Abstract
Nicotinic systems have been shown by a variety of studies to be involved in cognitive function. Nicotinic receptors have an inherent property to become desensitized after activation. The relative role of nicotinic receptor activation vs. net receptor inactivation by desensitization in the cognitive effects of nicotinic drugs remains to be fully understood. In these studies, we tested the effects of the α7 nicotinic receptor antagonist methyllycaconitine (MLA), the α4β2 nicotinic receptor antagonist dihydro-β-erythroidine (DHβE), the nonspecific nicotinic channel blocker mecamylamine and the α4β2 nicotinic receptor desensitizing agent sazetidine-A on learning in a repeated acquisition test. Adult female Sprague-Dawley rats were trained on a repeated acquisition learning procedure in an 8-arm radial maze. MLA (1–4 mg/kg), DHβE (1–4 mg/kg), mecamylamine (0.125–0.5 mg/kg) or sazetidine-A (1 and 3 mg/kg) were administered in four different studies either alone or together with the NMDA glutamate antagonist dizocilpine (0.05 and 0.10 mg/kg). MLA significantly counteracted the learning impairment caused by dizocilpine. The overall choice accuracy impairment caused by dizocilpine was significantly attenuated by co-administration of DHβE. Low doses of the non-specific nicotinic antagonist mecamylamine also reduced dizocilpine-induced repeated acquisition impairment. Sazetidine-A reversed the accuracy impairment caused by dizocilpine. These studies provide evidence that a net decrease in nicotinic receptor activity can improve learning by attenuating learning impairment induced by NMDA glutamate blockade. This adds to evidence in cognitive tests that nicotinic antagonists can improve cognitive function. Further research characterizing the efficacy and mechanisms underlying nicotinic antagonist and desensitization induced cognitive improvement is warranted.
Index words: Nicotinic, Learning, Antagonist, α4β2, α7, MLA, DHβE, Mecamylamine, Sazetidine-A, Dizocilpine
1. Introduction
Nicotinic acetylcholine receptors have been shown by a variety of studies to be critically involved in cognitive function (for review see (Levin et al., 2006)). These receptors are targets for cognitive enhancement research to help with diseases like Alzheimer’s disease, attention deficit hyperactivity disorder, and schizophrenia (Levin, 2002; Wallace et al., 2011). The critical actions of nicotinic agonists at nicotinic receptors for these effects are still not well understood.
It is important to note that an inherent property of nicotinic receptors is to become desensitized after activation (Ochoa et al., 1989). The relative role of nicotinic receptor activation vs. net inactivation by desensitization for cognitive enhancing as well as other functional effects of nicotinic agonists remains to be fully understood, but nicotinic receptor desensitization may provide therapeutic effects including cognitive improvement (Buccafusco et al., 2009; Levin et al., 2013; Picciotto et al., 2008) and nicotinic antagonists may also have therapeutic benefits (Dwoskin and Crroks, 2001).
Though high doses of nicotinic antagonists have been shown to impair memory (Levin et al., 1987), modestly decreased nicotinic receptor activation by receptor desensitization or blockade can improve cognition. Low doses of the nonspecific nicotinic antagonist mecamylamine had memory enhancing effects in rats and monkeys (Terry et al., 1999). Chronic infusions of mecamylamine improved working memory in the radial-arm maze (Levin et al., 1993). Relevant to the current tests of learning, we showed that low-dose acute administration of mecamylamine significantly reduced repeated acquisition errors (Levin and Caldwell, 2006). In a clinical study, low dose mecamylamine was found to improve recognition memory in adults with ADHD (Potter et al., 2009). These studies suggest that some cognitive improvement seen with nicotine and other agonists may be the result of receptor desensitization following activation, rather than the activation itself.
Previous studies have shown that attention can be improved through nicotinic receptor desensitization. Acute administration of the α4β2 nicotinic receptor desensitizing agent and partial agonist sazetidine-A improved attentional performance on an operant visual signal detection task, reversing the attentional impairments caused by either the NMDA glutamate antagonist dizocilpine or the muscarinic acetylcholine antagonist scopolamine (Rezvani et al., 2011). Chronic sazetidine-A infusions were also found to improve attentional performance on the same task and to significantly attenuate scopolamine-induced attentional impairment (Rezvani et al., 2012). To determine whether the sazetidine-A effects resulted from its desensitizing effect or from its partial agonist effect at α4β2 nicotinic receptors, we tested the effect of the α4β2 nicotinic receptor antagonist DHβE on the same task. Acute DHβE attenuated attentional impairment caused by dizocilpine (Levin et al., 2013). On the same task, the α7 nicotinic antagonist MLA also showed efficacy in reversing dizocilpine-induced attentional impairment. This finding is in line with previous research into the effect of low dose MLA on attentional enhancement (Hahn et al., 2011).
This further exploration of the efficacy of modestly decreasing nicotinic receptors for cognitive improvement was conducted to provide better understanding of the complex nature of nicotinic receptor involvement with cognitive function and to explore new avenues for development of nicotinic therapies for cognitive dysfunction.
2. Materials and Methods
2.1. Subjects
Young adult female Sprague-Dawley rats were used in the current set of studies (N=11–12/study). Female rats were selected for use in these studies to facilitate comparisons of the current results with previous results with nicotinic antagonist effects on other cognitive tasks like the attentional signal detection task. For an entire series of studies over 20 years of testing of nicotinic drug effects on cognitive function we have used female rats because they maintain relatively constant body weight throughout adulthood. Thus alterations in pharmacokinetics would not be a factor in drug effects on behavior. The rats were tested in a repeated measures counterbalanced design with the treatments given at multiple time points which would have been scattered throughout the estrus cycle so that estrus phase would not confound the drug effects. Separate sets of rats were used to test each of the three nicotinic antagonists. The rats were housed in groups of 2–3 with freely available water and feedings made each day to keep the subjects at approximately 85% of unrestricted feeding body weight adjusted for growth to provide motivation for the appetitively motivated repeated acquisition test. These studies were conducted under approval of the Duke University Institutional Animal Care and Use Committee.
2.2. Drug Treatments
In four different experiments the effects of the α7 nicotinic receptor antagonist methyllycaconitine (MLA), the α4β2 antagonist dihydro-β-erythroidine (DHβE), the nonspecific nicotinic antagonist mecamylamine and the nicotinic α4β2 desensitizing agent sazetidine-A (Georgetown University, Washington, DC, USA for sazetidine-A and Sigma, St. Louis, MO, USA for the other drugs) were tested for their effects in reversing the impairments caused by the NMDA antagonist dizocilpine on learning in a repeated acquisition test. Adult female Sprague-Dawley rats were trained on a repeated acquisition learning procedure in an 8-arm radial maze. Each day each rat was presented with a different array of three arms, which were rewarded with a food pellet for the first entry. The other five arms were not reinforced. The rats were tested for five trials per day to determine their learning of the new daily problem. Training continued until the rats reliably showed a learning curve when each daily new problem was presented. This took approximately 21 training sessions. Then three experiments were conducted in separate sets of rats in a repeated measures counter-balanced design with different dose sequences for each rat, a range of MLA doses (0, 1, 2 and 4 mg/kg), DHβE doses (0, 1, 2 and 4 mg/kg), mecamylamine doses (0, 0.125, 0.25 and 0.5 mg/kg) or sazetidine-A (0, 1 and 3 mg/kg) were administered either alone or together with the NMDA glutamate antagonist dizocilpine (0, 0.05 or 0.10 mg/kg) s.c. 20 min before the beginning of the test. The doses chosen were those that we previously found to effectively attenuate dizocilpine-induced impairment of accuracy on the attentional task. (Levin et al., 2013). The drug doses were given a repeated measures counterbalanced design.
