Abstract
Introduction
Pain management following major intracranial surgery is often limited by a presumed lack of need and a concern that opioids will adversely affect postoperative outcome and interfere with the neurologic examination. Nevertheless, evidence in adults is accumulating that these patients suffer moderate to severe pain and this pain is often under-treated. The purpose of this prospective, clinical observational cohort study was to assess the incidence of pain, prescribed analgesics, methods of analgesic delivery, and patient/parent satisfaction in pediatric patients undergoing cranial surgery at 3 major university children’s hospitals.
Methods
After obtaining IRB and parental consent (and when applicable, patient assent), children who underwent cranial surgery for cancer, epilepsy, vascular malformations, and craniofacial reconstruction were studied. Neither intraoperative anesthetic management nor postoperative pain management was standardized, but were based on institutional routine. Patients were evaluated daily by a study investigator and by chart review for pain scores using age appropriate, validated tools (FLACC, Faces Pain Scale-Revised, Wong Baker Faces Scale or Self-Report on a 0–10 scale), for patient/parent satisfaction using a subset of the NRC Picker satisfaction tool and in adolescents a modified QoR-40, and for the frequency, mode of administration, and type of analgesic provided. Finally, the incidence of opioid-induced side effects, specifically nausea, vomiting, pruritus, altered level of consciousness, and need for emergency diagnostic radiologic studies for altered neurologic examination were recorded. Data are provided as mean ± SD.
Results
Two hundred children (98:102 M:F), averaging 7.8 ± 5.8 years old (range 2 mos to 18.5 yr) and 32.2 ± 23.0 kg (range 4.5 to 111.6 kg) undergoing craniectomy (51), craniotomy (96), and craniofacial reconstruction (53) were studied. Despite considerable variation in mode and route of analgesic administration, there were no differences in average pain score, length of hospital stay, or parental satisfaction with care. Interestingly, opioid induced side effects were not related to total daily opioid consumption, site of surgery, or method of opioid delivery. The most common side effect was vomiting. No patient developed respiratory depression or altered mental status secondary to analgesic therapy. Regardless of age or procedure, once eating, most patients were treated with oral oxycodone and/or acetaminophen.
Conclusions
Despite considerable variation in modality and route of analgesic administration, there were no differences in average pain score, length of stay, or parental satisfaction with care. Pain scores were low, side effects were minimal, and parental satisfaction was high, providing equipoise for future blinded prospective randomized trials in this patient population.
Keywords: craniotomy, pediatric pain, pain control, analgesia, opioid
Introduction
Historically, the pain associated with intracranial surgery has been under treated because of the fear that the use of opioids may interfere with the neurologic examination or lead to its deterioration.1–4 Much of the information concerning the quality, duration, and treatment of pain following cranial surgery is anecdotal.5 Opioids, the analgesics most often prescribed for moderate to severe pain, may produce sedation and miosis and mask signs of intracranial catastrophe.6 Furthermore, opioids, even when administered in therapeutic doses, may depress minute ventilation leading to hypercapnia, increased intracerebral blood volume, and potentially increased intracranial pressure and cerebral edema.7 Moreover, why expose a patient to these risks when there is a presumed lack of need? Decades of training and anecdote have reinforced a widely held belief that patients do not experience intense pain following intracranial surgery, a belief supported by the fact that surgery on the brain parenchyma per se is not painful.
On the other hand, a growing body of published studies in adult patients report that pain following intracranial surgery is in fact common, often intense, and under treated.1;3;4;8–10 Failure to adequately treat pain in other postoperative conditions is associated with poor outcome.11;12 Indeed, aggressive assessment and treatment of pain for other conditions is now routine and the standard of care. In infants, children, and adolescents, we simply do not know the incidence of pain following cranial surgery, how it is treated, or the consequences of the extremes of no or overly aggressive pain management.
To improve the safety and efficacy of postoperative pain management after major elective cranial surgery, we undertook this prospective, clinical observational cohort trial to assess the incidence of pain, prescribed analgesics, methods of analgesic delivery, and patient/parent satisfaction in pediatric patients undergoing cranial surgery at 3 major university children’s hospitals in the United States. We sought to determine the incidence and intensity of postoperative pain, the surgical and demographic factors associated with pain intensity, and the analgesic therapies used to manage pain in infants, children, and adolescents. Additionally, we sought to assess parental and when possible patient satisfaction with analgesic therapy and identify any complications that might be associated with analgesic therapy that could conceivably affect recovery from neurosurgery, such as nausea and vomiting, excessive sedation, and respiratory depression.
Methods
After receiving approval by the institutional review board at each participating institution, we recruited male and female pediatric patients ages 2 months to ≤18 years presenting for elective craniotomy, craniectomy, or craniofacial surgery at the Boston Children’s Hospital (BCH), the Children’s Hospital of Philadelphia (CHOP), or the Charlotte R. Bloomberg Children’s Center at the Johns Hopkins Hospital (JHH). Patients were included in the study only if the procedure was performed by a participating surgeon, both parents spoke English, study personnel were available, and the case was scheduled during the daytime. Eligible subjects included patients undergoing surgery for brain tumors, vascular malformations, uncontrollable seizures, Chiari malformations, or craniofacial reconstruction. Patients were recruited by a study investigator prior to surgery and entered into this study in the immediate post-operative period. Exclusion criteria included patients who remained intubated and mechanically ventilated. Additionally, we excluded patients who were allergic to opioids or who had a known history of substance abuse. Patients were enrolled in the study only after obtaining parental written informed consent and when applicable, patient assent.
