Abstract
Objective
Radiation therapy (RT), with or without chemotherapy, can cause significant acute toxicity among patients treated for head & neck cancer (HNC), but predicting, before treatment, who will experience a particular toxicity or symptom is difficult. We created and evaluated two multivariate models and generated a nomogram to predict symptom severity during RT based on a patient-reported outcome (PRO) instrument, the MD Anderson Symptom Inventory–Head&Neck Module (MDASI-HN).
Study Design
This was a prospective, longitudinal, questionnaire-based study.
Setting
Tertiary cancer care center.
Subjects and Methods
Subjects were 264 patients with HNC (mostly oropharyngeal) who had completed the MDASI-HN before and during therapy. Pretreatment variables were correlated with MDASI-HN symptom scores during therapy with multivariate modeling and then correlated with composite MDASI-HN score during week 5 of therapy.
Results
A multivariate model incorporating pretreatment PROs better predicted MDASI-HN symptom scores during treatment than did a model based on clinical variables and physician-rated patient performance status alone (Aikake information criterion=1442.5 vs. 1459.9). In the most parsimonious model, pretreatment MDASI-HN symptom severity (P<0.001), concurrent chemotherapy (P=0.006), primary tumor site (P=0.016), and receipt of definitive (rather than adjuvant) RT (P=0.044) correlated with MDASI-HN symptom scores during week 5. That model was used to construct a nomogram.
Conclusion
Our model demonstrates the value of incorporating baseline PROs, in addition to disease and treatment characteristics, to predict patient symptom burden during therapy. Although additional investigation and validation are required, PRO-inclusive prediction tools can be useful for improving symptom interventions and expectations for patients being treated for HNC.
Keywords: Symptom burden, MD Anderson Symptom Inventory–Head & Neck Module, patient reported outcome, prediction tool, nomogram
Introduction
Radiation therapy (RT), with or without concurrent chemotherapy, is a critical component of curative treatment for most patients with head and neck cancer but may result in substantial treatment-related toxicity.1-4 The severity of these symptoms varies and depends on a series of complex interactions between patient-, disease-, and treatment-related factors. Thus in order to achieve adequate informed consent, manage expectations during the patient counseling/education process, and develop an individual symptom management plan prior to initiating RT, the treating physician is faced with the challenging task of estimating the potential for acute and late toxicity and quantifying the patient symptom experience.
Nomograms, which are a graphical representation of a multivariate prediction model, have been implemented in various clinical settings, and can serve as a “look-up” tools during the consultation process to derive individualized end-point-specific scores (i.e. toxicity or mortality) from a given patient’s clinical profile. Nomograms, including those with treatment-related toxicity endpoints, have been applied in many oncology practice settings and have been used to facilitate individualized consultations and to support clinical decision-making during cancer therapy.5 For example a nomogram can be used to predict the probability of developing grade 2 or 3 acute lower gastrointestinal toxicity by inputting a patient’s status regarding diabetes, hemorrhoids, hormonal therapy treatment, anticoagulation therapy, irradiation of pelvic lymph nodes as well as what the magnitude of the mean rectal dose during RT for prostate cancer.6
Patient-reported outcome (PRO) measures have been shown to add valuable prognostic information and several groups have called for improved implementation of non-survival patient reported outcome (PRO) endpoints in cancer therapy.7, 8 Indeed, strategies to reduce treatment-related toxicity have increasingly relied on PRO comparisons.9, 10 Few series have used a composite symptom burden metric. Moreover, despite the growing body of evidence supporting the prognostic capabilities of PRO, the data used to derive symptom prediction models have been limited to traditional clinical parameters and physician ratings of patient function.
To our knowledge, no formal PRO-focused prediction tools have been developed to facilitate risk stratification based on potential symptom severity during RT or concurrent chemoradiation for head and neck cancer.11-14 By comparison, widely used therapy-selection, prediction, and patient education tools such as Adjuvant! Online (http://www.adjuvantonline.com), the Memorial Sloan Kettering Cancer Center prediction models (http://www.mskcc.org/cancer-care/prediction-tools), and the Oregon Health and Science University Knight Cancer Institute Cancer Survival Prediction Calculators (http://skynet.ohsu.edu/nomograms) provide readily understandable clinical tools for predicting outcomes in other disease sites.