The drugs used like all others have complex actions. DHβE shows preference for blocking α4β2 nicotinic receptors, but also does some effects at α7 receptors (Papke et al., 2008). MLA is a competitive antagonist that has been found to have selectivity for α7 vs. α4β2 nicotinic receptors (Marks et al., 1999), but there is evidence that it also has activity at α4β2 nicotinic receptors as well (Karadsheh et al., 2004). Mecamylamine is a noncompetitive nicotinic channel blocker without much selectivity among nicotinic receptor subtypes (Papke et al., 2008). Sazetidine-A is a mixed agonist and desensitizing agent at α4β2 nicotinic receptors (Xiao et al., 2006; Zwart et al., 2008)and recently has been found to have some actions at α7 nicotinic receptors (Brown and Wonnacott, 2014).
An automated radial-arm maze (Med Associates Inc., Georgia, VT, USA) was used. The rats were trained on an automated 8-arm radial maze elevated 5 cm from the floor with a central platform of 30 cm in diameter and walls 32.5 cm height from which extend the arms with the dimensions of 17.5 × 12.5 × 67.5 cm. Clear Plexiglas walls are at the sides and on the top of each arm. Each arm is separated from the central platform by vertical aluminum gates. Feeders are located at the end of each arm and feed one pallet (P.J. Noyes Co Inc.) at a time. The maze was in a room that contained extra-maze visual cues. The cues were always kept in the same position when testing. The rats were first handled for 5 min for a few days to accustom them to human contact. They were then shaped by being placed in the center of the maze with 15 pellets and kept there until all the pieces had been eaten or a maximum of 15 min had ended. Once the rats had consumed the food reinforcers within the 15 min allocated, training on the maze was started. This involved baiting 3 of the 8 arms with reinforcers. The same 3 arms were kept baited for an individual rat for 5 continuous trials in which they chose arms until they had selected the three baited arms or a maximum of 3 min elapsed. Then the next trial was immediately started with the return of the rat to the center of the maze, and after 10 seconds the doors to the arms were opened. Different random combinations of arms were baited in different sessions. Not more than two adjacent arms would be baited. To start the session, the rat was placed in the central cylinder and the program would start after 10s. The gates open allowing rats free movement around the maze for 3 min or until all baited arms were chosen. To be considered an entry, the rat had to enter the arm and walk to the end. Entries to any arms other than the first time entry to the baited arms were counted as errors. The dependent measure for repeated acquisition was the number of errors per trial. Data from a trial were included in analysis if all three of the baited arms were selected within the 180 second time limit. If only two baited arms were selected, an error of omission was added to the error score for that trial. If only one or no baited arms were selected within the 180 second time limit then the error data were not included in the analysis and the subject was run with that treatment on another day. The rats were trained on the maze at least twice weekly until they reached a stable level of performance before drug administration was carried out.
2.3. Statistics
The choice accuracy (errors per trial) data were analyzed for statistical significance with ANOVA for two within subjects factors, nicotine antagonist dose levels and dizocilpine dose level. The learning rate was indexed by analysis of the linear trend (slope of errors across the five trials). This was done by assigning coefficients describing a 45-degree linear slope to each of the successive trials as described in Keppel (Keppel, 1982). Significant interactions were followed-up by tests of the simple main effects. Treatment comparisons were made to test the hypotheses that dizocilpine would impair performance accuracy and that the nicotinic drugs tested would counteract the dizocilpine-induced impairment. A P-value < 0.05 was used as the threshold for significance.
3. Results
3.1. MLA: α7 Nicotinic Blockade Interactions with NMDA Glutamate Blockade
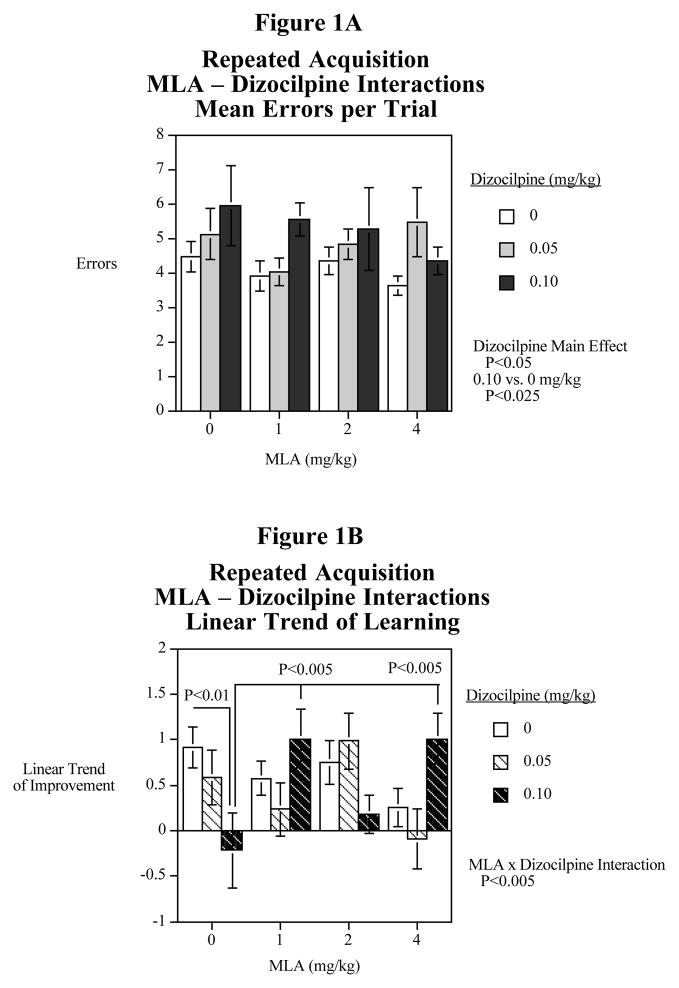
Dizocilpine caused a significant (F(2,22)=3.47, P<0.05) main effect of increased errors. The 0.10 dizocilpine dose caused a significant (F(1,22)=6.72, P<0.025) increase in errors vs. performance without dizocilpine. There was no significant effect of MLA or MLA x dizocilpine interaction detected with analysis of average performance in the repeated acquisition task (Fig. 1A). The linear function of improved performance (fewer errors) over the course of the five trials per session was used as the index of learning. A different pattern of effects was seen when considering learning performance (Fig. 1B). The main effects of MLA and dizocilpine were not significant. But there was a significant (F(6,66)=3.91, P<0.005) interactive effect of MLA x dizocilpine on learning in the repeated acquisition test. Tests of the simple main effects comparing the treatments showed that as expected dizocilpine (0.10 mg/kg) caused a significant (F(1,66)=7.95, P<0.01) learning impairment relative to control. With this dose of dizocilpine there was no evidence of learning over the five-trial session. The addition of MLA, 1 or 4 mg/kg (F(1,66)=9.43, P<0.005) significantly counteracted the learning impairment caused by dizocilpine. The middle MLA dose of 2 mg/kg provided a trend toward an improvement in learning, but a significant effect with this dose was not seen. When given alone, none of the MLA doses significantly affected learning rate relative to control.