Neither intraoperative anesthetic management nor postoperative pain management was standardized, but were based on institutional routine. At one institution (CHOP), 51 of the 75 patients enrolled in the study spent their entire postoperative in-hospital stay in the Pediatric Intensive Care Unit (PICU). At the other 2 institutions, patient disposition varied and was based on surgical procedure, underlying medical conditions, and institutional customary practice. Some, but not all of these patients spent their first postoperative day in the PICU and then, once considered no longer critically ill, were transferred to regular in-hospital wards.
Patients were evaluated for pain and for the incidence of side effects by their nurses and physicians based on the hospital unit protocol. On each postoperative day, a study investigator (either a nurse, research assistant and/or a physician), reviewed each patient’s medical record to obtain information related to analgesic use, antiemetic use, and any other complication that could be related to analgesics. The study investigator also interviewed each patient and his or her parent(s). Interview times were not standardized, but occurred mostly in late morning or early afternoon. The study investigators evaluated subjective pain scores at rest and with activity (coughing and deep inspiration), sedation scores, and a satisfaction score once daily during the patient’s hospitalization. The study investigators also recorded the method of pain assessment by the patient’s physician and nurses and where these scores were documented in the hospital record.
The study investigators independently made a pain assessment using one of several age appropriate pain scores; in younger children the FLACC scale13, in school age children the Wong-Baker Faces pain scale (BCH and CHOP) or the Faces Pain Scale–Revised (JHH)14, and in older patients a 0–10 scale. Sedation scores were measured using the University of Michigan Sedation Score (1–6).13;15 Finally, patients and their parents were evaluated daily until discharge from the hospital by a study investigator for patient/parent satisfaction using a subset of the NRC Picker satisfaction tool and in adolescents a modified QoR-40.
The study investigators also recorded what analgesics were ordered (and by whom) and which, if any, of these medications were administered. To facilitate comparison, opioid analgesics were converted to the equivalent amount of intravenous morphine sulfate; where 100 mcg/kg intravenous morphine was equivalent to 1mcg/kg intravenous fentanyl, 20 mcg/kg intravenous hydromorphone, 100 mcg/kg oral oxycodone, and 1 mg/kg oral codeine.16 Additionally we measured the frequency and method of analgesic administration and its effectiveness throughout the child’s hospitalization or up to 5 days postoperatively. Medications and therapies that might be associated with adverse effects of postoperative opioid analgesic use, such as pruritus, nausea and vomiting, urinary retention, and respiratory depression treated with opioid antagonists were determined during the interview and by reviewing the patient’s medical record. Finally, we recorded the need for readmission to the ICU and the need for emergency interventional radiologic or surgical procedures for unexpected alterations in consciousness in all of these patients.
Data Analysis
All data were entered into Microsoft Excel, de-identified, merged and exported to Stata 12 statistical software for analysis (STATA Corp, College Station, TX). Descriptive statistics were used to summarize patient characteristics and study measurements at all centers. Continuous variables were summarized using median and interquartile range or mean and standard deviation, as appropriate. Categorical variables were summarized using frequencies and percentages. The chi-square test was used for comparing proportions, and one-way analysis of variance (ANOVA) was used for comparing continuous variables between the centers. Step-wise multivariate logistic regression was used to identify predictors for opioid-induced side effects. All statistical tests were two-sided and were considered to be statistically significant at P<0.05.
Results
During the 15 month period from September 9, 2011 to January 2, 2013, 538 eligible patients presented for elective cranial surgery: 160 at BCH, 190 at CHOP, and 188 at JHH. Of these, 302 patients underwent surgery on days or at times that research study staff were unavailable (Figure 1). Of the remaining 236 patients, 62 met inclusion criteria at BCH, 89 at CHOP, and 85 at JHH. Of these 7, 9, and 4 refused to participate at BCH, CHOP, and JHH respectively. Reasons for refusal included parental anxiety, parents not wanting to have their child in a study, and the perception that the study was too burdensome or was of no direct benefit to the patient (Figure 1). Thus, data collection was performed on 200 children; 50 at BCH, 75 at CHOP, and 75 at JHH. These 200 patients (98 males, and 102 females) averaged 7.8 ± 5.8 years old (range 2 mos to 18.5 yr) and 32.2 ± 23.0 kg (range 4.5 to 111.6kg). They underwent craniectomy (51), craniotomy (96), and cranio-facial reconstruction (53) (Figure 2).
Figure 1.
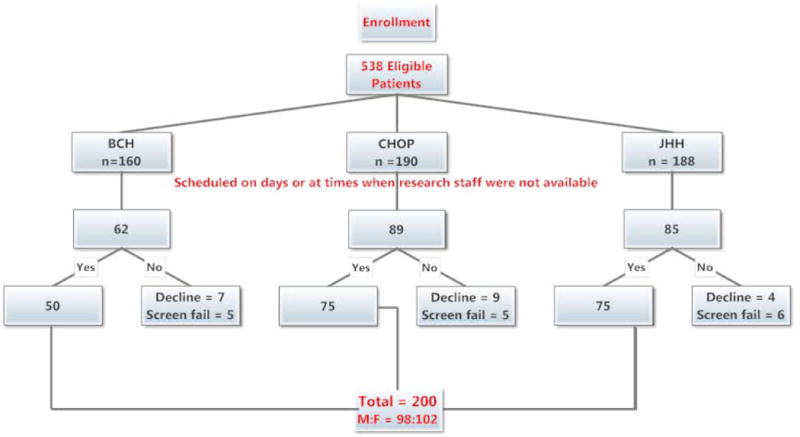
CONSORT (Consolidated Standards of Reporting Trials) flow chart of patient enrollment. BCH: Boston Children’s Hospital; CHOP: Children’s Hospital of Philadelphia; JHH: Charlotte R. Bloomberg Children’s Center of the Johns Hopkins Hospital.
