. 2013;62:41–47. doi: 10.1161/HYPERTENSIONAHA.113.01330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiong XQ, Chen WW, Han Y, Zhou YB, Zhang F, Gao XY, Zhu GQ. Enhanced adipose afferent reflex contributes to sympathetic activation in diet-induced obesity hypertension. Hypertension. 2012;60:1280–1286. doi: 10.1161/HYPERTENSIONAHA.112.198002. [DOI] [PubMed] [Google Scholar]
- 61.Grubbs V, Lin F, Vittinghoff E, Shlipak MG, Peralta CA, Bansal N, Jacobs DR, Siscovick DS, Lewis CE, Bibbins-Domingo K. Body Mass Index and Early Kidney Function Decline in Young Adults: A Longitudinal Analysis of the CARDIA (Coronary Artery Risk Development in Young Adults) Study. Am J Kidney Dis. 2014;63:590–597. doi: 10.1053/j.ajkd.2013.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal Denervation Attenuates the Sodium Retention and Hypertension Associated with Obesity. Hypertension. 1995;25:893–897. doi: 10.1161/01.hyp.25.4.893. [DOI] [PubMed] [Google Scholar]
- 63.Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, Fox CS. Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. Hypertension. 2011;58:784–790. doi: 10.1161/HYPERTENSIONAHA.111.175315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belin de Chantemele EJ, Ali MI, Mintz JD, Rainey WE, Tremblay ML, Fulton DJ, Stepp DW. Increasing peripheral insulin sensitivity by protein tyrosine phosphatase 1B deletion improves control of blood pressure in obesity. Hypertension. 2012;60:1273–1279. doi: 10.1161/HYPERTENSIONAHA.112.196295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beyer AM, Raffai G, Weinberg B, Fredrich K, Lombard JH. Dahl salt-sensitive rats are protected against vascular defects related to diet-induced obesity. Hypertension. 2012;60:404–410. doi: 10.1161/HYPERTENSIONAHA.112.191551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo DF, Beyer AM, Yang BL, Nishimura DY, Sheffield VC, Rahmouni K. Inactivation of Bardet-Biedl syndrome genes causes kidney defects. Am J Physiol Renal Physiol. 2011;300:F574–F580. doi: 10.1152/ajprenal.00150.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bloor ID, Sebert SP, Mahajan RP, Symonds ME. The influence of sex on early stage markers of kidney dysfunction in response to juvenile obesity. Hypertension. 2012;60:991–997. doi: 10.1161/HYPERTENSIONAHA.112.195412. [DOI] [PubMed] [Google Scholar]
- 68.Kennedy DJ, Chen Y, Huang W, Viterna J, Liu J, Westfall K, Tian J, Bartlett DJ, Tang WH, Xie Z, Shapiro JI, Silverstein RL. CD36 and Na/K-ATPase-alpha1 form a proinflammatory signaling loop in kidney. Hypertension. 2013;61:216–224. doi: 10.1161/HYPERTENSIONAHA.112.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lurbe E, Torro I, Garcia-Vicent C, Alvarez J, Fernandez-Fornoso JA, Redon J. Blood pressure and obesity exert independent influences on pulse wave velocity in youth. Hypertension. 2012;60:550–555. doi: 10.1161/HYPERTENSIONAHA.112.194746. [DOI] [PubMed] [Google Scholar]
- 70.Cao J, Peterson SJ, Sodhi K, Vanella L, Barbagallo I, Rodella LF, Schwartzman ML, Abraham NG, Kappas A. Heme oxygenase gene targeting to adipocytes attenuates adiposity and vascular dysfunction in mice fed a high-fat diet. Hypertension. 2012;60:467–475. doi: 10.1161/HYPERTENSIONAHA.112.193805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marshall NJ, Liang L, Bodkin J, Dessapt-Baradez C, Nandi M, Collot-Teixeira S, Smillie SJ, Lalgi K, Fernandes ES, Gnudi L, Brain SD. A role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension. 2013;61:246–252. doi: 10.1161/HYPERTENSIONAHA.112.201434. [DOI] [PubMed] [Google Scholar]
- 72.Beyer AM, Guo DF, Sheffield VC, Rahmouni K. Contrasting vascular effects caused by loss of Bardet-Biedl syndrome genes. Am J Physiol Heart Circ Physiol. 2010;299:H1902–H1907. doi: 10.1152/ajpheart.00336.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beyer AM, Guo DF, Rahmouni K. Prolonged treatment with angiotensin 1–7 improves endothelial function in diet-induced obesity. J Hypertens. 2013;31(4):730–738. doi: 10.1097/HJH.0b013e32835ecbe5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belin de Chantemele EJ, Mintz JD, Rainey WE, Stepp DW. Impact of leptin-mediated sympatho-activation on cardiovascular function in obese mice. Hypertension. 2011;58:271–279. doi: 10.1161/HYPERTENSIONAHA.110.168427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leopold JA. Cellular and molecular mechanisms of arterial stiffness associated with obesity. Hypertension. 2013;62:1003–1004. doi: 10.1161/HYPERTENSIONAHA.113.01872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vandell AG, Lobmeyer MT, Gawronski BE, Langaee TY, Gong Y, Gums JG, Beitelshees AL, Turner ST, Chapman AB, Cooper-DeHoff RM, Bailey KR, Boerwinkle E, Pepine CJ, Liggett SB, Johnson JA. G protein receptor kinase 4 polymorphisms: beta-blocker pharmacogenetics and treatment-related outcomes in hypertension. Hypertension. 2012;60:957–964. doi: 10.1161/HYPERTENSIONAHA.112.198721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McDonough CW, Gong Y, Padmanabhan S, Burkley B, Langaee TY, Melander O, Pepine CJ, Dominiczak AF, Cooper-DeHoff RM, Johnson JA. Pharmacogenomic association of nonsynonymous SNPs in SIGLEC12, A1BG, and the selectin region and cardiovascular outcomes. Hypertension. 2013;62:48–54. doi: 10.1161/HYPERTENSIONAHA.111.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Campbell CY, Blumenthal RS. Pharmacogenetics of antihypertensive response. Hypertension. 2012;59:1094–1096. doi: 10.1161/HYPERTENSIONAHA.112.192559. [DOI] [PubMed] [Google Scholar]


