Abstract
NADPH reductase NQO1 is needed to maintain a cellular pool of antioxidants and this enzyme may contribute to tumorigenesis, on the basis of studies in NQO1-deficient mice. In this work, we sought deeper insights into how NQO1 contributes to prostate carcinogenesis, a setting where oxidative stress and inflammation are established contributors to disease development and progression. In the TRAMP mouse model of prostate cancer, NQO1 was highly expressed in tumor cells. NQO1 silencing in prostate cancer cells increased levels of nuclear IKK-α and NF-κB while decreasing the levels of p53, leading to interactions between NF-κB and p300 that reinforce survival signaling. Gene expression analysis revealed upregulation of a set up immune-associated transcripts associated with inflammation and tumorigenesis in cells where NQO1 was attenuated, with IL-8 confirmed functionally in cell culture as one key NQO1-supported cytokine. Notably, NQO1-silenced prostate cancer cells were more resistant to androgen deprivation. Further, NQO1 inhibition increased migration including under conditions of androgen deprivation. These results reveal a molecular link between NQO1 expression and pro-inflammatory cytokine signaling in prostate cancer. Further, our results suggest that altering redox homeostasis through NQO1 inhibition might promote androgen-independent cell survival via opposing effects on NF-κB and p53 function.
Introduction
Oxidative stress and the associated pathological conditions are considered as risk factors in the initiation, development and progression of many cancers including prostate cancer (1–3). NAD(P)H:quinone oxidoreductase 1 (NQO1) is a homodimeric flavoprotein that catalyzes the reduction of quinones to hydroquinones and is a key component of cellular antioxidant defense system. NQO1 regulates stability of p53 protein and cell fate decisions in response to endogenous and exogenous stress. Mice with targeted disruption of NQO1 were highly susceptible to chemical-induced mouse skin carcinogenesis suggesting its potential role in carcinogenesis (6, 7). On the other hand, deletion of NQO1 gene in keratinocytes leads to cell death by inhibiting NF-κB activation (8) and NQO1 induced melanoma progression via upregulating NF-κB-p50 (9). NQO1-mediated reduction of quinones to hydroquinones is essential for anti-tumor activity of chemotherapeutic quinone drugs signifying the importance of NQO1 in cancer therapy (10). A single nucleotide polymorphism (SNP) in NQO1 cDNA results in a proline to serine substitution (C609T) and production of an unstable protein that undergoes rapid degradation (10). This SNP has been associated with increased risk for various cancers (11, 12). However, association of this SNP with prostate cancer risk is unclear although recent meta-analysis data show no significant association in Caucasians (13). The molecular pathogenesis and consequences of NQO1 changes in prostate cancer have not been explored. Here, we determined the basic function of NQO1 using prostate cancer as a model system.
Prostate cancer is the most commonly diagnosed cancer among men in the United States (14). Cancer progresses from prostatic intraepithelial neoplasia (PIN) to hormone-responsive locally invasive adenocarcinoma, to hormone-independent metastatic carcinoma. Perturbation in redox balance in PIN and prostate cancer has been attributed to elevated oxidative stress (15, 16). However, the role of redox imbalance in the development of androgen-independent prostate cancer is anecdotal and based on increased levels of cytokines and chemokines (17, 18). The C-C chemokine ligand 2 (CCL2), CXCL12, and interleukin-8 (IL-8) are major cytokines associated with prostate cancer (19–21).
NF-κB upregulates cytokines and chemokines providing a critical mechanistic link between inflammation and cancer (22). Several reports establish an inverse correlation between NQO1 and NF-κB activation (23). In addition to IKK-mediated regulation of IκB-NF-κB interaction, its crosstalk with other cell-signaling networks determine cell fate (24). Crosstalk between NF-κB and p53 can play a pivotal role in determining the cellular response to stress (24, 25). IKKα in the nucleus plays a key role in NF-κB activation in response to stress or inflammation (26, 27). Interaction between IKKα and transcriptional co-activator proteins CBP or p300 has been reported as a mechanism of NF-κB activation to promote cell proliferation and tumor growth (28). Moreover, higher levels of nuclear IKKα in human and mouse prostate carcinomas is correlated with prostate cancer progression and metastatic phenotype (29).
We show that NQO1-mediated p53 inhibition activates NF-κB/p300 association, which appears to be associated with nuclear IKKα. We report for the first time that NQO1 knockdown LNCaP cells are more resistant to androgen deprivation-induced growth arrest, in which an increased fraction of NQO1 knockdown cells survive under reduced hormone conditions. Together our results provide a mechanism that implicates NQO1 as an important player in the development of hormone-independent prostate cancer.
Materials and Methods
Cell culture
RWPE-1, LNCaP, PC3 and DU145 purchased from ATCC (Rockville, MD) were cultured according to vendor recommendations. C4-2B cells obtained from Dr. T Dorai, New York Medical College (Valhalla, NY) were maintained in T-medium containing 5% FBS and 100 IU/mL penicillin and 100 μg/mL streptomycin. BPH-1 cells obtained from Dr. MS Lucia, CU-Denver were maintained in RPMI 1640 supplemented with 10% FBS, and penicillin/streptomycin.
Real Time quantitative PCR
RNA was reverse transcribed using SuperScript VILO cDNA Synthesis Kit according to manufacturer’s instructions (Invitrogen, Carlsbad, CA) and gene expression was analyzed by qPCR on ABI 7300 (Applied Biosystems). β-actin was used as reference.
Immunoblotting
Whole cell, cytoplasmic or nuclear extracts were resolved by SDS-PAGE and immunoblotted. β-actin and Lamin B1 were used as loading controls. Immunoprecipitation kit from eBioscience (San Diego, CA) was used to determine protein interactions.
NQO1 knockdown
Cells were transduced lentiviral particles against control (NTC) or NQO1 (shNQ; Dharmacon, Lafayette, CO) and selected with 1 μg/mL puromycin. Knockdown was verified by qPCR and immunoblot analysis.