As part of an overall goal to reduce treatment-related symptoms for patients with head and neck cancer, the specific goals of this proof of principle study are to (1) construct two testable and iteratively adaptable models for predicting symptoms during treatment using either prospective PRO data or more traditional metrics of patient function, (2) generate a symptom prediction nomogram from the more robust model, and (3) demonstrate how individualized estimates of a patient’s symptom burden during therapy could be generated and used for risk stratification.
Methods
Patients and MDASI-HN Data Collection
This study was approved the University of Texas MD Anderson Cancer Center Institutional Review Board. Patients with previously untreated locoregional head and neck cancer who were to be initiated on RT or concurrent chemoradiation as part of therapy with curative intent between March 2005 and July 2007 were eligible for study inclusion. Participants were provided study-specific informed consent and enrolled before initiation of RT. Patient-reported information on symptom incidence and severity was collected from all patients as part of an ongoing prospective, longitudinal, questionnaire-based study. Patients’ performance status was scored at study enrollment by the treating radiation oncologists using the Eastern Cooperative Oncology Group (ECOG) performance status scale. Demographic and clinical variables were collected from medical records. Tumor and lymph node status were recorded according to the 2002 (6th edition) American Joint Committee on Cancer (AJCC), which was the working staging system at the time of data collection. Primary tumor site and receipt of surgery as part of this curative treatment were coded likewise.15
The MD Anderson Symptom Inventory-Head and Neck Module (MDASI-HN) is a brief, psychometrically validated patient-reported multisymptom assessment questionnaire containing 13 “core” items representing symptoms common across all cancer types and 9 “head and neck cancer‒specific” items representing important disease-specific and treatment-related symptoms. The 22 MDASI-HN symptom items are rated on numeric 0-to-10 scales from “not present” to “as bad as you can imagine.”16 Participating patients self-administered the MDASI-HN before starting radiation-based treatments (week 0) and then during the 6- to 7-week course of RT or concurrent chemoradiation. Two MDASI-HN time points were considered in this analysis: “baseline” (before the initiation of radiation-based treatments) and during RT (week 5 [±1 week], when symptom severity would be expected to be near its peak and compliance with MDASI-HN survey completion high). Participants were excluded from this analysis if they failed to complete at least 75% of the MDASI-HN survey items.
Statistical Analyses
All statistical calculations were done with STATA 12 (College Station, TX). Descriptive statistics were used to summarize patient and clinical characteristics and MDASI-HN scores. Missing symptom items were linearly interpolated by using the average change between the patient’s baseline and mid-treatment MDASI-HN overall scores. Ordinary least-squares linear regression was used to determine the correlation between pretreatment (baseline) clinical variables and MDASI-HN composite score during therapy. Clinical variables known to be associated with increased treatment-related toxicity or symptoms were collected and include age,17-21 sex,22 T category,17,19,23 N category,20 primary tumor site (hypopharynx/larynx, skin/major salivary gland, paranasal sinus/nasal cavity, oropharynx/nasopharynx, oral cavity/minor salivary gland, or thyroid/trachea, versus unknown primary site),20, 23 receipt of induction chemotherapy (yes or no), 22 receipt of concurrent systemic therapy (yes or no), 22 radiation therapy intent/timing (“upfront”/definitive versus postoperative/adjuvant), 21 and performance status (ECOG 0-1 versus 2-3).17 These were defined as a priori independent variables. The composite MDASI-HN score (possible range 0‒220; the sum of the scores for the 22 individual items) during week 5 of radiation therapy was identified as a dependent variable for univariate and multivariate modeling.
Distinct multivariate models were formulated by using accepted statistical criteria for covariate selection by forward-selection and backward-elimination using a stepwise regression algorithm within STATA 12. For the forward-selection process, the individual covariates were independently assessed with linear regression against week 5 MDASI-HN score by using P value minimization criteria. The remaining covariates were then serially added to the model, with iteratively added covariates resulting in the lowest P value included, until the model reached a P>0.05. Afterwards, a serial backward-elimination sequence was performed, with all covariates initially included and then serially eliminated performed, again by using a P value threshold of 0.05.