Figure 1.
Fig. 1A: Interactive effects of the nicotinic α7 antagonist MLA and the NMDA glutamate antagonist dizocilpine on average errors in the radial-arm maze repeated acquisition task (mean±S.E.M.) N=12
Fig. 1B: Interactive effects of the nicotinic α7 antagonist MLA and the NMDA glutamate antagonist dizocilpine on the linear decrease in errors in the radial-arm maze repeated acquisition task (mean± S.E.M.) N=12
Response latency was not significantly affected by drug treatment in this study.
3.2. DHβE: α4β2 Nicotinic Blockade Interactions with NMDA Glutamate Blockade
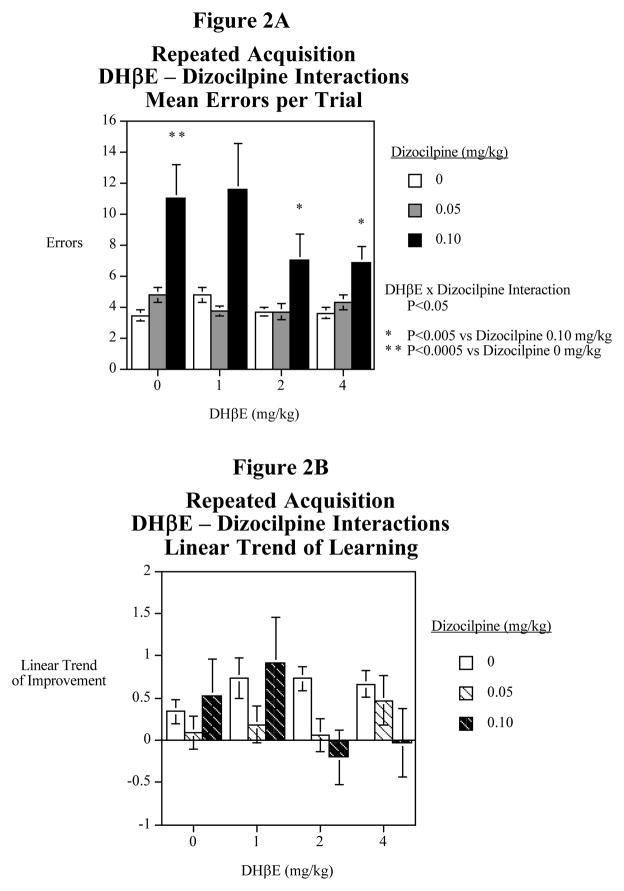
There was a significant (F(2,22)=10.02, P<0.01) main effect of dizocilpine. There was also a significant (F(6,66)=2.48, P<0.05) interaction of dizocilpine and DHβE with regard to errors per trial. Follow-up tests of the simple main effects were conducted. The significant (F(1,66)=34.94, P<0.0005) choice accuracy impairment caused by dizocilpine was significantly attenuated by co-administration of either 2 mg/kg (F(1,66)=9.76, P<0.005) or 4 mg/kg (F(1,66)=10.67, P<0.005) of DHβE (Fig. 2A). Unlike MLA, DHβE did not significantly affect the linear trend of improvement over the five trials of training on the repeated acquisition task (Fig. 2B).
Figure 2.
Fig. 2A: Interactive effects of the nicotinic α4β2 antagonist DHβE and the NMDA glutamate antagonist dizocilpine on average errors in the radial-arm maze repeated acquisition task (mean± S.E.M.) N=12
Fig. 2B: Interactive effects of the nicotinic α4β2 antagonist DHβE and the NMDA glutamate antagonist dizocilpine on the linear decrease in errors in the radial-arm maze repeated acquisition task (mean± S.E.M.) N=12
With latency (seconds per entry) there was a significant main effect of dizocilpine (F(2,22)=7.90, P<0.005). Comparisons of the 0.05 mg/kg (F(1,22)=15.33, P<0.005) and 0.10 mg/kg (F(1,22)=−6.50, P<0.05) vs. 0 mg/kg of dizocilpine showed significant quickening of response. There was also a significant dizocilpine x sazetidine-A interaction (F(6,66)=3.78, P<0.005). Individual means comparisons showed that neither dizocilpine dose when given alone significantly affected response latency compared with vehicle control. The only dose of DHβE that affected response latency was a slowing in response by the 2 mg/kg dose (F(1,66)=16.56, P<0.005), and effect that was revered by both dizocilpine doses (P<0.0005).
3.3. Mecamylamine: Nicotinic Blockade Interactions with NMDA Glutamate Blockade
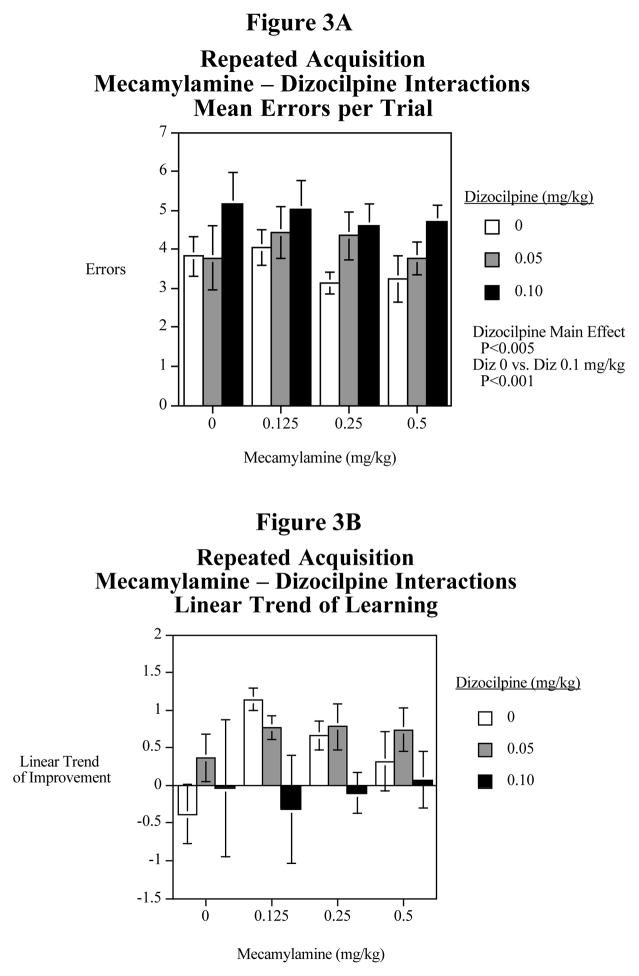
As shown in Fig. 3A, dizocilpine caused a significant (F(2,11)=8.24, P<0.005) main effect increasing errors. The 0.1 mg/kg dizocilpine dose caused a significant (F(1,11)=15.80, P<0.001) increase in errors. The 0.05 mg/kg dizocilpine dose did not cause a significant effect. Fig. 3B shows the results of mecamylamine and dizocilpine treatment on improvement over the repeated acquisition session. No significant drug effects were seen in this experiment with this measure.