Functional assays
Trypan blue, crystal violet staining and MTT assays were used to determine growth and survival. Wound scratch was used to determine migration. Peroxy Orange (PO1) was used for H2O2 generation (30).
Microarray analysis
Agilent 8×60K Whole Human Genome platform was used to compare NQO1 knockdown and non-targeted control LNCaP cells. Data has been deposited in the GEO database (accession: GSE58336.
Luciferase assay
Luciferase activity was determined using the dual luciferase reporter assay (Promega, Madison, WI),
Cytokine array
Antibody-based array was used to determine cytokines in culture supernatant and lysates according to manufacturer’s protocol (R&D Systems, Minneapolis, MN)..
Immunofluorescence
Immunostained cells were examined on a Sweptfield confocal system (Prairie Technologies, Middleton, WI).
Animal experiments
NQO1 and IKKα levels were examined in TRAMP tumors and tissues obtained from a repository available from previous studies in our laboratory (31, 32). These animals display high-grade PIN (HGPIN) at 28 weeks and poorly differentiated carcinoma by 34 weeks. Blinded pathologist (RLR) scored samples. Scoring was based on proportion of cells stained+intensity of staining. For HGPIN n=7 and carcinoma n=8.
Statistical analysis
Data are expressed as mean ± SD. Student’s t-test or one-way ANOVA was used to calculate P values. Statistically significant differences between experimental results were established as P < 0.05 (GraphPad Prism v5 software, San Diego, CA).
Results
NQO1 in the prostate
Basal levels of NQO1 protein, transcript and enzymatic activity was higher in all prostate cancer cells tested (LNCaP, C4-2B, PC3 and DU145) compared with non-cancerous cells, RWPE-1, BPH-1 (Fig. 1A, Fig. 1B and Fig. 1C). We examined basal transcriptional regulation of NQO1 using the NQO1-promoter-luciferase construct containing NRF2 binding sites. Consistent with the transcript level, NQO1 promoter activity was higher in cancer cell lines compared to the non-cancerous cell lines. Higher expression of NQO1 was observed in cancer cells of bladder, pancreas and melanocyte origin (Supplementary Fig. S1). Higher levels of NQO1 may be because of adaptive mechanism against persistent oxidative stress in most cancer cells (1). It is interesting to note, however, that the two aggressive prostate cancer cells (PC3 and DU145) have different levels of NQO1. Further, a representative image of a 28-week old TRAMP animal shows HGPIN while in the 34-week old animal the histological finding was the presence of poorly differentiated carcinoma (Fig. 1E). Immunohistochemical detection of NQO1 showed cytoplasmic staining in the animal with HGPIN with a marked increase in cytoplasmic staining of tumor cells in the animal with poorly differentiated carcinoma (Fig. 1F). The association observed between high NQO1 levels in the absence of p53 in the TRAMP tumors (SV40 T-antigen used in generating TRAMP animals inactivates p53 in this model) is in agreement with the mutant p53 DU145 cells.
Figure 1. Higher NQO1 levels in prostate cancer cells.
(A) Whole cell lysates were prepared and basal NQO1 levels were determined in the panel of prostate cell lines using immunoblot analysis. Similar blots were obtained in three independent experiments. (B) Bar graphs were obtained from the qRT-PCR analysis and expressed as normalized NQO1 expression relative to RWPE-1 cells. (C) NQO1 activity was measured as the Dicoumarol-sensitive reduction of DCPIP. Values normalized to equal amount of proteins are expressed as NQO1 activity relative to RWPE-1 cells. The bar graph shows means ± SD from four experiments. (D) Basal NQO1 activity was assessed in the panel of prostate cells using dual luciferase reporter assay with the human NQO1-luc plasmid. Values are means ± SD from four measurements. (E) H&E staining of 28 weeks-old TRAMP prostate show proliferation of cells consistent with high grade PIN and 34 weeks old TRAMP shows nuclear polymorphisms under higher magnification. (F) IHC staining to detect NQO1 showed faint cytoplasmic staining in most cells of HGPIN lesions (n=7) and prominent cytoplasmic staining in poorly differentiated carcinoma (n=8). Scale bar, 1.0 mm for low magnification and 100 μm for high magnification.