To evaluate the potential gain in model performance observed with inclusion of baseline PROs (vs. a model that did not include such PROs), an identical variable selection procedure was performed with and without the pretreatment (baseline) composite MDASI-HN score in the variable pool. The resultant multivariate models were compared by using the Aikake information criterion (AIC). The most parsimonious model that resulted (i.e., that with the lowest AIC value) was converted into a nomogram by using the open-source statistical software R (http://www.R-project.org) with the Regression Modeling Strategies package of Harrell (http://biostat.mc.vanderbilt.edu/rms).24
Results
Patient Characteristics
Of the 274 patients enrolled in the longitudinal survey, 10 were excluded because of incomplete MDASI-HN data, leaving 264 evaluable patients for this analysis. Patient, disease, and treatment characteristics are summarized in Table 1. Median patient age was 59.4 years. Induction chemotherapy regimens were typically platinum and taxane-based. Of the 112 patients who received concurrent chemotherapy with radiation, single-agent cisplatin was the most commonly used (59%), followed by carboplatin (13%) and cetuximab (13%). Eighty-five percent of patients received intensity-modulated RT.
Table 1.
Patient Characteristics.
| Characteristic | No. of Patients (%) |
Characteristic | No. of Patients (%) |
|---|---|---|---|
| Sex | Disease Site | ||
| Male | 202 (77) | Hypopharynx | 7 (4) |
| Female | 62 (23) | Larynx | 18 (9) |
| ECOG performance status | Major salivary gland | 12 (6) | |
| 0 | 155 (59) | Nasal cavity | 2 (1) |
| 1 | 87 (33) | Nasopharynx | 8 (4) |
| 2 | 21 (8) | Oral cavity | 21 (11) |
| 3 | 1 (<1) | Oropharynx | 88 (44) |
| T category | Paranasal sinus | 13 (7) | |
| 0 | 17 (6) | Skin | 9 (5) |
| 1 | 52 (20) | Thyroid | 9 (5) |
| 2 | 74 (28) | Unknown primary | 11 (6) |
| 3 | 41 (16) | Treatment Sequence | |
| 4 | 47 (18) | RT | 89 (34) |