Figure 3.
Fig. 3A: Interactive effects of the non-specific nicotinic antagonist mecamylamine and the NMDA glutamate antagonist dizocilpine on average errors in the radial-arm maze repeated acquisition task (mean± S.E.M.) N=12
Fig. 3B: Interactive effects of the non-specific nicotinic antagonist mecamylamine and the NMDA glutamate antagonist dizocilpine on the linear decrease in errors in the radial-arm maze repeated acquisition task (mean± S.E.M.) N=12
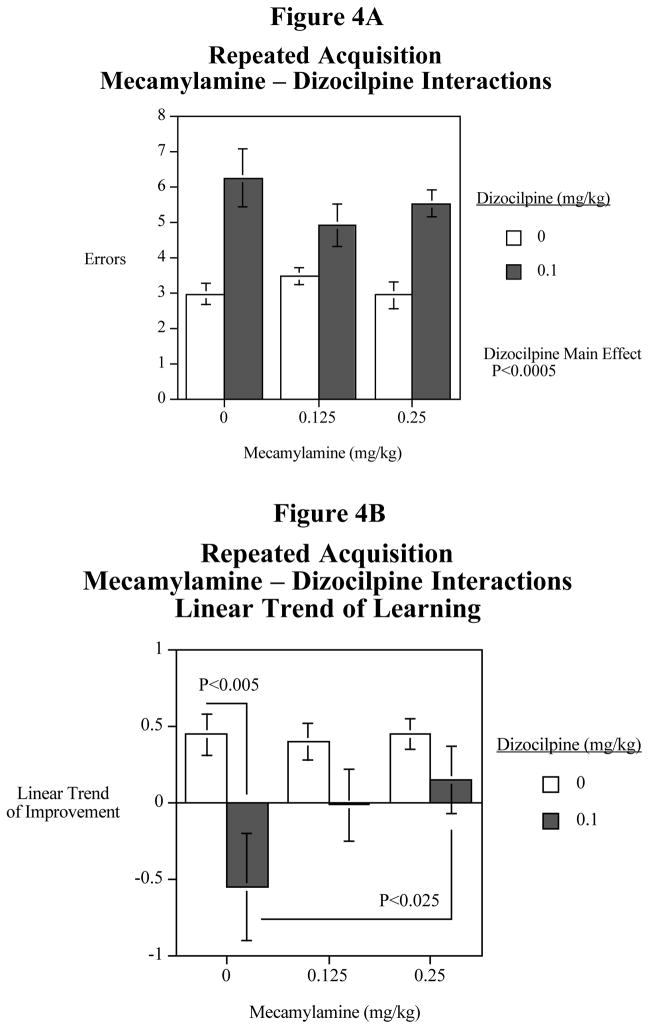
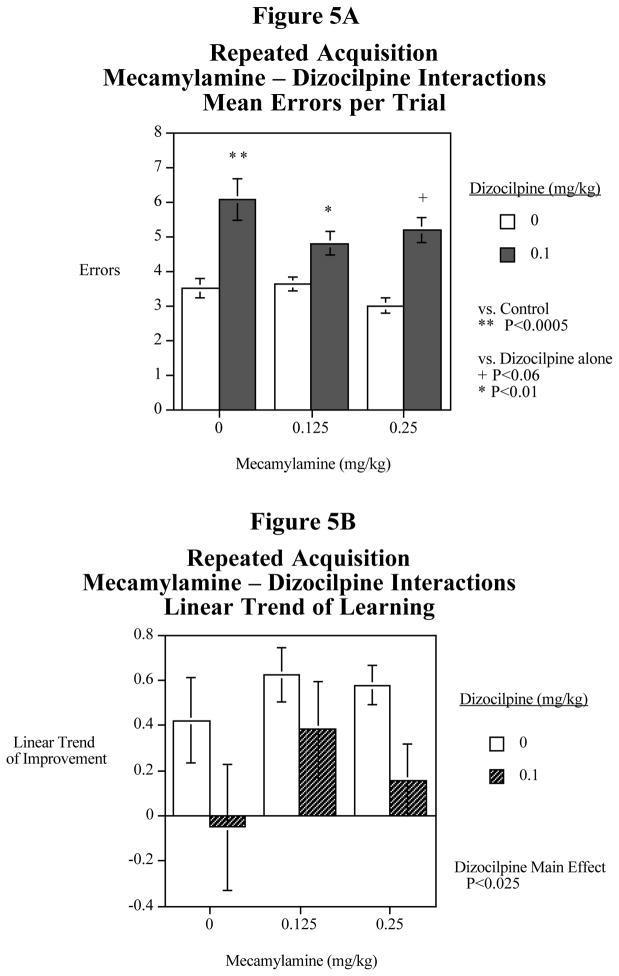
A follow-up study was conducted to verify the suggestions of interactive effects of mecamylamine and dizocilpine. The main effect of dizocilpine increasing errors was quite significant (F(1,11)=38.36, P<0.0005). This effect is shown in Fig. 4A. There was a significant three-way interaction of dizocilpine x mecamylamine x trial (F(8,88)=2.06, P<0.05). As a follow-up the linear trend of improvement over the five trials (Fig. 4B), dizocilpine caused a significant (F(8,88)=2.06, P<0.005) decrease in learning across the five trials. Analysis of the linear trend of learning showed that dizocilpine caused a significant (F(1,22)=14.29, P<0.005) learning impairment. The 0.25 mg/kg mecamylamine dose significantly (F(1,22)=7.04, P<0.025) attenuated the dizocilpine-induced learning impairment. The lower 0.125 mg/kg mecamylamine dose showed some indication of improvement of the dizocilpine-induced learning impairment, but this was not quite significant (F(1,22,)=4.13, P<0.06).
Figure 4.
Fig. 4A: The repeat experiment, interactive effects of the non-specific nicotinic antagonist mecamylamine and the NMDA glutamate antagonist dizocilpine on mean errors across the session in the radial-arm maze repeated acquisition task (mean± S.E.M.) N=12
Fig. 4B: The repeat experiment, interactive effects of the non-specific nicotinic antagonist mecamylamine and the NMDA glutamate antagonist dizocilpine on linear trend of improvement errors across the session in the radial-arm maze repeated acquisition task (mean± S.E.M.) N=12
The two phases of the mecamylamine-dizocilpine experiment were considered together. The main effect showed that 0.1 mg/kg of dizocilpine caused a significant (F(1,11)=42.29, P<0.0005) increase in errors. There was a two-way interaction of dizocilpine x mecamylamine (F(1,22)=2.57, P<0.10) that prompted follow-up tests of the simple main effects. When dizocilpine was administered alone it caused a significant (F(1,22)=33.36, P<0.0005) impairment relative to vehicle control. The 0.125 mg/kg mecamylamine dose caused a significant (F(1,22)=8.18, P<0.01) attenuation of the dizocilpine-induced impairment. The 0.25 mg/kg mecamylamine dose caused a nearly significant (F(1,22)=4.05, P<0.06) attenuation of the dizocilpine-induced impairment (Fig. 5A). Analysis of the combined data from the two phases of the mecamylamine-dizocilpine study with regard to the linear improvement of accuracy over the session showed a significant (F(1,11)=7.05, P<0.025) dizocilpine-induced impairment. There was a suggestion of an attenuated impairment with the addition of mecamylamine, but the mecamylamine x dizocilpine interaction did not prompt tests of the simple main effects (Fig. 5B).