Effects of stable inhibition of basal level of NQO1 in prostate cancer cells
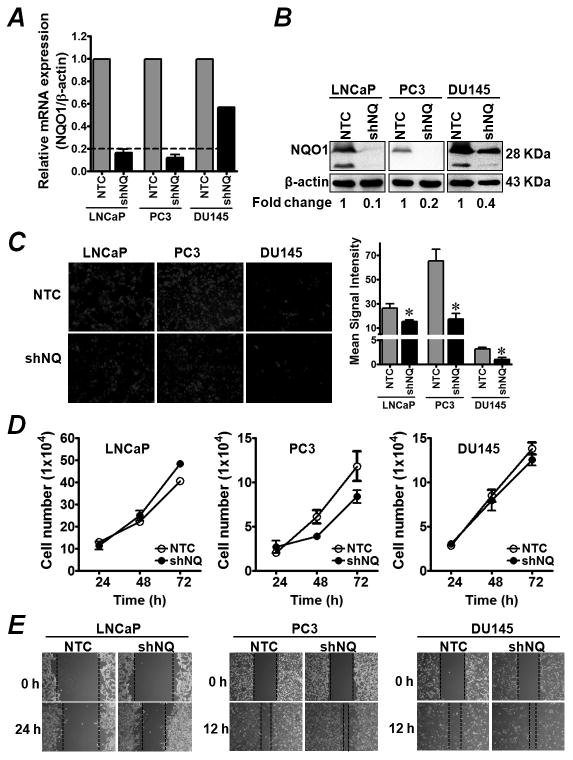
We asked whether higher levels and activities of NQO1 affect growth, proliferation and migration of prostate cancer cells. To characterize these functions, we generated NQO1-knockdown stable clones of hormone responsive (LNCaP) and hormone-independent (PC3 and DU145) prostate cancer cells. LNCaP- and PC3-shNQ-transduced cells showed a highly significant reduction in NQO1 mRNA expression, while DU145-shNQ was reduced by ~50% compared with the non-targeted control cells (NTC) (Fig. 2A). NQO1 silencing in these cells was further confirmed by Western blot (Fig. 2B). Immunocytochemistry was used to examine NQO1 localization and efficiency of stable knockdown in LNCaP cells (Supplementary Fig. S2). Interestingly, stable NQO1 knockdown cells maintained lower basal levels of H2O2 as determined by PO1 (H2O2-specific dye; Fig. 2C) and intracellular ROS (determined by CellROX; data not shown). Although, these findings suggest a critical role of NQO1 in maintaining the balance between ROS production and detoxification, we found small but significant changes in cellular oxidative stress markers such as NAD(P)H/NAD(P) ratio, total GSH and antioxidant level in a cell type-specific manner with DU145 cells remaining largely unaffected (Supplementary Fig. S3). NQO1 knockdown had a modest differential effect on in vitro growth characteristics; with a small but significant increase in cell proliferation in LNCaP cells and a non-significant decrease in PC3 and DU145 cells (Fig. 2D). A similar trend was observed in cell proliferation monitored in long-term culture to clonogenic density (data not shown). In contrast, NQO1 silencing increased cell migration in all cells (Fig. 2E). Our data suggest that under basal growth conditions inhibition of NQO1 does not largely alter cellular redox state and growth of prostate cancer cells. Cell type-specific effects may be due to alteration in intracellular ROS levels given that ROS can induce growth arrest, death, and growth/proliferation signals depending on the cellular context of redox adaptation, threshold to stress and redox-mediated signaling (33). In addition, p53 status may be an important factor to define cell type-specific fate during NQO1-mediated inhibition of ROS (34). These results emphasize the need for a deeper understanding of the role of NQO1 in regulating genes under basal redox homeostasis as well as in oxidative stress-induced conditions in prostate cancer.
Figure 2. Inhibition of NQO1 decreases endogenous ROS in prostate cancer cells.
(A) LNCaP, PC3 and DU145 cells were transduced with lentiviral particles containing non-target control (NTC) or NQO1 specific shRNA (shNQ). Clones were stably selected using puromycin. Inhibition of NQO1 was confirmed by measuring mRNA expression using qRT-PCR. Values are means ± SD from 3 experiments. *, P<0.05 compared with the NTC control. (B) Levels of NQO1 and β-actin in whole cell lysates were analyzed by Western blot. Fold change shows the average of three independent experiments. (C) NTC and shNQ cells were plated in 8-well chambered slides for 24 h. Cells were incubated with 5 μmol/L PO1. Intracellular level of H2O2 was determined by imaging with fluorescence microscope. Signal intensity was analyzed by NIH ImageJ software. Bar graph shows mean signal intensity (average of multiple images from each group). *, P<0.05 compared to the respective NTC control. (D) Cell proliferation was measured using trypan-blue. Values are average cell number ± SD from three samples. *, P<0.05 compared with the NTC control. (E) Migration ability of NQO1 knockdown cells vs. respective NTC cells in the presence of FBS was assessed using wound scratch assay at 24 h (for LNCaP) and 12 h (for PC3 and DU145). Dotted lines indicate approximate wound closure distance.
NQO1 knockdown does not alter AR signaling but increases resistance to hormone-deprivation mediated cell death in vitro
We investigated the effect of NQO1 inhibition on hormone response because oxidative stress, AR signaling and their interactions are regarded as oncogenic drivers in prostate tumorigenesis. However compared to NTC cells, LNCaP-shNQ cells showed no significant changes in AR signaling as determined by AR-promoter luciferase activity, and AR responsive gene expression (AR and PSA; Supplementary Fig. S4). Furthermore, DHT treatment increased proliferation of both LNCaP-NTC and LNCaP-shNQ cells in a dose-dependent manner (Fig. 3A). Similar to basal growth condition there was a marginal increase in DHT-induced cell proliferation in LNCaP-shNQ cells. When cells were cultured in charcoal-stripped serum (CSS) media (mimics hormone deprivation condition) both LNCaP-NTC and LNCaP-shNQ responded with significantly decreased survival (Fig. 3B). However, upon closer observation we found that under CSS conditions, a population of LNCaP-shNQ cells escaped from hormone dependency. We observed clear differences in morphology with differentiated growth and survival rates over time (day 2 to day 4) under hormone deprivation condition (Fig. 3B). Live-Dead cell count supported the above observations (Fig. 3B, right panel). Although long-term culture confirmed increased cell survival in hormone-independent manner (Fig. 3C), we do not rule out the possibility that increased survival is due to a marginal increase in proliferation rate under CSS conditions. Confluent monolayers of LNCaP-NTC and LNCaP-shNQ cells were switched to CSS medium and observed for wound closure. LNCaP-shNQ cells migrated faster and significantly decreased wound size compared with NTC control at 24 h (Fig. 3D). These observations suggest that NQO1 repression may induce cellular signaling that supports survival and adaptation to hormone depletion conditions.
Figure 3. Increased survival of NQO1 knockdown LNCaP cells under hormone-deprived condition.
(A) NTC and shNQ cells were cultured in serum-free medium with or without indicated concentration of DHT for 72 h. Representative microscopic images show DHT-induced cell survival (Left panel). Graph shows DHT-induced cell proliferation measured by MTT assay (Right panel). Values are mean ± SD from 4 wells. (B) NTC and shNQ cells grown in either FBS- or CSS-supplemented medium were assessed for growth characteristics at various time points. Live-dead cells were estimated using trypan-blue assay. Bar graphs show the percentage live and dead cells counted at 72 h and 96 h. Values are mean ± SD from 4 wells. (C) NTC and shNQ cells were cultured in CSS-supplemented medium for 12 days and cells were stained with crystal violet. A representative enlarged microscopy image from each well is shown. (D) Confluent monolayer of LNCaP cells were switched to CSS-supplemented medium and wound scratch assay was done as described previously.