| Recurrent or | 33 (13) | ChemoRT | 67 (25) |
| N/A | |||
| N category | Surgery → RT | 32 (12) | |
| 0 | 81 (31) | Surgery → CRT | 7 (3) |
| 1 | 28 (11) | Ind. chemo → RT | 31 (12) |
| 2 | 18 (45) | Ind. chemo → CRT | 38(14) |
| 3 | 17 (6) | ||
| Recurrent or | 20 (8) | ||
| N/A |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; N/A, not available; RT, radiation therapy; ChemoRT, concurrent chemoradiation therapy; Ind. chemo, induction chemotherapy
MDASI‒HN Data Collection and Symptom Severity
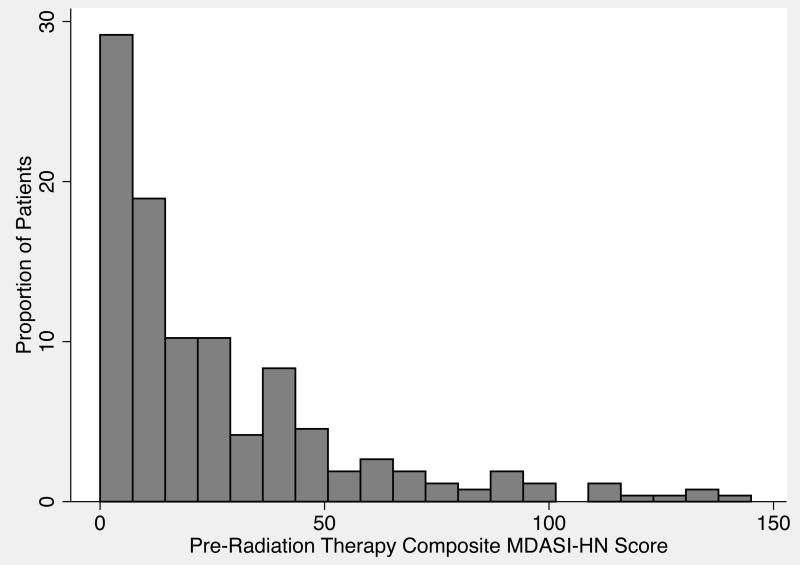
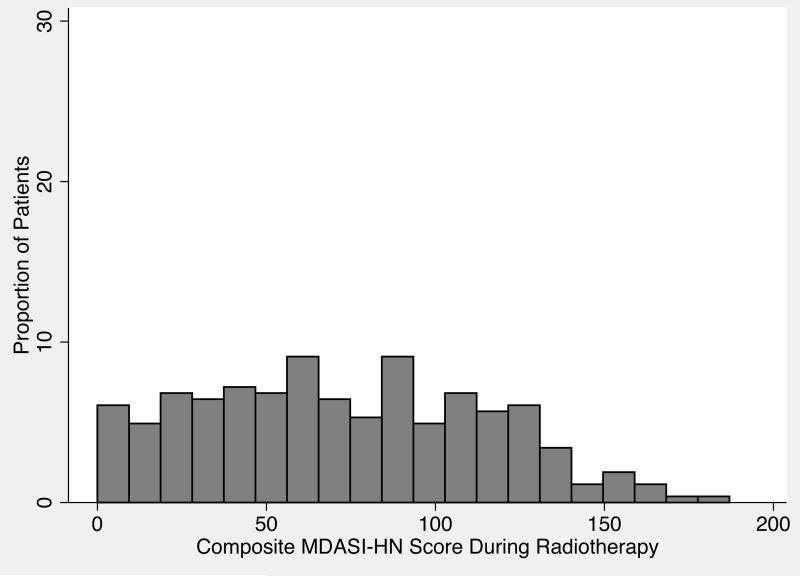
The baseline MDASI-HN data were collected at median of 10.2 days (SD 5.6) before the initiation of RT; the second MDASI-HN time point analyzed was collected at a median of 29.9 days (SD 4.1) into treatment. The median composite MDASI-HN score before the start of radiation-based treatment was 16 (range 0-145, mean±SEM 26.19±1.77) (Figure 1), and the median composite MDASI-HN score during treatment was 69 (range 0-187, mean±SEM 72.36±4.45) (Figure 2).
Figure 1.
Distribution of composite symptom scores on the MD Anderson Symptom Inventory–Head and Neck Module (MDASI-HN) before radiation therapy for head and neck cancer.
Figure 2.
Distribution of composite symptom scores on the MD Anderson Symptom Inventory–Head and Neck Module (MDASI-HN) during radiation therapy for head and neck cancer.
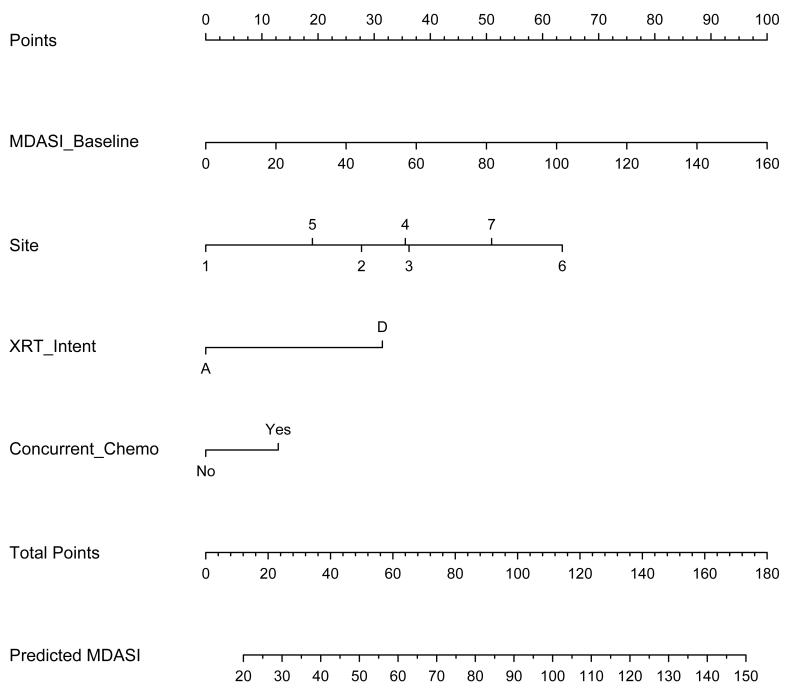
Results of the univariate and multivariate analyses are shown in Tables 2 and 3. Notably, inclusion of baseline MDASI-HN PRO data significantly improved the model’s AIC, with an evidence ratio of 6002, suggesting that, at least within our dataset with selected variables, the MDASI-inclusive model has a ≥95% probability of being correct as compared to the base model.25 The nomogram constructed with the normalized significant covariates of the model that incorporated baseline MDASI-HN score is shown in Figure 3.