Figure 5.
Fig. 5A: Combined data from the first and second experiments concerning mecamylamine - dizocilpine interactions and mean errors across the session in the radial-arm maze repeated acquisition task (mean± S.E.M.) N=12
Fig. 5B: Combined data from the first and second experiments concerning mecamylamine - dizocilpine interactions and the linear trend of improvement errors across the session in the radial-arm maze repeated acquisition task (mean± S.E.M.) N=12
Response latency was significantly reduced by dizocilpine (F(1,11)=8.50, P<0.025). However, this low mecamylamine dose range did not cause a significant effect on response latency, nor did it interact with the dizocilpine effect.
3.4. Sazetidine: Nicotinic Desensitization Interactions with NMDA Glutamate Blockade
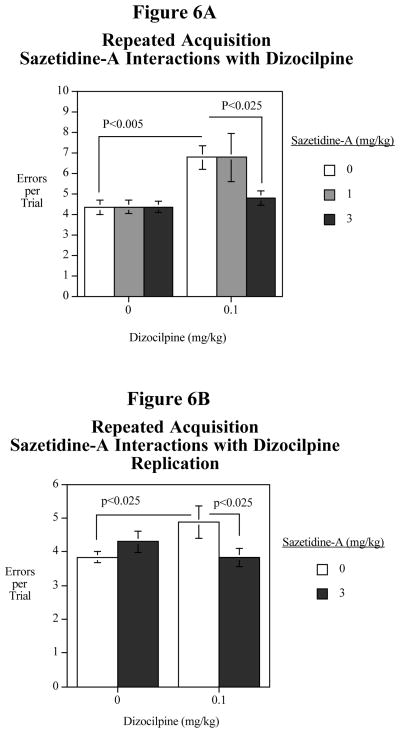
There was a significant (F(1,10)=13.48, P<0.005) main effect of dizocilpine impairing accuracy. There was also a sazetidine-A x dizocilpine interaction (F(2,20)=2.93, P<0.08), which prompted examination of the simple main effects. Dizocilpine caused a significant (F(1,20)=13.01, P<0.005) increase in the number of errors per trial (Fig. 6A). The 3 mg/kg dose of sazetidine-A significantly (F(1,20)=8.64, P<0.025) reversed the dizocilpine-induced impairment. The lower dose of 1 mg/kg was not effective in attenuating the dizocilpine-induced impairment. On its own, sazetidine-A was not seen to have any hint of an effect on choice accuracy.
Figure 6.
Fig. 6A: Interactive effects of acute sazetidine-A (0, 1 and 3 mg/kg) on dizocilpine (0 and 0.1 mg/kg) induced increase errors in the radial-arm maze repeated acquisition task, errors per trial (mean± S.E.M.).
Fig. 6B: Replication of the efficacy of 3 mg/kg of sazetidine-A for reversing the dizocilpine (0.1 mg/kg) impairment in repeated acquisition accuracy (mean± S.E.M.).
To be sure of the effectiveness of the 3 mg/kg of sazetidine-A its interaction with dizocilpine was tested again. There was a significant sazetidine-A x dizocilpine interaction (F(1,10)=8.89, P<0.025). As seen previously, 0.1 m/kg of dizocilpine caused a significant (F(1,10)=8.38, P<0.025) accuracy impairment and 3 mg/kg of sazetidine-A significantly (F(1,10)=8.68, P<0.025) reversed the dizocilpine-induced impairment (Fig. 6B). Also, as seen in the earlier experiment, sazetidine-A by itself had no significant effect relative to vehicle administration.
With response latency, there was a significant sazetidine-A x dizocilpine interaction (F(1,10)=8.85, P<0.025). Follow-up means comparisons showed that both sazetidine-A (F(1,10)=10.41, P<0.01) and dizocilpine (F(1,10)=9.99, P<0.025) significantly quickened response. But these two treatments did not mutually augment each other’s effects.
4. Discussion
These studies provide evidence that a net decrease in nicotinic receptor activity can significantly attenuate the choice accuracy and learning impairment induced by NMDA glutamate blockade as measured in the radial-arm maze repeated acquisition test. This adds to other evidence with tests of learning, memory and attention that nicotinic antagonists can improve cognitive function.
We tested the efficacy of nicotinic antagonists, either α7 selective (MLA), α4β2 selective (DHβE) or non-specific (mecamylamine), in reversing the impairment caused by the NMDA glutamate receptor antagonist dizocilpine on the radial-arm maze repeated acquisition task. Dizocilpine has been shown to impair cognitive function in rats. Dizocilpine can induce a good model of cognitive impairment with up to 0.1mg/kg subcutaneous doses without causing motor issues or intoxication (van der Staay et al., 2011). Of specific interest to our radial-arm repeated acquisition task, dizocilpine has been shown to increase errors on a radial-arm task (Levin et al., 1998; Ward et al., 1990) as well as impair acquisition of behavior in a novel repeated acquisition nose poke test (Pitts et al., 2006). Nicotine was previously shown to attenuate the impairment caused by this NMDA antagonist in a radial-arm maze task (Levin et al., 1998). The repeated acquisition paradigm allowed us to measure performance of learning and working memory.
There were differential effects of the various nicotinic antagonists, MLA, DHβE and mecamylamine and the nicotinic receptor desensitizing agent sazetidine-A. The α7 antagonist MLA was effective in significantly reversing learning impairment induced by dizocilpine (MK-801) as measured by the linear trend of improvement over the five trials of the session. In contrast, the α4β2 nicotinic antagonist DHβE significantly attenuated the overall choice accuracy impairment caused by dizocilpine without a significant effect on the linear trend of improvement of the course of the session. The nonspecific nicotinic antagonist mecamylamine showed some efficacy in reversing both the overall error rate increase caused by dizocilpine and the learning impairment caused by dizocilpine.
This adds to other evidence with tests of learning, memory and attention and others that nicotinic antagonists and desensitizing agents can improve cognitive function. In previous studies we have found that mecamylamine at a low dose can significantly improve repeated acquisition in the same radial-arm maze repeated acquisition procedure as used in the current study (Levin and Caldwell, 2006). The most efficacious mecamylamine dose in that study was 0.125 mg/kg, the same dose range as seen to be effective in the current study. However, dizocilpine was not used in the previous study. Significant improvement in accuracy was not seen in the current study with mecamylamine alone. This may have been due to the intercurrent dosing with dizocilpine in the repeated measures design which occurred in the current study but not the earlier one. Interestingly, we also found that the α7 nicotinic agonist ARR-17779 to significantly improve choice accuracy in the radial-arm maze repeated acquisition procedure (Levin et al., 1999). However, it should be remembered that nicotinic receptors, particularly the α7 receptor is very easily desensitized and functional effects of an agonist drug can also be due to its desensitizing and net antagonist effects.