Effects of silencing NQO1 on global gene expression
To answer the fundamentally important question regarding how LNCaP-shNQ cells survive under hormone-depleted conditions, we performed genome wide molecular profiling using the Agilent platform. Three different clones from each LNCaP-NTC and LNCaP-shNQ cells were subject to whole genome microarray. Expression profiles are shown in clustered heatmaps (Supplementary Fig. S5). Analysis of up- and down-regulated transcripts showed that NQO1 knockdown affected several hundred genes (Supplementary Tables 2 and 3) involved in receptor signaling, inflammation, stress, invasion, migration as well as angiogenesis suggesting NQO1 is a top discriminatory gene (Fig. 4A and Fig. 4B). We analyzed the database for targets of signaling pathways and noted a major upregulation in immune and cytokine signaling pathway targets as summarized in Fig. 4C. Of note, genes related to inflammatory signaling, notably target signaling of IL-1 (CCL20, CXCL2, CCL2, BIRC3, TNFAIP3, IL-8, SAA2) and TNFα (CXCR7, CXCL2, UBD, CCL2, BIRC3, IL-8, CD70) were among the most differentially overexpressed targets in NQO1 knockdown cells (Fig. 4C and Fig. 4D). In addition, EGFR targets, such as LCN2, GJB2, IER3, also increased upon NQO1 knockdown (Fig. 4C and Fig. 4D). Many of the deregulated genes including pro-inflammatory mediators have previously been demonstrated to induce prostate tumorigenesis and androgen independence (18, 35, 36). We also validated microarray data on gene expression using qPCR. Affected genes and their functions are listed in Table 1.
Figure 4. NQO1 silencing induces inflammatory pathways.
(A–B) Global gene expression changes upon NQO1 silencing. Bar graphs show frequency of upregulated (left) and downregulated (right) gene clusters. Heatmaps and a complete list of gene expression differences are provided in the supplementary data. (C) Search of functional grouping using various database sources was carried out to identify targets of important signaling pathways (both induced and repressed target genes). (D) Heatmap expression profiling of significantly induced pathway targets.
Table 1.
Expression levels of selected genes in LNCaP (shNQ) cells compared to NTC analyzed by Real Time qPCR
| Genes | Relative levels in shNQ cells (shNQ/NTC) |
|---|---|
| Chemokines and cytokines | |
| IL-8 | 3.11 |
| IL-32 | 1.47 |
| IL17C | 1.31 |
| IL-10RA | 1.51 |
| CCL2 | 5.31 |
| Cell Growth & Proliferation | |
| BMP7 | 3.85 |
| AKT3 | 2.55 |
| LCN2 | 11.85 |
| NOS3 | 3.21 |
| PAGE4 | 10.29 |
| Receptor Signaling | |
| CXCR2 | 6.22 |
| CXCR7 | 1.27 |
| IRS1 | 1.83 |
| Stress response | |
| NQO1 | 0.15 |
| Downregulated genes | |
| NPM2 | 0.76 |
Link between NQO1 and inflammatory mediators
The link between NQO1 and inflammatory signaling was reinforced in an independent clone following transient knockdown with siRNA smartpool against NQO1 in LNCaP cells. Expression of NQO1, IL-8 and CCL2 was analyzed by qPCR. As shown in Fig. 5A, expression of IL-8 and CCL2 was significantly increased in NQO1 knockdown cells. To further validate the effect of NQO1 in IL-8 expression, pharmacological inhibitors (Dicoumarol and MAC220) that inhibit the catalytic activity of NQO1 were used. Consistent with stable (shRNA) and transient (siRNA) genetic inhibition, both agents increased the expression of IL-8 and CCL2 in LNCaP cells (Fig. 5B and Fig. 5C). We used a cytokine array to test if increased expression leads to higher secreted levels of these cytokines. We found that IL-8 induction was clearly observed in conditioned media from LNCaP-shNQ cells (Fig. 5D). While migration inhibitory factor (MIF) was found in both conditioned media and cell lysates, the interleukin-1 receptor antagonist (IL-1Ra) was only detected in cell lysates with slightly higher levels in LNCaP-shNQ cells (Fig. 5D). Supplementation of hormone-deprived media with IL-8 partially rescued LNCaP-NTC and LNCaP-shNQ cells from cell death (Fig. 5E). We used siRNA to attenuate IL-8 expression in LNCaP-shNQ clones to examine the role of elevated IL-8 production and IL-8-induced signaling cascade in promoting androgen-independent survival of prostate cancer cells. IL-8 knockdown partially abrogated the NQO1 knockdown-mediated increased cell survival (Fig. 5F).
Figure 5. NQO1 knockdown-mediated IL-8 production in LNCaP cells.
(A) Transient knockdown of NQO1 using specific siRNA increased CCL2 and IL-8 expression. (B–C) NQO1 inhibitors induced cytokine expression in LNCaP cells. (D) Conditioned media or cell lysates from NTC and shNQ cells were subjected to cytokine expression profiling using human cytokine expression array. (E) GFP-tagged stable NTC and shNQ cells were cultured in CSS supplemented media with or without IL-8 for 3 days and fluorescence images were recorded. (F) Stable NTC and shNQ cells were transfected with scrambled (Scr) and IL-8 siRNA smartpool (si-IL8). These cells were cultured in CSS-supplemented medium for 9 days. Cell growth was assessed by trypan-blue assay and colony formation assay. *, P<0.05 Scr vs. si-IL8 in shNQ cells.