Table 2.
Correlates of Symptom Severity During Radiation Therapy for Head and Neck Cancer: Results of Univariate Analysis.
| Covariate | Coefficient | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age | −0.514 | −0.033 to −0.959 | 0.016 |
| Sex | 5.340 | −6.669 to 17.348 | 0.382 |
| ECOG performance status | 19.342 | 1.049 to 37.637 | 0.038 |
| Baseline MDASI–HN | 0.528 | 0.362 to 0.693 | <0.001 |
| T category | 11.818 | 1.010 to 22.536 | 0.031 |
| N category | 17.045 | 7.060 to 27.031 | 0.001 |
| Primary site | 6.582 | 3.516 to 9.647 | <0.001 |
| Radiation therapy intent | 21.27 | 10.48 to 32.05 | <0.001 |
| Induction chemotherapy | 15.465 | 4.016 to 26.914 | 0.008 |
| Concurrent chemotherapy | 22.264 | 12.311 to 32.217 | <0.001 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; MDASI-HN, MD Anderson Symptom Inventory–Head and Neck Module
Table 3.
Correlates of Symptom Severity During Radiation Therapy for Head and Neck Cancer: Results of Multivariate Analyses and Model Comparison
| Covariate | Coefficient | 95% CI | P Value | Model AIC |
|---|---|---|---|---|
| Model With Baseline MDASI-HN in Covariate Pool | ||||
| Baseline MDASI-HN | 0.553 | 0.328 to 0.777 | <0.001 | 1442.5 |
| Concurrent chemo | 18.778 | 5.557 to32.000 | 0.006 | |
| Primary tumor site | 5.030 | 0.945 to 9.114 | 0.016 | |
| RT intent | 15.013 | 0.380 to 29.646 | 0.044 | |
| Model Without Baseline MDASI-HN in Covariate Pool | ||||
| ECOG PS | 46.040 | 15.655 to 76.426 | 0.003 | 1459.9 |
| Concurrent chemo | 25.364 | 45.752 to 64.218 | <0.001 | |
Abbreviations: CI, confidence interval; AIC, Aikake information criterion; ECOG PS, Eastern Cooperative Oncology Group performance status; MDASI-HN, MD Anderson Symptom Inventory–Head and Neck Module
Figure 3.
Nomogram predicting the composite MDASI-HN symptom score during radiation therapy (RT). (Site: 1=hypopharynx/larynx; 2=skin/major salivary gland; 3=paranasal sinus/nasal cavity; 4=oropharynx/nasopharynx; 5=thyroid/trachea; 6=oral cavity/minor salivary gland; and 7=unknown primary site).