Nicotinic α4β2 and α7 are two receptor subtypes highly expressed in the central nervous system and important for a variety of cognitive functions (Gotti et al., 2006; Leiser et al., 2009; Levin et al., 2002). Frontal cortex and hippocampal α4β2 and α7 receptors have been shown to be critically involved in memory function (Chan et al., 2007; Felix and Levin, 1997; Nott and Levin, 2006; Pocivavsek et al., 2006). Mice with α7 receptor knockouts have impaired sustained attention (Hoyle et al., 2006) and impaired working memory (Fernandes et al., 2006; Levin et al., 2009), further emphasizing the importance of this receptor subtype for normal cognitive function. Systemic α7 agonist treatment has been found to improve cognitive function in a variety of ways. Studies have shown them to attenuate symptoms of schizophrenia (Hauser et al., 2009; Pichat et al., 2007), as well as improve memory (Boess et al., 2007; Prickaerts et al., 2012; Tietje et al., 2008; Van Kampen et al., 2004). The α7 agonist AR-R17779 has been shown to improve learning and memory in rats (Levin et al., 1999). Because the α7 receptor subtype desensitizes particularly rapidly, our current study tested the effect of modest decreases in activity of the α7 receptor with low doses of antagonist MLA on learning and memory. This experiment allowed to us see if the cognitive enhancing effects seen with α7 agonists can in part be attributed to a net antagonist effect of the post activation desensitization.
The α4β2 subtype has also been a promising target of research into cognitive function. The agonist ABT-418 has shown preclinical memory enhancing effects (Buccafusco et al., 1995), as well as some clinical efficacy in treating Alzheimer’s disease and attention deficit hyperactivity disorder (Potter et al., 1999; Wilens et al., 1999). The agonist metanicotine improved working memory in rats on a radial-arm maze (Levin and Christopher, 2002). Mice with knockout of β2-containing receptors show significant memory impairments (Levin et al., 2009). As with the α7 agonists, we want to test whether the effects seen with α4β2 agonists can be at least in part attributed to the post-activation desensitization. Studies of α4β2 partial agonists and desensitizing agents have shown beneficial cognitive effects. In addition to efficacy at reducing nicotine self-administration (Johnson et al., 2012; Levin et al., 2010; Rezvani et al., 2010) and treating smoking cessation (Coe et al., 2005), there is further preclinical evidence of these compounds having positive effects on cognition and depression (Caldarone et al., 2011; Rezvani et al., 2011; Rezvani et al., 2012; Rollema et al., 2009). However, many of the desensitizing agents also have some agonist properties. Thus, our current study with the use of α4β2 selective antagonist DHβE allowed us to see if learning and memory improvements can be attributed to the desensitization of the receptor and not the agonist properties of agonists and desensitizing agents.
The nicotinic α4β2 desensitizing agent sazetidine-A was shown in our recent studies to significantly improve attentional performance in an operant signal detection task. Acute sazetidine-A injections significantly reversed attentional impairments caused by either dizocilpine or the muscarinic acetylcholine antagonist scopolamine (Rezvani et al., 2011). In addition, chronic infusions of sazetidine-A have also been found to improve attentional performance and reverse scopolamine-induced attentional impairment (Rezvani et al., 2012). Recently, we showed that MLA and DHβE both effectively attenuate attentional impairment caused by dizocilpine (Levin et al., 2013).
Mice with knockouts of α7 or β2 containing nicotinic receptors have been found to have impaired choice accuracy in the radial-arm maze (Levin et al., 2009). However, when interpreting the effects of knockout studies, it is important to keep in mind that missing these receptors during development can cause important dysfunction in the construction of the brain and cognitive impairments could result from this abnormal development as well as the absence of particular at the time of testing. Even conditional knockout studies cause complete inactivation of particular nicotinic receptors rather than less than complete temporary blockade with antagonists. The fact that we seen significant attenuation of dizocilpine-induced impairments with outright nicotinic receptor antagonists indicates that desensitizing effects of sazetidine-A rather than agonist effects likely underlies its efficacy in reversing dizocilpine and by extension that there could be therapeutic value in the nicotinic receptor desensitization caused by nicotine and other nicotinic agonists.
The nature of the participation of anatomically distinct nicotinic neural systems in cognitive function is likely to be significant. Data show that local infusions of α7 and α4β2 nicotinic antagonists (MLA and DHβE) into the dorsal or ventral hippocampus or basolateral amygdala in rats significantly impaired working memory function (Addy et al., 2003; Levin et al., 2002; Nott and Levin, 2006). In contrast, acute or chronic local infusion of the α4β2 antagonist DHβE into the dorsomedial thalamic nucleus caused a significant improvement in working memory function (Cannady et al., 2009).
The complex relationship of nicotinic receptor actions and cognitive function includes the findings that for learning, memory and attention modest decreases in nicotinic receptor action can improve performance. Clearly, from the nicotinic receptor knockout studies, assessment of cholinergic neurodegeneration and high dose nicotinic antagonist effects, substantial nicotinic receptor underactivity impairs cognitive function. This non-monotonic relationship of nicotinic receptor activity and cognitive function can also explain the reason why one finds that the cognitive enhancing effects of nicotinic agonists can be reversed by nicotinic antagonists. These results are commonly interpreted as supporting the conclusion that it is the agonist effect of a nicotinic agonist that underlies the cognitive improvement. Another possibility is that the addition of an antagonist to the desensitization caused by the nicotinic agonist makes the nicotinic receptor population underactivity too great to be of benefit.
These results suggest that the improvements seen with nicotinic agonists may at least in part be due to the receptor desensitizing effects and net antagonist effects of these drugs. Desensitization of nicotinic receptors may play important roles in a variety of physiological functions. Nicotinic receptor desensitization appears to be more than merely the cessation of agonist action. Further research characterizing the efficacy and mechanisms underlying nicotinic antagonist and desensitization induced cognitive improvement is warranted. This may lay the foundation for new paths for developing nicotinic receptor antagonists and desensitizing drugs to improve cognitive function.