M:F: Male to female ratio.
Figure 2.
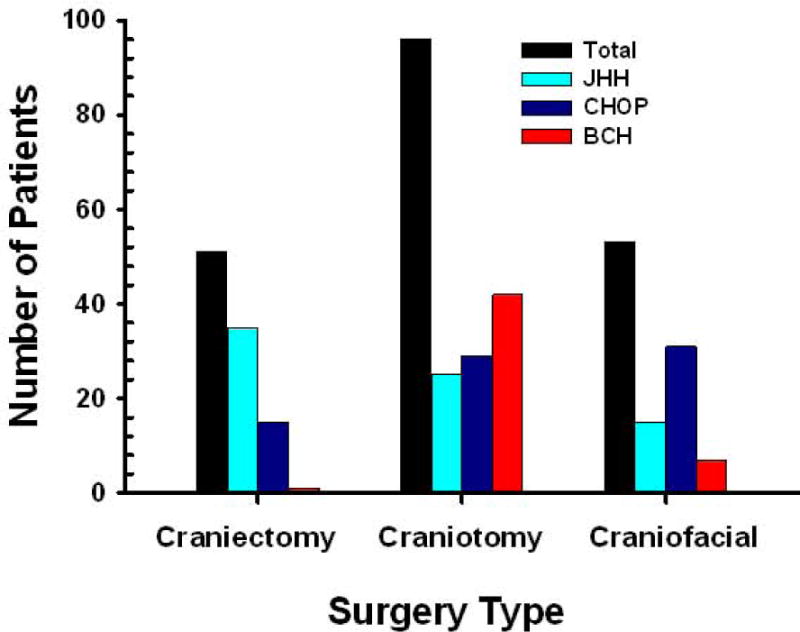
Frequency distribution by surgery type and institution. BCH: Boston Children’s Hospital; CHOP: Children’s Hospital of Philadelphia; JHH: Charlotte R. Bloomberg Children’s Center of the Johns Hopkins Hospital.
Patient demographics at each institution are summarized in Table 1. There was considerable variation in the opioid administered and method of analgesic administration based on type of surgery and institutional preference. For JHH patients undergoing craniotomy surgery, on postoperative day zero, 96% were treated with either IV Patient Controlled Analgesia (PCA) (n = 21) or with IV Parent/Nurse Controlled Analgesia (PNCA) (“PCA by proxy” or “surrogate PCA”) (n = 3). At CHOP 83% (n = 24) of craniotomy patients were treated with opioids administered intravenously by a nurse on an as needed basis (“PRN”, pro re nata). The remaining patients (n = 4) were treated with oral opioids or no opioids (n=1). Similarly at BCH, 81% (n = 34) of craniotomy patients were treated with IV PRN opioids and only one patient was treated with PNCA. Six other patients at BCH were treated with oral opioids. For patients that underwent craniectomy or craniofacial reconstruction the results were similar. Patients at JHH were predominantly treated with IV PCA or PNCA and patients at CHOP and BCH were predominantly treated with nurse administered IV PRN opioids. For patients undergoing craniectomy surgery, on postoperative day zero, 94% of patients treated at JHH were treated with either IV PCA (n = 27) or with IV PNCA (n = 6). On the other hand, PCA was used for only one patient at CHOP and 73% (n = 11) of patients were treated with IV PRN opioids. The remaining patients (n = 4) were treated with oral opioids. For patients undergoing craniofacial reconstruction surgery, on postoperative day zero, 60% of patients treated at JHH were treated with either IV PCA (n = 1) or with IV PNCA (n = 8). On the other hand, PCA and PNCA were not used at CHOP and 81% (n = 25) of patients were treated with PRN opioids. Including some overlap with the PRN patients, nine patients were treated with oral opioids. At BCH, one patient was treated with PNCA, 86% (n = 6) of patients were treated with IV PRN opioids, and only two patients were treated with oral opioids.
Table 1.
Patient Demographics by Institution
| Characteristics | JHH (n=75) AVG (SD or %) |
Institution CHOP (n=75) AVG (SD or %) |
BCH (n=50) AVG (SD or %) |
|---|---|---|---|
| Age (years) | 8.4 (6.0) | 6.7 (5.6) | 8.3 (5.3) |
| Gender | |||
| Female | 38 (50.6) | 38 (50.6) | 26 (52.0) |
| Race | |||
| African American | 9 (12.0) | 10 (13.3) | 2 (4.0) |
| Asian | 0 | 1 (1.3) | 1 (2.0) |
| Caucasian | 61 (81.3) | 57 (76.0) | 40 (80.0) |
| Hispanic | 2 (2.6) | 2 (2.6) | 0 |
| Other | 3 (4.0) | 5 (2.5) | 7 (14.0) |
| Weight (kg) | 32.8 (23.4) | 30.7 (24.9) | 33.5 (19.6) |
| Length of Stay (days) | 4.8 (2.0) | 5.3 (2.9) | 5.3 (3.2) |
| Pain scores (Postop day 1) | 2.6 (2)** | 2.9 (1.3) | 3.4 (1.9) |
| Morphine dose (Postop day 1) | 878 (620)** | 101 (123) | 247 (190) |
Opioid doses were converted to morphine equivalents (mcg/kg)
p < 0.05
Patients treated at JHH with PCA or PNCA received a continuous background opioid infusion of either hydromorphone (n = 47), fentanyl (n = 14), or morphine (n = 5). The PCA pump allowed up to 5 intermittent demand doses by the patient or by a surrogate with a lock out period of 8–10 minutes. Additionally, all of these patients were treated with a concomitant low dose naloxone infusion (1–1.5 mcg/kg/hour) to prophylactically limit opioid-induced side effects.16,17 Continuous opioid background infusions and low dose prophylactic naloxone infusions were not used in any enrolled patient at CHOP or BCH. The intermittent, PRN IV opioids administered at CHOP were either hydromorphone (n = 1), fentanyl (n = 21), or morphine (n = 38). The intermittent and PRN IV opioids administered at BCH were either hydromorphone (n = 1), fentanyl (n = 1), or morphine (n = 38).