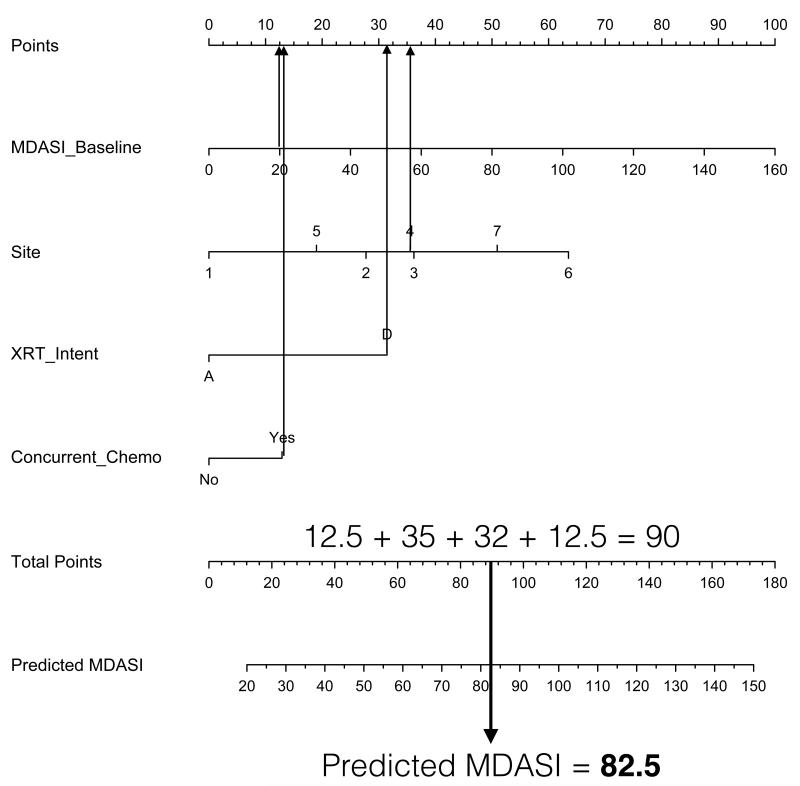
Using the nomogram involves drawing a vertical line from the values of each of the covariates up to the “Points” line at the top of the page. The sum of the points is determined and another vertical line is drawn from the “Total Points” line to the bottommost line (Predicted MDASI) to give the predicted composite MDASI-HN symptom score during RT. For example, a patient treated at our institution with definitive concurrent chemoradiation for oropharyngeal cancer with a pretreatment MDASI-HN score of 20 would have a total of 90 points, corresponding to a predicted MDASI-HN score of 82.5 (Figure 4). Referencing this predicted MDASI-HN score and the histogram in Figure 2 leads to the estimate that this patient would have more severe symptoms compared with this study cohort on the whole, despite having a relatively low pretreatment symptom burden.
Figure 4.
A patient undergoing definitive concurrent chemoradiation for oropharyngeal cancer with a pretreatment MDASI-HN score of 20 would have a total of 90 points and a predicted MDASI-HN score of 82.5.
Discussion
Assessment, prevention, and reduction of patient symptoms and treatment-related toxicity are increasingly important during RT for head and neck cancer in order to: minimize patient distress and suffering; minimize the need to interrupt treatment (which can negatively affect disease control); 26 optimize use of health care resources; and minimize the need for invasive interventions (e.g., feeding tubes) and hospitalizations.3 Although other investigators have correlated clinical variables with quality of life during the course of head and neck cancer22 and after RT,27 our findings represent, to our knowledge, the first implementation of a multivariate model derived from prospectively collected baseline PRO data that can be used to predict composite PRO-assessed symptom burden in patients while they undergo RT for head and neck cancer.
We modeled composite symptom severity from prospectively collected, patient-reported symptom data, and routinely available pretreatment clinical variables. Consistent with other reports, the use of chemotherapy concurrent with RT, a treatment with greater acute toxicity than RT alone,1,2 was correlated with higher symptom severity in both models. Notably, during the step-wise selection of covariates, the inclusion of baseline PROs in the pool of covariates yielded the more robust model of composite symptom burden that also incorporated more treatment and disease-related parameters. Improved model performance with the inclusion of baseline MDASI-HN data is likely due to the scalar granularity of the MDASI-HN versus that of the ECOG performance status scale, which is a fairly nonspecific, discriminator of an individual’s ability to function.