Acknowledgments
The authors thank Karen Gordon for her help with testing and the laboratory of Dr. Milton Brown of Georgetown University School of Medicine for providing the sazetidine-A. This research was supported by a grant from the National Institute of Drug Abuse DA027990.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addy NA, Nakijama A, Levin ED. Nicotinic mechanisms of memory: effects of acute local DHβE and MLA infusions in the basolateral amygdala. Cognitive Brain Research. 2003;16:51–57. doi: 10.1016/s0926-6410(02)00209-4. [DOI] [PubMed] [Google Scholar]
- Boess FG, De Vry J, Erb C, Flessner T, Hendrix M, Luithle J, Methfessel C, Riedl B, Schnizler K, van der Staay FJ, van Kampen M, Wiese WB, Koenig G. The novel alpha7 nicotinic acetylcholine receptor agonist N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1-benzofuran-2-carboxamide improves working and recognition memory in rodents. J Pharmacol Exp Ther. 2007;321:716–725. doi: 10.1124/jpet.106.118976. [DOI] [PubMed] [Google Scholar]
- Brown JA, Wonnacott S. Sazetidine-A activates and desensitizes native a7 nicotinic acetylcholine receptors. Neurochemical Research. 2014 doi: 10.1007/s11064-11014-11302-11066. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Brach JW, Terry AV. Desensitization of nicotinic acetylcholine receptors as a stategy for drug development. The Journal of Pharmacology and Experimental Therapeutics. 2009;328:364–370. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Jackson WJ, Terry AV, Marsh KC, Decker MW, Arneric SP. Improvement in performance of a delayed matching-to-sample task by monkeys following ABT-418: a novel cholinergic channel activator for memory enhancement. Psychopharmacology. 1995;120:256–266. doi: 10.1007/BF02311172. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, Wang D, Paterson NE, Manzano M, Fedolak A, Cavino K, Kwan M, Hanania T, Chellappan SK, Kozikowsk iAP, Olivier B, Picciotto MR, Ghavami A. Dissociation between duration of action in the forced swim test in mice and nicotinic acetylcholine receptor occupancy with sazetidine, varenicline, and 5-I-A85380. Psychopharmacology. 2011;217:199–210. doi: 10.1007/s00213-011-2271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, Weir R, Wee B, Gotschlich E, Kolia N, Lau E, Brotherton J, Levin ED. Nicotinic antagonist effects in the mediodorsal thalamic nucleus: regional heterogeneity of nicotinic receptor involvement in cognitive function. Biochem Pharmacol. 2009;78:788–794. doi: 10.1016/j.bcp.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Chan WK, Wong PT, Sheu FS. Frontal cortical alpha7 and alpha4beta2 nicotinic acetylcholine receptors in working and reference memory. Neuropharmacology. 2007;52:1641–1649. doi: 10.1016/j.neuropharm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crroks PA. Competitive neuronal nicotinic receptor antagonists: A new direction for drug discovery. The Journal of Pharmacology and Experimental Therapeutics. 2001;298:395–402. [PubMed] [Google Scholar]
- Felix R, Levin ED. Nicotinic antagonist administration into the ventral hippocampus and spatial working memory in rats. Neuroscience. 1997;81:1009–1017. doi: 10.1016/s0306-4522(97)00224-8. [DOI] [PubMed] [Google Scholar]
- Fernandes C, Hoyle E, Dempster E, Schalkwyk LC, Collier DA. Performance deficit of alpha7 nicotinic receptor knockout mice in a delayed matching-to-place task suggests a mild impairment of working/episodic-like memory. Genes, Brain, & Behavior. 2006;5:433–440. doi: 10.1111/j.1601-183X.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Bohr I, Ziabreva I, Vailati S, Longhi R, Riganti L, Gaimarri A, McKeith IG, Perry RH, Aarsland D, Larsen JP, Sher E, Beattie R, Clementi F, Court JA. Selective nicotinic acetylcholine receptor subunit deficits identified in Alzheimer’s disease, Parkinson’s disease and dementia with Lewy bodies by immunoprecipitation. Neurobiology of Disease. 2006;23:481–489. doi: 10.1016/j.nbd.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Selective nicotinic receptor antagonists: effects on attention and nicotine-induced attentional enhancement. Psychopharmacology (Berl) 2011;217:75–82. doi: 10.1007/s00213-011-2258-8. [DOI] [PubMed] [Google Scholar]
- Hauser TA, Kucinski A, Jordan KG, Gatto GJ, Wersinger SR, Hesse RA, Stachowiak EK, Stachowiak MK, Papke RL, Lippiello PM, Bencherif M. TC-5619: an alpha7 neuronal nicotinic receptor-selective agonist that demonstrates efficacy in animal models of the positive and negative symptoms and cognitive dysfunction of schizophrenia. Biochem Pharmacol. 2009;78:803–812. doi: 10.1016/j.bcp.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle E, Genn RF, Fernandes C, Stolerman IP. Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology. 2006;189:211–223. doi: 10.1007/s00213-006-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Slade S, Wells C, Petro A, Sexton H, Rezvani AH, Brown ML, Paige MA, McDowell BE, Xiao Y, Kellar KJ, Levin ED. Chronic sazetidine-A infusion reduces nicotine self-administration in male and female rats. Psychopharmacology. 2012;222:269–276. doi: 10.1007/s00213-012-2642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadsheh MS, Shah MS, Tang X, Macdonald RL, Stitzel JA. Functional characterization of mouse a4b2 nicotinic acetylcholine receptors stably expressed in HEK293T cells. Journal of Neurochemistry. 2004;91:1138–1150. doi: 10.1111/j.1471-4159.2004.02801.x. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher’s Handbook. 2. Prentice-Hall, Inc; Englewood Cliffs, New Jersey: 1982. [Google Scholar]
- Leiser SC, Bowlby MR, Comery TA, Dunlop J. A cog in cognition: how the alpha 7 nicotinic acetylcholine receptor is geared towards improving cognitive deficits. Pharmacol Ther. 2009;122:302–311. doi: 10.1016/j.pharmthera.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. Journal of Neurobiology. 2002;53:633, 640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bettegowda C, Blosser J, Gordon J. AR-R17779, an α7 nicotinic agonist, improves learning and memory in rats. Behavioural Pharmacology. 1999;10:675–680. doi: 10.1097/00008877-199911000-00014. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bettegowda C, Weaver T, Christopher NC. Nicotine-dizocilpine interactions and working and reference memory performance of rats in the radial-arm maze. Pharmacology, Biochemistry and Behavior. 1998;61:335–340. doi: 10.1016/s0091-3057(98)00109-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bradley A, Addy N, Sigurani N. Hippocampal α7 and α4β2 nicotinic receptors and working memory. Neuroscience. 2002;109:757–765. doi: 10.1016/s0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Chronic nicotinic stimulation and blockade effects on working memory. Behavioural Pharmacology. 1993;4:179–182. [PubMed] [Google Scholar]