NQO1-mediated negative regulation of p53 promotes NF-κB activation and IL-8 induction
Regulation of IL-8 gene expression by transcriptional activation of NF-κB is widely reported, and many of the IL-1β and TNFα signaling targets shown in Fig. 4C and Fig. 4D are regulated by NF-κB. This is consistent with the possibility that NF-κB activation may mediate IL-8 expression for increased cell survival and migration, either directly through redox imbalance or indirectly by negative regulation of p53. We found that NQO1 knockdown increased basal constitutive NF-κB luciferase activity by ~3 fold (Fig. 6A). Interestingly, treatment with TNFα induced NF-κB activation in LNCaP-NTC but not in LNCaP-shNQ cells. This differential response suggests that NQO1 might be an important determinant of NF-κB activation via the classical pathway. Previously, Aggarwal and colleagues reported that NQO1-deficient keratinocytes have impaired TNFα-mediated NF-κB activation (8). We consistently observed higher constitutive nuclear p65 level in LNCaP-shNQ cells (Fig. 6B, bottom panel). NF-κB translocation to the nucleus in response to TNFα was only noticeable in LNCaP-NTC but not in LNCaP-shNQ cells. In a parallel experiment, NQO1-specific inhibitor MAC220 also increased basal nuclear p65 levels (Supplementary Fig. S6). Given that crosstalk between NF-κB and p53 is probably most relevant to oxidative stress (24) and NQO1-deficiency induces p53 degradation (4), we hypothesized that NQO1 knockdown-mediated p53 inhibition enhances NF-κB activation by competing for limited pools of p300 or CBP co-activators. We found that NQO1 knockdown resulted in decreased p53 levels and increased nuclear levels of NF-κB and IKKα (Fig. 6C and Supplementary Fig. S7). IKKα has been reported to tip the binding preference from p53 to NF-κB by activating these co-activators (28). Our aforementioned hypothesis was confirmed by the findings that NQO1-knockdown decreased p53 level (Fig. 6C) and increased NF-κB/p300 association (Fig. 6D). Further, when cells were treated with the p53-specific inhibitor PFTα and nuclear lysates were immunoprecipitated with anti-p300 followed by immunoblotting with anti-NF-κB, we found NQO1 knockdown indeed increased p300 binding to NF-κB and this effect was further enhanced by p53 inhibitor (Fig. 6D). IKKα immunostaining showed no nuclear staining in either HGPIN or poorly differentiated carcinoma samples. There was some IKKα cytoplasmic staining in HGPIN lesions of TRAMP animals (Fig. 6E). In poorly differentiated tumors, IKKα staining was sparse to absent. However, material within the lumen of glands showed positive staining (Fig. 6E). Overall, our data suggest that nuclear IKKα might be involved in increased interaction of NF-κB/p300 and activation of NF-κB.
Figure 6. NQO1-mediated p53 inhibition activates NF-κB/p300 association via IKKα.
(A) NF-κB luciferase activity was analyzed by Dual-Luciferase Assay system. (B) NTC and shNQ LNCaP cells were treated with TNFα and cytoplasmic and nuclear fractions were prepared. Western blot shows changes in nuclear NF-κB and IKKα. (C) NTC and shNQ cells were fixed and immunostained with anti-NQO1, anti-p53, anti-NF-κB p65 and anti-IKKα. Representative images are shown from two independent experiments performed in duplicate wells. (D) NTC and shNQ cells were incubated with or without PFTα (20 μmol/L) and lysates were immunoprecipitated with anti-p300 followed by immunoblotting with anti-NF-κB. (E) Representative IHC staining of IKKα in HGPIN lesions (n=7) and poorly differentiated carcinoma (n=8) are shown. Scale bar, 1.0 mm for low magnification and 100 μm for high magnification. (F) Proposed mechanism of hormone-independent survival of NQO1 knockdown prostate cancer cells. NQO1 suppression reduces endogenous ROS and p53 levels that facilitate increased NF-κB-p300 interaction. These cells appear to adapt to hormone-deprivation condition by the activation of pro-inflammatory and survival signaling molecules.
Discussion
NQO1 contributes to anti-inflammation, p53 stability, chemo-sensitivity and hence it has tumor-suppressor properties (4, 10, 37). On the other hand, high level of NQO1 expression in cancer cells can create a microenvironment favorable for growth and survival during elevated oxidative stress, via induction of oncogenic signaling pathways. Although, cytoprotective effects of NQO1 have been confirmed by several studies, there is increasing evidence that NQO1 may interact with other molecules to integrate their activities with signaling networks in cancer cell biology. These new roles of NQO1 may be direct effects or consequences of crosstalk between signaling pathways that ultimately determines cell fate. In this study, we attempted to delineate the biological significance and mechanism through which NQO1 is involved in prostate cancer. We report for the first time, that NQO1 may be protective against prostate cancer since its suppression increases inflammatory mediators that lead to increased resistance to hormone deprivation-induced cell death. Furthermore, we have defined a molecular mechanism that links NQO1 to IL-8 expression via control of IKKα-mediated p300 recruitment to NF-κB.
Accumulating evidence shows that NQO1 is overexpressed in most cancer cells as well as solid tumors (10, 37). Our screening results suggest that basal NQO1 protein levels may be under the control of NRF2, a master regulator that binds to the ARE-sites on the NQO1 promoter. In fact, the levels of NQO1 expression in these prostate cancer cells correlated with constitutively higher level of NRF2 activity bearing somatic mutations in NRF2 and KEAP1 genes suggesting that basal expression is regulated by KEAP1/NRF2 pathway (38). Although NQO1 level is induced during cancer initiation and progression compelling evidence from several studies suggest that NQO1 suppression or deletion leads to adverse biological consequences. For example, epigenetic downregulation or loss of NQO1 by NQO1*2, the C609T substituted polymorphic form of NQO1, has been correlated with strong adverse prognosis in various cancers such as hepatocellular carcinoma, breast etc. (39, 40).