Several groups have called for improved implementation of non-survival PRO endpoints in cancer therapy7,8; indeed, strategies to reduce treatment-related toxicity have increasingly relied on PRO comparisons.9,10 Nevertheless, few series have used a composite symptom burden metric, as we did in this study, as a variable of interest. Use of the composite MDASI-HN at week 5 allows an assessment of global and head-and-neck‒specific symptom burdens that is readily calculated and allows comparisons to be made within groups and within individuals in terms of symptom burden. Additional efforts are underway to apply similar modeling with specific MDASI-HN subscales (e.g., symptom interference) and to use a similar approach to define or refine models.
Creation and use of pretreatment models that can reliably define the risk of composite symptom burden during treatment, and translating them into usable nomograms permits the formulation of individualizing care plans and strategic allocation of resources. Knowledge of a patient’s symptom burden risk ensures that supportive care consultations can be implemented proactively, and nutritional and functional assessments and interventions can be intensified during periods of therapy when the patient is predicted to have an elevation in composite symptom burden.28 Additionally, accurate symptom prediction can be used to stratify patients for specific care pathways that are tailored toward certain at-risk groups or to stratify patients in clinical trials with symptom reduction endpoints. Finally, such models can be used to provide an individualized, realistic, evidence-based expectation of the nature and degree of toxicities that may arise during treatment.
Several caveats must be noted in interpreting our results. The limitations inherent in any single-institution series apply: the generated models are not necessarily generalizable and will require validation before they can be routinely used in other clinical facilities. In addition certain patient groups and tumors at specific organ sites were also proportionally underrepresented in this series.
These findings reflect an initial effort; and validation, refinement, and further investigation of these and future models are needed before they can be used in a broad variety of clinical settings. Ongoing PRO collection will allow iterative improvements to ensure that institution-specific models, such as those presented here, are modified to account for differences in patient populations, treatment paradigms, and refinements in treatment approach (e.g. the use of endoscopic and transoral surgery, novel chemotherapy regimens, or proton beam therapy). As an initial step toward these goals, we have demonstrated that the integration of PRO measures with the development of patient-centered outcome prediction tools provides a simple platform with which to optimize the care of patients with head and neck cancer.
Acknowledgements
Supported in part by National Cancer Institute grants CA026582 to Charles Cleeland, PhD and by Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- 1.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081–6. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 3.Murphy BA, Beaumont JL, Isitt J, et al. Mucositis-related morbidity and resource utilization in head and neck cancer patients receiving radiation therapy with or without chemotherapy. J Pain Symptom Manage. 2009;38:522–32. doi: 10.1016/j.jpainsymman.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Xiao C, Hanlon A, Zhang Q, et al. Symptom clusters in patients with head and neck cancer receiving concurrent chemoradiotherapy. Oral Oncol. 2013;49:360–6. doi: 10.1016/j.oraloncology.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lughezzani G, Briganti A, Karakiewicz PI, et al. Predictive and prognostic models in radical prostatectomy candidates: A critical analysis of the literature. Eur Urol. 2010;58:687–700. doi: 10.1016/j.eururo.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valdagni R, Kattan MW, Rancati T, et al. Is it time to tailor the prediction of radio-induced toxicity in prostate cancer patients? Building the first set of nomograms for late rectal syndrome. Int J Radiat Oncol Biol Phys. 2012;82:1957–66. doi: 10.1016/j.ijrobp.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Turk DC, Dworkin RH, McDermott MP, et al. Analyzing multiple endpoints in clinical trials of pain treatments: Immpact recommendations. Initiative on methods, measurement, and pain assessment in clinical trials. Pain. 2008;139:485–93. doi: 10.1016/j.pain.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Cleeland CS, Sloan JA, Cella D, et al. Recommendations for including multiple symptoms as endpoints in cancer clinical trials: A report from the ascpro (assessing the symptoms of cancer using patient-reported outcomes) multisymptom task force. Cancer. 2013;119:411–20. doi: 10.1002/cncr.27744. [DOI] [PubMed] [Google Scholar]