The average daily opioid consumption, expressed in morphine equivalents, varied by surgical site, institution, and postoperative day (Figure 3). Regardless of site of surgery, patients treated at JHH with PCA or PNCA received significantly more opioid than patients treated with IV PRN medications at BCH and CHOP (p < 0.001). This increase in opioid consumption was greatest on the first postoperative day and persisted as long as the parenteral route was the primary method of opioid administration. There were no differences in the amount of opioid administered to patients at BCH and CHOP receiving IV PRN analgesics over the first 2 postoperative days (p=0.16).
Figure 3.
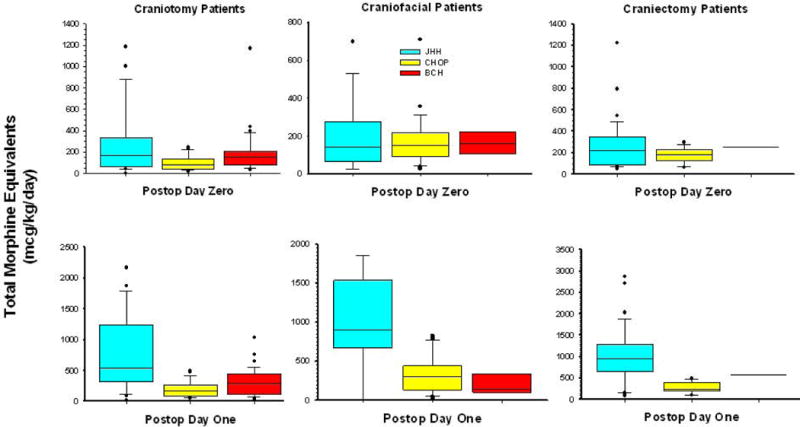
Comparison of total intravenous morphine equivalents (mcg/kg/day) by surgery type, postoperative day, and institution. Data are represented by box plot. BCH: Boston Children’s Hospital; CHOP: Children’s Hospital of Philadelphia; JHH: Charlotte R. Bloomberg Children’s Center of the Johns Hopkins Hospital.
Over 87% (156/181) of patients transitioned to oral analgesics (oral opioids alone, acetaminophen alone, or both) by postoperative day 2 or 3. Oxycodone was the predominant opioid prescribed and acetaminophen the most common non-opioid analgesic. No patient was treated with codeine. Oxycodone doses averaged 447 ± 229, 244 ± 158, and 318 ± 165 mcg/kg/day at JHH, CHOP, and BCH respectively (Figure 4). Other, less commonly prescribed oral analgesics included morphine, hydromorphone, and methadone. Acetaminophen was prescribed and administered to almost all patients: 76, 93, and 89 percent of patients at JHH, CHOP, and BCH respectively. No patients received anti-epileptic medications such as gabapentin or NSAIDS such as ketorolac.
Figure 4.
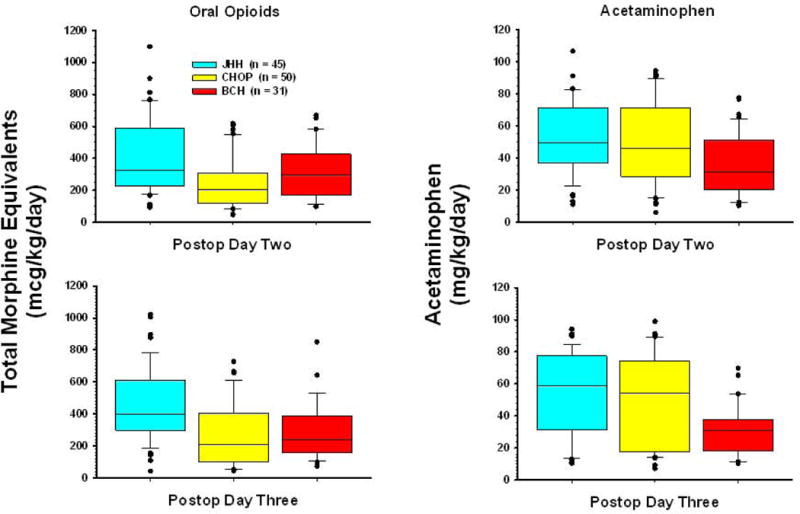
Comparison of total oral analgesics (opioid and acetaminophen) by surgery type, postoperative day, and institution. Data are represented by box plot. BCH: Boston Children’s Hospital; CHOP: Children’s Hospital of Philadelphia; JHH: Charlotte R. Bloomberg Children’s Center of the Johns Hopkins Hospital.