Our findings indicate that NQO1 may play a critical role in maintaining redox balance between intracellular ROS and endogenous oxidative stress stimuli. NQO1 has been shown to generate ROS from NQO1-dependent futile redox cycling of certain substrates including endogenous quinones (10). Based on this fact, we speculate that stable inhibition of NQO1 may hinder constitutive ROS generation. However at the basal level, DU145 cells expressing high levels of NQO1 show significant low levels of H2O2. It is possible that because DU145 cells have the highest basal level of NQO2, it is able to quench H2O2 by converting to less reactive metabolites and therefore display the low levels observed. Further, our data also show high basal levels of GSH. These observations along with published literature that shows constitutive high levels of NRF2 and its target antioxidant genes in DU145 may also contribute to the significant lower basal H2O2 level. Further work in this area is required to establish how specific inhibition of NQO1 decreases intracellular ROS, as this may help provide novel strategies for prostate cancer management (41). Surprisingly, decrease in cellular ROS by NQO1 knockdown increased cell migration. Although elevated ROS levels is directly associated with development and progression of various cancers is (2, 3), ROS detoxification by antioxidant supplementation can also promote tumor aggressiveness in some genetic settings (42). Recently, in Nkx3.1 mutant mice, the ROS scavenger NAC increased pro-tumorigenic gene signature, increased prostate epithelial cell proliferation and promoted the expression of a pro-proliferative gene signature. More importantly, ROS did not promote cell proliferation in the prostate of Nkx3.1-null mice (43). Furthermore, ROS inhibition by NAC and vitamin E prevented p53 activation and markedly increased tumor cell proliferation in mouse and human lung tumor cells (34).
Several studies suggest that hormone deregulation, elevated oxidative stress and chronic inflammation in the prostatic microenvironment are connected through orchestrated ways to cause prostate cancer progression from androgen dependence to castration resistance (15, 18, 44). The exploitation of complex relationships between these factors will facilitate increased efficacy of hormone ablation allow design of novel combinatorial therapeutic approaches for advanced disease. Saylor and colleagues measured inflammatory and angiogenic biomarkers in men undergoing ADT treatment and found induction of inflammatory and angiogenic biomarkers including IL-8 in the treatment group compared with controls (45). We unexpectedly observed that NQO1 knockdown cells survived in hormone-deprived condition using CSS supplemented medium. However, multiple approaches failed to show significant differences between LNCaP-NTC and LNCaP-shNQ cells in hormone responsiveness and androgen signaling. These data suggest that the LNCaP-shNQ cells may have acquired changes, which contribute to their ability to survive under hormone-deprived condition. Genome wide microarray revealed that the absence of NQO1 allows orchestration of inflammatory signaling that sustains cell survival and androgen-independence. In this study, we identified a link between NQO1 and genes involved in different signaling pathways (Fig. 4), and were intrigued as to how these transcripts could be deregulated by NQO1 knockdown, which after all is best known as a key antioxidant enzyme. We hypothesized that it could be due to crosstalk with the activity of many signaling pathways and transcription factors that control their expression. Our analysis of targets of signaling pathway and their validation suggest an inverse relationship of NQO1 and pro-inflammatory targets. In vivo studies from our laboratory and others have found an inverse correlation between NQO1 and pro-inflammatory markers (46, 47). Here we report that pro-inflammatory cytokines including IL-8 are significantly highly expressed in androgen-independent cells, while being barely detectable in androgen-responsive prostate cancer cells (Supplementary Fig. S8). Our observation shows increased IL-8 production in LNCaP-shNQ cells and that induction at least in part, contributes to cell survival under hormone-deprived stress condition. Furthermore, we noted that IL-8 silencing partially reversed NQO1 knockdown-mediated survival under hormone depleted condition, indicating that IL-8 may be an important but not exclusive mediator of hormone-independent growth. Given the documented roles of these inflammatory mediators in prostate cancer progression and androgen-independent survival (35, 44, 48), it is possible that NQO1-mediated alterations may play a role in prostate pathology and the increased resistance of polymorphic individuals lacking NQO1 to standard treatments.
In keeping with our observations Rushworth et al (49) reported increased TNFα and IL-1β production upon NQO1 silencing while NQO1 overexpression inhibited inflammatory responses without affecting NF-κB activation in LPS-activated monocytes. Additionally, an increased level of nuclear IKKα, p300 binding to NF-κB and its transcriptional activation was confirmed in our NQO1 knockdown model system (Fig. 6). Several mechanisms have been proposed to define the role of nuclear IKKα in NF-κB activation. For instance, IKKα is recruited to NF-κB responsive promoters by interacting with CBP, which contributes to NF-κB-mediated gene expression through phosphorylation of histone H3 (26, 27). IKKα promotes prostate tumor metastasis by downregulating tumor suppressor Maspin (29). The underlying mechanisms of increased nuclear shuttling of IKKα in NQO1 knockdown LNCaP cells remain unclear. First, loss of NQO1 may result in excessive inflammatory response by releasing the feedback inhibition of NQO1, which in turn activates various cytokine-induced responses including NF-κB transactivation by nuclear IKKα activation. Second, in response to NQO1 blockade, p53 degradation is induced. We propose that NQO1 antagonizes the pro-apoptotic function of p53 and increases the access of shared transcription co-factors, CBP or p300 to NF-κB to induce cytokines and cell survival genes.
Our observations regarding nuclear IKKα in LNCaP cells and lack thereof in TRAMP tissues suggest that p53 status may be an important determinant for the observed inconsistency. As discussed, p53 was inactivated in the creation of this model therefore, the lack of p53 could explain the difference between our in vitro and in vivo work. We tested this hypothesis in mutant-p53 DU145 cells and found that nuclear IKKα was absent in both NTC and shNQ DU145 cells (data not shown). Further studies are on going to understand this mechanism.