- 9.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (parsport): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–36. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le QT, Kim HE, Schneider CJ, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: A randomized, placebo-controlled study. J Clin Oncol. 2011;29:2808–14. doi: 10.1200/JCO.2010.32.4095. [DOI] [PubMed] [Google Scholar]
- 11.Cleeland CS, Sloan JA. Assessing the symptoms of cancer using patient-reported outcomes (ascpro): Searching for standards. J Pain Symptom Manage. 2010;39:1077–85. doi: 10.1016/j.jpainsymman.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: Immpact recommendations. Pain. 2003;106:337–45. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Reeve BB, Burke LB, Chiang YP, et al. Enhancing measurement in health outcomes research supported by agencies within the us department of health and human services. Qual Life Res. 2007;16(Suppl 1):175–86. doi: 10.1007/s11136-007-9190-8. [DOI] [PubMed] [Google Scholar]
- 14.Gotay CC, Kawamoto CT, Bottomley A, et al. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26:1355–63. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- 15.Gunn GB, Mendoza TR, Fuller CD, et al. High symptom burden prior to radiation therapy for head and neck cancer: A patient-reported outcomes study. Head Neck. 2012:1490–98. doi: 10.1002/hed.23181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal DI, Mendoza TR, Chambers MS, et al. Measuring head and neck cancer symptom burden: The development and validation of the m. D. Anderson symptom inventory, head and neck module. Head Neck. 2007;29:923–31. doi: 10.1002/hed.20602. [DOI] [PubMed] [Google Scholar]
- 17.Salama JK, Stenson KM, List MA, et al. Characteristics associated with swallowing changes after concurrent chemotherapy and radiotherapy in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134:1060–5. doi: 10.1001/archotol.134.10.1060. [DOI] [PubMed] [Google Scholar]
- 18.Bond SM, Dietrich MS, Murphy BA. Association of age and symptom burden in patients with head and neck cancer. ORL Head Neck Nurs. 2011;29:8–14. [PubMed] [Google Scholar]
- 19.Chen SC, Liao CT, Chang JT. Orofacial pain and predictors in oral squamous cell carcinoma patients receiving treatment. Oral Oncol. 2011;47:131–5. doi: 10.1016/j.oraloncology.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Best SR, Ha PK, Blanco RG, et al. Factors associated with pharyngoesophageal stricture in patients treated with concurrent chemotherapy and radiation therapy for oropharyngeal squamous cell carcinoma. Head Neck. 2011;33:1727–34. doi: 10.1002/hed.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machtay M, Moughan J, Farach A, et al. Hypopharyngeal dose is associated with severe late toxicity in locally advanced head-and-neck cancer: An rtog analysis. Int J Radiat Oncol Biol Phys. 2012;84:983–9. doi: 10.1016/j.ijrobp.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terrell JE, Ronis DL, Fowler KE, et al. Clinical predictors of quality of life in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:401–8. doi: 10.1001/archotol.130.4.401. [DOI] [PubMed] [Google Scholar]
- 23.Borggreven PA, Verdonck-de Leeuw IM, Muller MJ, et al. Quality of life and functional status in patients with cancer of the oral cavity and oropharynx: Pretreatment values of a prospective study. Eur Arch Otorhinolaryngol. 2007;264:651–7. doi: 10.1007/s00405-007-0249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrell FE. Regression modeling strategies. Springer-Verlag; New York: 2001. [Google Scholar]
- 25.Motulksky H, Christopoulos A. Fitting models to biological data using linear and nonlinear regression: A practical guide to curve fitting. Oxford University Press; New York: 2004. [Google Scholar]
- 26.Bese NS, Hendry J, Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys. 2007;68:654–61. doi: 10.1016/j.ijrobp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Hunter KU, Schipper M, Feng FY, et al. Toxicities affecting quality of life after chemo-imrt of oropharyngeal cancer: Prospective study of patient-reported, observer-rated, and objective outcomes. Int J Radiat Oncol Biol Phys. 2013;85:935–40. doi: 10.1016/j.ijrobp.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oskam IM, Verdonck-de Leeuw IM, Aaronson NK, et al. Prospective evaluation of health-related quality of life in long-term oral and oropharyngeal cancer survivors and the perceived need for supportive care. Oral Oncol. 2013;49:443–8. doi: 10.1016/j.oraloncology.2012.12.005. [DOI] [PubMed] [Google Scholar]