Despite the considerable variation in method of delivery and amount of opioid administered, there were no significant differences between institutions in average pain scores (p=0.71), length of hospital stay (p=0.45), or parental satisfaction with care (p=0.23). The majority of patients experienced mild pain regardless of surgical procedure or method of pain management. Interestingly, there was a small but significant difference in parental perceptions of the amount of opioid their child received between the PCA/PNCA subjects and those who received IV PRN medications in the post-operative period, with a higher level of dissatisfaction (14%) among the PCA/PNCA group (p=.03). Eleven percent thought it was insufficient and 3% thought it was too much (Figure 5).
Figure 5.
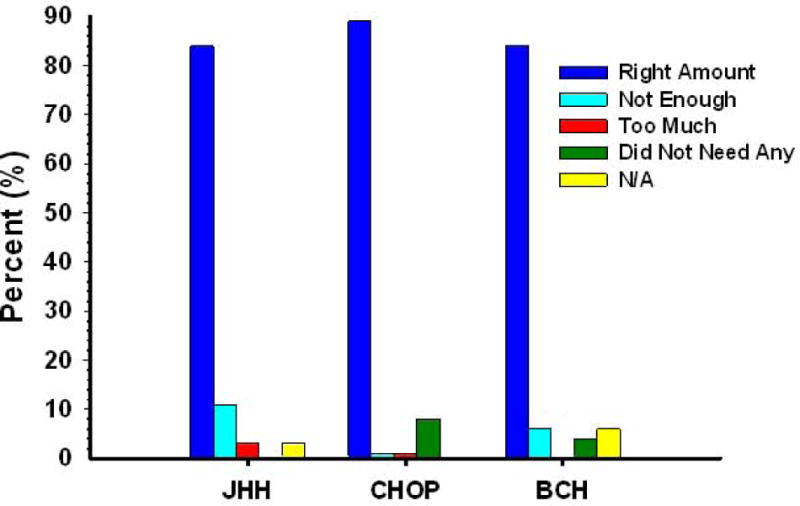
Parental perception of the amount of pain medication given to their child to control pain. BCH: Boston Children’s Hospital; CHOP: Children’s Hospital of Philadelphia; JHH: Charlotte R. Bloomberg Children’s Center of the Johns Hopkins Hospital.
Opioid-induced side effects were not related to total daily opioid consumption, site of surgery, or method of opioid delivery. The most common side effects were vomiting and nausea (Table 2). No subject at BCH reported pruritus and there was no difference in the incidence of pruritus between CHOP (7%) and JHH (10%), (p=0.07). Neither respiratory depression nor altered mental status was observed secondary to analgesic therapy at any of the centers. Although there was no difference in the proportion of patients with nausea between institutions, BCH and CHOP had a significantly higher proportion (p<0.02) of patients with vomiting during the post-operative period (58% and 66%, respectively) when compared to JHH (33%). This association was consistent after adjusting for age, gender, length of stay, average morphine dose and site of surgery in a multivariable logistic regression model, where institution was the only positive predictor for vomiting. In the adjusted model, the odds of having vomiting as a side effect of opioid analgesia was 4.3 times higher at CHOP and 2.7 times higher at BCH when compared to JHH. Nausea is reported only for children > 5 years of age because its presence is difficult to assess in younger children. (Table 2)
Table 2.
Opioid-Induced Side Effects by Institution
| Side Effects | JHH (n=75) | Institution CHOP (n=75) |
BCH (n=50) |
|---|---|---|---|
| Vomiting | 25 (33%)* | 50 (66%) | 29 (58%) |
| Craniectomy | 15 (60%) | 7 (14%) | 1 (4%) |
| Craniotomy | 8 (32%)* | 21 (42%) | 25 (86%) |
| Cranioplasty | 2 (8%)* | 22 (44%) | 3 (10%) |
| Pruritus | 7 (9%) | 5 (7%) | 0 (0)* |
| Craniectomy | 3 (43%) | 1 (20%) | 0 (0) |
| Craniotomy | 4 (57%) | 2 (40%) | 0 (0)* |
| Cranioplasty | 0 (0) | 2 (40%) | 0 (0) |
| Nausea (> 5yo) | 29/52 (56%) | 18/42 (42%) | 17/32 (53%) |
| Naloxone Infusion | 69 (92%)* | 0 (0) | 6 (12%) |
| Altered Mental Status | 0 (0) | 2 (2.5%) | 1 (2%) |
| Respiratory Depression | 0 (0) | 0 (0) | 0 (0) |
p < 0.05
Discussion
Few studies of any size have focused specifically on the incidence and severity of postoperative pain in pediatric patients after cranial surgery or on how this pain is treated.18 In adult populations, some investigators have questioned the need for analgesia, believing pain was minimal, whereas others recommended PCA.19–21 In this prospective, clinical observational cohort study conducted at 3 academic children’s hospitals, we found that pain is common after major elective cranial surgery, but can be managed with opioids administered intravenously either by PCA, PNCA, or by nurses on an as needed basis. Patients receiving PCA or PNCA received nearly three to five times as much opioid as did patients receiving nurse administered PRN opioids. Unlike studies in adult patients, this increase in opioid consumption did not result in differences in pain scores or in patient satisfaction.3;4 Indeed, in this study, there were no differences in average pain score, length of stay, or parental satisfaction with care regardless of the method of analgesic therapy. Finally, IV opioids neither altered the neurologic exam nor increased the incidence of neurologic deterioration regardless of the method of administration. These results belie the widely held belief that opioid administration is without benefit in this patient population.