- Levin ED, Caldwell DP. Low-dose mecamylamine improves learning of rats in the radial-arm maze repeated acquisition procedure. Neurobiol Learn Mem. 2006;86:117–122. doi: 10.1016/j.nlm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Levin ED, Castonguay M, Ellison GD. Effects of the nicotinic receptor blocker mecamylamine on radial-arm maze performance in rats. Behavioral & Neural Biology. 1987;48:206–212. doi: 10.1016/s0163-1047(87)90752-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Cauley M, Rezvani AH. Improvement of attentional function with antagonism of nicotinic receptors in female rats. Eur J Pharmacol. 2013;702:269–274. doi: 10.1016/j.ejphar.2013.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Christopher NC. Persistence of nicotinic agonist RJR 2403 induced working memory improvement in rats. Drug Develop Res. 2002;55:97–103. [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, Avery J, Nicholson J, Rose JE. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behavioural Brain Research. 2009;196:207–213. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Xiao Y, Slade S, Cauley M, Wells D, Hampton D, Petro A, Rose JE, Brown ML, Paige MA, McDowell BE, Kellar K. Sazetidine-A, a selective α4β2 nicotinic receptor desensitizing agent and partial agonist reduces nicotine self-administration in rats. Journal of Pharmacology and Experimental Therapeutics. 2010;332:933–939. doi: 10.1124/jpet.109.162073. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR, Picciotto MR, Changeux JP, Collins AC. Two pharmacologically distinct components of nicotinic receptor-mediated rubidium efflux in mouse brain require the beta 2 subunit. The Journal of Pharmacology and Experimental Therapeutics. 1999;289:1090–1103. [PubMed] [Google Scholar]
- Nott A, Levin ED. Dorsal hippocampal α7 and α4β2 nicotinic receptors and memory. Brain Res. 2006;1081:72–78. doi: 10.1016/j.brainres.2006.01.052. [DOI] [PubMed] [Google Scholar]
- Ochoa ELM, Chattopadhyay A, McNamee MG. Desensitization of the nicotinic acetylcholine receptor: Molecular mechanisms and effect of modulators. Cell Mol Neurobiol. 1989;9:141–178. doi: 10.1007/BF00713026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA, Zheng G, Zhang Z, McIntosh JM, Stokes C. Extending the analysis of nicotinic receptor antagonists with the study of alphs6 nicotinic receptor subunit chimeras. Neuropharmacology. 2008;54:1189–1200. doi: 10.1016/j.neuropharm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichat P, Bergis OE, Terranova JP, Urani A, Duarte C, Santucci V, Gueudet C, Voltz C, Steinberg R, Stemmelin J, Oury-Donat F, Avenet P, Griebel G, Scatton B. SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (II) efficacy in experimental models predictive of activity against cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2007;32:17–34. doi: 10.1038/sj.npp.1301188. [DOI] [PubMed] [Google Scholar]
- Pitts RC, Buda DR, Keith JR, Cerutti DT, Galizio M. Chlordiazepoxide and dizocilpine, but not morphine, selectively impair acquisition under a novel repeated-acquisition and performance task in rats. Psychopharmacology (Berl) 2006;189:135–143. doi: 10.1007/s00213-006-0538-5. [DOI] [PubMed] [Google Scholar]
- Pocivavsek A, Icenogle L, Levin ED. Hippocampal α7 and α4β2 nicotinic receptors and clozapine effects on memory. Psychopharmacology. 2006;188:596–604. doi: 10.1007/s00213-006-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter A, Corwin J, Lang J, Piasecki M, Lenox R, Newhouse PA. Acute effects of the selective cholinergic channel activator (Nicotinic agonist) ABT-418 in Alzheimer’s disease. Psychopharmacology. 1999;142:334–342. doi: 10.1007/s002130050897. [DOI] [PubMed] [Google Scholar]
- Potter AS, Ryan K, Newhouse PA. Effects of acute ultra-low dose mecamylamine on cognition in adult attention-deficit/hyperactivity disorder (ADHD) Human Psychopharmacology: Clinical and Experimental. 2009;24:309–317. doi: 10.1002/hup.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickaerts J, van Goethem NP, Chesworth R, Shapiro G, Boess FG, Methfessel C, Reneerkens OA, Flood DG, Hilt D, Gawryl M, Bertrand S, Bertrand D, Konig G. EVP-6124, a novel and selective alpha7 nicotinic acetylcholine receptor partial agonist, improves memory performance by potentiating the acetylcholine response of alpha7 nicotinic acetylcholine receptors. Neuropharmacology. 2012;62:1099–1110. doi: 10.1016/j.neuropharm.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Cauley M, Sexton H, Xiao X, Brown ML, Paige MA, McDowell BE, Kellar KL, Levin ED. Sazetidine-A, a selective α4β2 nicotinic acetylcholine receptor desensitizing agent reverses dizocilpine and scopolamine-induced attentional impairments in rats. Psychopharmacology. 2011;215:621–630. doi: 10.1007/s00213-010-2161-8. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Cauley M, Xiao Y, Kellar KJ, Levin ED. Effects of chronic sazetidine-A, a selective β2* nicotinic receptor desensitizing agent on pharmacologically-induced impaired sustained attention in rats. Psychopharmacology. 2012;222:269–276. doi: 10.1007/s00213-012-2895-6. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Slade S, Wells C, Petro A, Li TK, Lumeng L, Xiao Y, Brown ML, Paige MA, McDowell BE, Kellar KJ, Rose JE, Levin ED. Sazetidine-A, a selective α4β2 nicotinic acetylcholine receptor desensitizing agent and partial agonist reduces both alcohol and nicotine self-administration in selectively-bred alcohol preferring (P) rats. Psychopharmacology. 2010;211:161–174. doi: 10.1007/s00213-010-1878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, Horner WE, Chapin DS, Hoffmann WE, Johnson DE, McLean S, Freeman J, Williams KE. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 2009;78:813–824. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- Terry AV, Buccafusco JJ, Prendergast MA. Dose-specific improvements in memory-related task performance by rats and aged monkeys administered the nicotinic-cholinergic antagonist mecamylamine. Drug Develop Res. 1999;47:127–136. [Google Scholar]
- Tietje KR, Anderson DJ, Bitner RS, Blomme EA, Brackemeyer PJ, Briggs CA, Browman KE, Bury D, Curzon P, Drescher KU, Frost JM, Fryer RM, Fox GB, Gronlien JH, Hakerud M, Gubbins EJ, Halm S, Harris R, Helfrich RJ, Kohlhaas KL, Law D, Malysz J, Marsh KC, Martin RL, Meyer MD, Molesky AL, Nikkel AL, Otte S, Pan L, Puttfarcken PS, Radek RJ, Robb HM, Spies E, Thorin-Hagene K, Waring JF, Ween H, Xu H, Gopalakrishnan M, Bunnelle WH. Preclinical characterization of A-582941: a novel alpha7 neuronal nicotinic receptor agonist with broad spectrum cognition-enhancing properties. CNS Neuroscience & Therapeutics. 2008;14:65–82. doi: 10.1111/j.1527-3458.2008.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Staay FJ, Rutten K, Erb C, Blokland A. Effects of the cognition impairer MK-801 on learning and memory in mice and rats. Behav Brain Res. 2011;220:215–229. doi: 10.1016/j.bbr.2011.01.052. [DOI] [PubMed] [Google Scholar]
- Van Kampen M, Selbach K, Schneider R, Schiegel E, Boess F, Schreiber R. AR-R 17779 improves social recognition in rats by activation of nicotinic alpha7 receptors. Psychopharmacology. 2004;172:375–383. doi: 10.1007/s00213-003-1668-7. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Ballard TM, Pouzet B, Riedel WJ, Wettstein JG. Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacol Biochem Behav. 2011;99:130–145. doi: 10.1016/j.pbb.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Ward L, Mason SE, Abraham WC. Effects of the NMDA antagonists CPP and MK-801 on radial arm maze performance in rats. Pharmacol Biochem Behav. 1990;35:785–790. doi: 10.1016/0091-3057(90)90359-p. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Bostic J, Prince J, Monuteaux MC, Soriano J, Fine C, Abrams A, Rater M, Polisner D. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. American Journal of Psychiatry. 1999;156:1931–1937. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Molecular Pharmacology. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, Broad LM, Williams AC, Zhang D, Ding C, Heinz BA, Sher E. Sazetidine-A is a potent and selective agonist at native and recombinant alpha 4 beta 2 nicotinic acetylcholine receptors. Mol Pharmacol. 2008;73:1838–1843. doi: 10.1124/mol.108.045104. [DOI] [PubMed] [Google Scholar]