In summary, our findings provide substantial insight into the intricate roles of NQO1 in prostate cancer and how NQO1 inhibition leads to the induction of inflammatory mediators in prostate cancer cells. Our data show that NQO1 blockade decreases p53 levels and activates NF-κB and its targets including IL-8, implying it is a crucial step leading to hormone-independent survival. NQO1 knockdown-induced nuclear IKKα may promote NF-κB activation by recruiting p300 to mediate pro-inflammatory gene expression. These findings may also provide a rationale for the development of new combination treatments using hormone ablation or chemotherapy and modifiers of oxidative stress for prostate cancer management.
Supplementary Material
Acknowledgments
We thank Dr. Ross (CU-Denver) for NQO1 reporter, anti-NQO2 antibody and MAC220; Dr. Tindall (Mayo) for AR reporter; Dr. Chang at UC-Berkeley for PO1 dye; Mrs. Hambright for cancer cell cDNA; Mr. Rivas for maintaining animal tissue repository and Optical Imaging shared resource at UTHSCSA.
Grant Support: This work was supported by 5R01CA149516 (RG), CTSA UL1 TR000149 (RG), 1R01AT7448 (APK) and CPRIT PDF RP101491 (DT).
Footnotes
Conflict of interest: The authors have no conflict of interest to report.
References
- 1.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–85. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 2.Thapa D, Ghosh R. Antioxidants for prostate cancer chemoprevention: challenges and opportunities. Biochem Pharmacol. 2012;83:1319–30. doi: 10.1016/j.bcp.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Letts. 2009;282:125–36. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asher G, Lotem J, Kama R, Sachs L, Shaul Y. NQO1 stabilizes p53 through a distinct pathway. Proc Natl Acad Sci U S A. 2002;99:3099–3104. doi: 10.1073/pnas.052706799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anwar A, Dehn D, Siegel D, Kepa JK, Tang LJ, Pietenpol JA, Ross D. Interaction of human NAD(P)H:quinone oxidoreductase 1 (NQO1) with the tumor suppressor protein p53 in cells and cell-free systems. J Biol Chem. 2003;278:10368–73. doi: 10.1074/jbc.M211981200. [DOI] [PubMed] [Google Scholar]
- 6.Shen J, Barrios RJ, Jaiswal AK. Inactivation of the quinone oxidoreductases NQO1 and NQO2 strongly elevates the incidence and multiplicity of chemically induced skin tumors. Cancer Res. 2010;70:1006–14. doi: 10.1158/0008-5472.CAN-09-2938. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Patrick BA, Gong X, Jaiswal AK. Disruption of NAD(P)H:quinone oxidoreductase 1 gene in mice leads to 20S proteasomal degradation of p63 resulting in thinning of epithelium and chemical-induced skin cancer. Oncogene. 2011;30:1098–1107. doi: 10.1038/onc.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Ahn KS, Sethi G, Jain AK, Jaiswal AK, Aggarwal BB. Genetic deletion of NAD(P)H:quinone oxidoreductase 1 abrogates activation of nuclear factor-kappaB, IkappaBalpha kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. J Biol Chem. 2006;281:19798–808. doi: 10.1074/jbc.M601162200. [DOI] [PubMed] [Google Scholar]
- 9.Garate M, Wani AA, Li G. The NAD(P)H:Quinone Oxidoreductase 1 induces cell cycle progression and proliferation of melanoma cells. Free Radic Biol Med. 2010;48:1601–1609. doi: 10.1016/j.freeradbiomed.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Siegel D, Yan C, Ross D. NAD(P)H:quinone oxidoreductase 1 (NQO1) in the sensitivity and resistance to antitumor quinones. Biochem Pharmacol. 2012;83:1033–40. doi: 10.1016/j.bcp.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao C, Zhang ZF, Berthiller J, Boffetta P, Hashibe M. NAD(P)H:quinone oxidoreductase 1 (NQO1) Pro187Ser polymorphism and the risk of lung, bladder, and colorectal cancers: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:979–87. doi: 10.1158/1055-9965.EPI-05-0899. [DOI] [PubMed] [Google Scholar]
- 12.Yuan W, Xu L, Chen W, Wang L, Fu Z, Pang D, et al. Evidence on the association between NQO1 Pro187Ser polymorphism and breast cancer risk in the current studies: a meta-analysis. Breast Cancer Res Treat. 2011;125:467–72. doi: 10.1007/s10549-010-0966-0. [DOI] [PubMed] [Google Scholar]
- 13.Sun Z, Cui Y, Pei J, Fan Z. Association between NQO1 C609T polymorphism and prostate cancer risk. Tumour Biol. 2014 doi: 10.1007/s13277-014-2051-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Siegel R, Naishadham D, Jemal A. Cancer statistics. 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 15.Shiota M, Song Y, Takeuchi A, Yokomizo A, Kashiwagi E, Kuroiwa K, et al. Antioxidant Therapy Alleviates Oxidative Stress by Androgen Deprivation and Prevents Conversion From Androgen Dependent to Castration Resistant Prostate Cancer. The Journal of Urology. 2012;187:707–14. doi: 10.1016/j.juro.2011.09.147. [DOI] [PubMed] [Google Scholar]
- 16.Jorgenson TC, Zhong W, Oberley TD. Redox Imbalance and Biochemical Changes in Cancer. Cancer Res. 2013;73:6118–23. doi: 10.1158/0008-5472.CAN-13-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vindrieux D, Escobar P, Lazennec G. Emerging roles of chemokines in prostate cancer. Endocr Relat Cancer. 2009;16:663–73. doi: 10.1677/ERC-09-0109. [DOI] [PubMed] [Google Scholar]
- 18.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60:199–215. doi: 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21:41–48. doi: 10.1016/j.cytogfr.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–94. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 21.Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67:6854–62. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- 22.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamshidi M, Bartkova J, Greco D, Tommiska J, Fagerholm R, Aittomaki K, et al. NQO1 expression correlates inversely with NFkappaB activation in human breast cancer. Breast Cancer Res Treat. 2012;132:955–68. doi: 10.1007/s10549-011-1629-5. [DOI] [PubMed] [Google Scholar]