In the traditional PRN approach, a patient must complain of pain and ask for pain relief. The responding nurse must respond to and assess the patient, then locate, prepare, and administer the ordered medication at a fixed dose and time interval.3;4 Thus, even in the best of circumstances, there is a lag time inherent in asking for and receiving analgesia. In addition, dosing and time intervals are often inadequate because of prescriber’s lack of familiarity with the pharmacology of opioids, fear of opioid-induced side effects, an under-appreciation of pain, and an irrational fear of causing drug addiction. Patient controlled aqnalgesia permits patients to treat their pain by direction activation of a device that administers predetermined, intermittent aliquots of analgesics. In this study, how opioids were administered was in large part institutionally driven. Opioids were administered intravenously either by PCA, PNCA, or by nurses on an as needed basis. Perhaps not surprisingly, pediatric patients who received IV PCA or PNCA received more opioid analgesics than those who received nurse administered PRN analgesics. These results are the same as previously reported in adult patients undergoing craniotomy surgery.3;4 However, unlike the adult studies, this increase in opioid consumption seen with PCA or PNCA did not result in differences in pain scores or in patient satisfaction. In the adult studies, patients treated with PRN opioids received significantly less opioid and were in more pain and significantly less satisfied with their care than patients receiving IV PCA therapy3;4, while in this study pain scores were comparable in the PCA/PNCA and PRN opioid populations. Perhaps pediatric nurses were more vigilant and responsive to patients’ early complaints of pain. When only patients or their surrogates are permitted to self-administer opioids with IV PCA, they receive higher hourly and daily doses than when analgesics needed to be requested from, or offered by, a nurse who may have been unwilling or unavailable to meet patient needs.
In the patients in the current study who received PCA, the basal infusion accounted for 15% of the total intravenous opioid administered. Although all patients at JHH receiving PCA or PNCA had co-administration of a naloxone infusion, the higher total intravenous opioid dosing in these patients should not be attributed to the naloxone. Multiple blinded, randomized controlled studies have demonstrated that low dose naloxone infusions (0.25–1.65 mcg/kg/hour) neither increased total opioid consumption nor pain scores.17,18
Despite 3–5 fold higher opioid dosing in the JHH PCA patients, those patients did not experience any sedation or difficulty in assessment of neurologic status. They did not have a higher incidence of opioid-related side effects, although the concomitant naloxone infusion may have mitigated possible side effects. The lack of difference in pain scores, side effects or satisfaction calls into question the superiority of PCA in this patient population, especially the benefit of a basal infusion. Interestingly, in this study, despite complete access to the PCA demand dose trigger device and a three to five fold difference in opioid delivery, the parents of patients being treated with PCA or PNCA were more likely to think that their child was receiving inadequate analgesia compared to the parents of patients being treated with nurse administered PRN medication. We have no explanation for why the parents of the children who received the largest opioid doses administered in an ad lib fashion were more likely to feel that their children received too little pain medication.
Opioids are the “gold standard” for the treatment of moderate to severe pain regardless of the modality of administration. In this study, three parenteral opioids were used, morphine, fentanyl, and hydromorphone. Why one drug was chosen over the others and what if any differences in pain relief or side effects profiles one achieves compared to the others cannot be detected in this study but may be worthy of future investigation. The predominant oral opioid was oxycodone and not codeine. Oxycodone has supplanted codeine in current pediatric pain practice, because oxycodone, unlike codeine, is not a prodrug and does not require the cytochrome P450 2D6 isoenzyme for metabolism to active drug.22;23 Some (3–7%) patients are either slow or rapid metabolizers, which can result in either under-dosing or catastrophic overdosing when codeine is administered. On the other hand, oxycodone is an active analgesic itself. Though commonly prescribed in children, it isnot labeled by the United States Food and Drug Administration for use in pediatric patients and illustrates the widespread off-label use of this drug in pediatric practice and the urgent need for pediatric labeling to guide practitioners.24
However, opioids should not and do not constitute the only therapy. As seen in this study, opioids have common side effects regardless of the method of administration including pruritus, nausea, vomiting, and constipation. The incidence of nausea and vomiting at the three institutions presents a mixed picture, but the use of naloxone at JHH was associated with both a high incidence of vomiting in the craniectomy patients but a low overall incidence of vomiting in the total patient group. The dose-ranging study by Monitto et al.18 did not show an increased incidence of nausea and vomiting associated with higher doses of opioid when 1 mcg/kg/hr or more naloxone was administered. Naloxone mitigates the occurrence of nausea and vomiting even in the presence of increased opioid dosing (which in this study was partly due to the basal infusion received by the JHH patients). The BCH patients had a very low incidence of vomiting for craniectomy and cranioplasty despite not receiving naloxone.
The two most feared adverse consequences of intravenous opioid management of post-cranial surgery pain (respiratory depression and excessive sedation) did not occur in this study. Thus, one of the most important findings of this study was our ability to improve analgesia by providing opioids via several routes including PCA and PNCA even though these latter patients received nearly twice as much opioid as their counterparts receiving PRN therapy. Although we did not observe any respiratory events requiring naloxone administration or neurological events attributable to opioid administration, these data should be interpreted cautiously as the study was not powered with respect to safety.
Indeed, because side effects are so common and expected, in this study all three institutions used a multimodal approach to maximize analgesia and to minimize opioid-related side effects.2 In this study, almost all patients were treated with acetaminophen in addition to opioids (BCH 80%, CHOP 91%; JHH 75% on POD1)(Figure 4, right panel). Other techniques including the use of local anesthetic neural blockade, and anti-inflammatory steroids (to minimize cerebral edema) may also be used. In the current study, most patients had local anesthetic infiltration of the site of the incision but not specific nerve blocks, and steroid administration was not documented. Only through a multimodal analgesic strategy can the side effects of opioids be limited while providing patients recovering from craniotomy with effective pain relief.2 Larger, prospective studies, powered to detect these differences will be needed in the future. Finally, not all side effects attributed to analgesics may actually be caused by opioids. Indeed, nausea and vomiting, the most common side effect seen in this study, may not be caused by opioids but may be directly related to the surgical procedure and the underlying disease process itself.25
Of note, our results may not be readily duplicated in other institutions and postoperative environments. This study was conducted at three tertiary care academic medical centers specializing in the care of infants, children, and adolescents. All three have dedicated acute pediatric pain services responsible for pain management in many postoperative patients. These pain services are staffed by anesthesiologists with subspecialty training in either pediatric anesthesia and/or pain management, as well as by specially educated advanced practice nurses.26 However, only the study patients at JHH where PCA or PNCA was administered had their care directed by the pain service. Pain management orders for the patients at BCH and CHOP were written either by the surgical or critical care service. In those settings, the pain service has often been instrumental in the development of safe protocols for pain management of postoperative patients but is not directly involved in their care.
Additionally, all three had dedicated pediatric intensive care units (PICU) that are staffed 24 hours a day, 365 days a year by specialty trained nurses and physicians. Following surgery, all patients enrolled in this study, were admitted to the PICU. Indeed, at one site (CHOP), the majority of patients remained in the PICU throughout their hospital course. The ready availability of PICU nurses at this site to treat pain on a PRN basis may explain some of our results especially when compared to previous studies of adult craniotomy patients.1;2 Institutional culture concerning pain and its management as well as parental expectation of how much pain their children will experience and how it will be treated may have affected our results.
A shortcoming of this study was the inconsistent availability of study personnel to recruit patients on the mornings of surgery, at night and on weekends. This may have resulted in failure to enroll patients that could have possibly influenced the study findings.
The study of post-cranial surgical pain is challenging because of several confounding variables including age, sex, site of surgery, institutional bias, the lack of standardized of intra- or postoperative pain management protocols, subjectivity of the pain assessment techniques, and where patients were received care postoperatively. Further, although many studies have reported that the location of surgery (infratentorial v. supratentorial) does not have an effect on the incidence and severity of postoperative pain, others have found site to be of importance.1;3;4 In this study, we looked at how pain was treated and assessed both in intracranial surgery, craniofacial surgery, and craniectomy surgery. Future studies may need to investigate each group separately.
Conclusion
Despite considerable variation in modality and route of analgesic administration, we found no differences in average pain score, length of stay, or parental satisfaction with care. Pain scores were low, side effects were minimal, and parental satisfaction was high, providing equipoise for future blinded prospective randomized trials in this patient population.
Acknowledgments
Disclosure: This study was registered prior to enrollment of the first subject at ClinicalTrials.gov (NCT01576601).
Drs. Morad and Yaster were supported by a grant from the Blaustein Foundation.
Dr. Kudchadkar was supported by the Johns Hopkins CTSA Award Number 5KL2RR025006 from the National Center for Advancing Translational Sciences of the National Institutes of Health.
The studies at BCH and CHOP were carried out without funding.
Footnotes
No conflicts of interest declared.
References
- 1.Gottschalk A, Berkow LC, Stevens RD, Mirski M, Thompson RE, White ED, Weingart JD, Long DM, Yaster M. Prospective evaluation of pain and analgesic use following major elective intracranial surgery. J Neurosurg. 2007;106:210–6. doi: 10.3171/jns.2007.106.2.210. [DOI] [PubMed] [Google Scholar]
- 2.Gottschalk A, Yaster M. The perioperative management of pain from intracranial surgery. Neurocrit Care. 2009;10:387–402. doi: 10.1007/s12028-008-9150-3. [DOI] [PubMed] [Google Scholar]
- 3.Morad A, Winters B, Stevens R, White E, Weingart J, Yaster M, Gottschalk A. The efficacy of intravenous patient-controlled analgesia after intracranial surgery of the posterior fossa: a prospective, randomized controlled trial. Anesth Analg. 2012;114:416–23. doi: 10.1213/ANE.0b013e31823f0c5a. [DOI] [PubMed] [Google Scholar]