- 24.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 25.Tergaonkar V, Perkins ND. p53 and NF-kappaB crosstalk: IKKalpha tips the balance. Mol Cell. 2007;26:158–59. doi: 10.1016/j.molcel.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 2003;423:655–59. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 27.Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003;423:659–63. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- 28.Huang WC, Ju TK, Hung MC, Chen CC. Phosphorylation of CBP by IKKalpha promotes cell growth by switching the binding preference of CBP from p53 to NF-kappaB. Mol Cell. 2007;26:75–87. doi: 10.1016/j.molcel.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–94. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 30.Dickinson BC, Huynh C, Chang CJ. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J Am Chem Soc. 2010;132:5906–15. doi: 10.1021/ja1014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar AP, Bhaskaran S, Ganapathy M, Crosby K, Davis MD, Kochunov P, et al. Akt/cAMP-responsive element binding protein/cyclin D1 network: a novel target for prostate cancer inhibition in transgenic adenocarcinoma of mouse prostate model mediated by Nexrutine, a Phellodendron amurense bark extract. Clin Cancer Res. 2007;13:2784–94. doi: 10.1158/1078-0432.CCR-06-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganapathy M, Ghosh R, Jianping X, Zhang X, Bedolla R, Schoolfield J, et al. Involvement of FLIP in 2-methoxyestradiol-induced tumor regression in transgenic adenocarcinoma of mouse prostate model. Clin Cancer Res. 2009;15:1601–11. doi: 10.1158/1078-0432.CCR-08-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Policastro LL, Ibanez IL, Notcovich C, Duran HA, Podhajcer OL. The Tumor Microenvironment: Characterization, Redox Considerations, and Novel Approaches for Reactive Oxygen Species-Targeted Gene Therapy. Antioxid Redox Signal. 2013;19:854–95. doi: 10.1089/ars.2011.4367. [DOI] [PubMed] [Google Scholar]
- 34.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6:221ra215. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka H, Kono E, Tran CP, Miyazaki H, Yamashiro J, Shimomura T, et al. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med. 2010;16:1414–20. doi: 10.1038/nm.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maxwell PJ, Coulter J, Walker SM, McKechnie M, Neisen J, McCabe N, et al. Potentiation of inflammatory CXCL8 signalling sustains cell survival in PTEN-deficient prostate carcinoma. Eur Urol. 2013;64:177–88. doi: 10.1016/j.eururo.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinkova-Kostova AT, Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys. 2010;501:116–23. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang P, Singh A, Yegnasubramanian S, Esopi D, Kombairaju P, Bodas M, et al. Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Mol Cancer Ther. 2010;9:336–46. doi: 10.1158/1535-7163.MCT-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu YL, Wang D, Peng XE, Chen YL, Zheng DL, Chen WN, et al. Epigenetic silencing of NAD(P)H:quinone oxidoreductase 1 by hepatitis B virus X protein increases mitochondrial injury and cellular susceptibility to oxidative stress in hepatoma cells. Free Radic Biol Med. 2013;65C:632–44. doi: 10.1016/j.freeradbiomed.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 40.Fagerholm R, Hofstetter B, Tommiska J, Aaltonen K, Vrtel R, Syrjakoski K, et al. NAD(P)H:quinone oxidoreductase 1 NQO1*2 genotype (P187S) is a strong prognostic and predictive factor in breast cancer. Nat Genet. 2008;40:844–53. doi: 10.1038/ng.155. [DOI] [PubMed] [Google Scholar]
- 41.Huang X, Dong Y, Bey EA, Kilgore JA, Bair JS, Li LS, et al. An NQO1 substrate with potent antitumor activity that selectively kills by PARP1-induced programmed necrosis. Cancer Res. 2012;72:3038–47. doi: 10.1158/0008-5472.CAN-11-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeNicola GM, Karreth FA, Humpton TJ, Gopinatha A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez EE, Anderson PD, Logan M, Abdulkadir SA. Antioxidant treatment promotes prostate epithelial proliferation in Nkx3.1 mutant mice. PLoS One. 2012;7:e46792. doi: 10.1371/journal.pone.0046792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dutt SS, Gao AC. Molecular mechanisms of castration-resistant prostate cancer progression. Future Oncol. 2009;5:1403–13. doi: 10.2217/fon.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saylor PJ, Kozak KR, Smith MR, Ancukiewicz MA, Efstathiou JA, Zietman AL, et al. Changes in biomarkers of inflammation and angiogenesis during androgen deprivation therapy for prostate cancer. Oncologist. 2012;17:212–19. doi: 10.1634/theoncologist.2011-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghosh R, Schoolfield J, Yeh IT, Smith ML, Hursting SD, Chan DC, et al. Loss of NADPH quinone oxidoreductase in the prostate and enhanced serum levels of cytokine-induced neutrophil chemoattractant 2alpha in hormone-stimulated noble rats: potential role in prostatic intraepithelial neoplasia development. Transl Oncol. 2009;2:65–72. doi: 10.1593/tlo.08214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheung KL, Lee JH, Khor TO, Wu TY, Li GX, Chan J, et al. Nrf2 knockout enhances intestinal tumorigenesis in Apc(min/+) mice due to attenuation of anti-oxidative stress pathway while potentiates inflammation. Mol Carcinog. 2014;53:77–84. doi: 10.1002/mc.21950. [DOI] [PubMed] [Google Scholar]
- 48.Lin TH, Liu HH, Tsai TH, Chen CC, Hsieh TF, Lee SS, et al. CCL2 increases alphavbeta3 integrin expression and subsequently promotes prostate cancer migration. Biochim Biophys Acta. 2013;1830:4917–27. doi: 10.1016/j.bbagen.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 49.Rushworth SA, MacEwan DJ, O’Connell MA. Lipopolysaccharide-induced expression of NAD(P)H:quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J Immunol. 2008;181:6730–37. doi: 10.4049/jimmunol.181.10.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.