- 4.Morad AH, Winters BD, Yaster M, Stevens RD, White ED, Thompson RE, Weingart JD, Gottschalk A. Efficacy of intravenous patient-controlled analgesia after supratentorial intracranial surgery: a prospective randomized controlled trial. J Neurosurg. 2009;111:343–50. doi: 10.3171/2008.11.JNS08797. [DOI] [PubMed] [Google Scholar]
- 5.Stoneham MD, Walters FJ. Post-operative analgesia for craniotomy patients: current attitudes among neuroanaesthetists. Eur J Anaesthesiol. 1995;12:571–5. [PubMed] [Google Scholar]
- 6.Ostermann ME, Keenan SP, Seiferling RA, Sibbald WJ. Sedation in the intensive care unit: a systematic review. JAMA. 2000;283:1451–9. doi: 10.1001/jama.283.11.1451. [DOI] [PubMed] [Google Scholar]
- 7.Cold GE, Felding M. Even small doses of morphine might provoke “luxury perfusion” in the postoperative period after craniotomy. Neurosurgery. 1993;32:327. doi: 10.1097/00006123-199302000-00032. [DOI] [PubMed] [Google Scholar]
- 8.De Benedittis G, Lorenzetti A, Migliore M, Spagnoli D, Tiberio F, Villani RM. Postoperative pain in neurosurgery: a pilot study in brain surgery. Neurosurgery. 1996;38:466–9. doi: 10.1097/00006123-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 9.de Gray LC, Matta BF. Acute and chronic pain following craniotomy: a review. Anaesthesia. 2005;60:693–704. doi: 10.1111/j.1365-2044.2005.03997.x. [DOI] [PubMed] [Google Scholar]
- 10.Talke PO, Gelb AW. Postcraniotomy pain remains a real headache! Eur J Anaesthesiol. 2005;22:325–7. doi: 10.1017/s0265021505000542. [DOI] [PubMed] [Google Scholar]
- 11.Liu SS, Carpenter RL, Mackey DC, Thirlby RC, Rupp SM, Shine TS, Feinglass NG, Metzger PP, Fulmer JT, Smith SL. Effects of perioperative analgesic technique on rate of recovery after colon surgery. Anesthesiology. 1995;83:757–65. doi: 10.1097/00000542-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Dahl JB, Kehlet H. Preventive analgesia. Curr Opin Anaesthesiol. 2011;24:331–8. doi: 10.1097/ACO.0b013e328345afd9. [DOI] [PubMed] [Google Scholar]
- 13.Malviya S, Voepel-Lewis T, Burke C, Merkel S, Tait AR. The revised FLACC observational pain tool: improved reliability and validity for pain assessment in children with cognitive impairment. Paediatr Anaesth. 2006;16:258–65. doi: 10.1111/j.1460-9592.2005.01773.x. [DOI] [PubMed] [Google Scholar]
- 14.Stinson JN, Kavanagh T, Yamada J, Gill N, Stevens S. Systematic review of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain. 2006;125:143–157. doi: 10.1016/j.pain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Malviya S, Voepel-Lewis T, Ludomirsky A, Marshall J, Tait AR. Can we improve the assessment of discharge readiness?: A comparative study of observational and objective measures of depth of sedation in children. Anesthesiology. 2004;100:218–24. doi: 10.1097/00000542-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell LG, Kaufmann SC, Bitzer S, Jackson EV, Jr, McGready J, Kost-Byerly S, Kozlowski L, Rothman SK, Yaster M. The effects of a small-dose naloxone infusion on opioid-induced side effects and analgesia in children and adolescents treated with intravenous patient-controlled analgesia: a double-blind, prospective, randomized, controlled study. Anesth Analg. 2005;100:953–8. doi: 10.1213/01.ANE.0000148618.17736.3C. [DOI] [PubMed] [Google Scholar]
- 17.Monitto CL, Kost-Byerly S, White E, Lee CK, Rudek MA, Thompson C, Yaster M. The Optimal Dose of Prophylactic Intravenous Naloxone in Ameliorating Opioid-Induced Side Effects in Children Receiving Intravenous Patient-Controlled Analgesia Morphine for Moderate to Severe Pain: A Dose Finding Study. Anesth Analg. 2011;113:834–42. doi: 10.1213/ANE.0b013e31822c9a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teo JH, Palmer GM, Davidson AJ. Post-craniotomy pain in a paediatric population. Anaesth Intensive Care. 2011;39:89–94. doi: 10.1177/0310057X1103900115. [DOI] [PubMed] [Google Scholar]
- 19.Dunbar PJ, Visco E, Lam AM. Craniotomy procedures are associated with less analgesic requirements than other surgical procedures. Anesth Analg. 1999;88:335–40. doi: 10.1097/00000539-199902000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Quiney N, Cooper R, Stoneham M, Walters F. Pain after craniotomy. A time for reappraisal? Br J Neurosurg. 1996;10:295–9. doi: 10.1080/02688699650040179. [DOI] [PubMed] [Google Scholar]
- 21.Vorenkamp KE, Durieux ME. Patient-controlled analgesia. J Neurosurg. 2009;111:340–2. doi: 10.3171/2008.12.JNS081529. [DOI] [PubMed] [Google Scholar]
- 22.Racoosin JA, Roberson DW, Pacanowski MA, Nielsen DR. New evidence about an old drug–risk with codeine after adenotonsillectomy. N Engl J Med. 2013;368:2155–7. doi: 10.1056/NEJMp1302454. [DOI] [PubMed] [Google Scholar]
- 23.Kelly LE, Rieder M, van den Anker J, Malkin B, Ross C, Neely MN, Carleton B, Hayden MR, Madadi P, Koren G. More codeine fatalities after tonsillectomy in North American children. Pediatrics. 2012;129:e1343–e1347. doi: 10.1542/peds.2011-2538. [DOI] [PubMed] [Google Scholar]
- 24.Smith MC, Williamson J, Yaster M, Boyd GJ, Heitmiller ES. Off-label use of medications in children undergoing sedation and anesthesia. Anesth Analg. 2012;115:1148–54. doi: 10.1213/ANE.0b013e3182501b04. [DOI] [PubMed] [Google Scholar]
- 25.Furst SR, Sullivan LJ, Soriano SG, McDermott JS, Adelson PD, Rockoff MA. Effects of ondansetron on emesis in the first 24 hours after craniotomy in children. Anesth Analg. 1996;83:325–8. doi: 10.1097/00000539-199608000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Nelson KL, Yaster M, Kost-Byerly S, Monitto CL. A national survey of American Pediatric Anesthesiologists: patient-controlled analgesia and other intravenous opioid therapies in pediatric acute pain management. Anesth Analg. 2010;110:754–60. doi: 10.1213/ANE.0b013e3181ca749c. [DOI] [PubMed] [Google Scholar]


