Abstract
Pregnancy and childbirth are associated with hemodynamic changes and vascular remodeling. It is not known whether parity is associated with later adverse vascular properties such as larger arterial diameter, wall thickness and lower distensibility.
We used baseline data from 3283 women free of cardiovascular disease aged 45-84 years enrolled in the population based Multi-Ethnic Study of Atherosclerosis. Participants self-reported parity status. Ultrasound derived carotid artery lumen diameters and brachial artery blood pressures were measured at peak-systole and end-diastole. Common carotid intima media thickness (cIMT) was also measured. Regression models to determine the association of carotid distensibility coefficient, lumen diameter, and cIMT with parity were adjusted for age, race, height, weight, diabetes, current smoking, BP medication use, total and high density lipoprotein cholesterol levels.
The prevalence of nulliparity was 18%. In adjusted models, carotid distensibility coefficient was 0.09 × 10−5Pa−1 lower (p = 0.009) in parous vs. nulliparous women. Among parous women, there was a nonlinear association with the greatest carotid DC seen in women with 2 live births, and significantly lower distensibility seen in primiparas (p=0.04) or with higher parity > 2 (p=0.005). No such pattern of association with parity was found for lumen diameter or cIMT.
Parity is associated with lower carotid artery distensibility, suggesting arterial remodeling that lasts beyond childbirth. These long-term effects on the vasculature may explain the association of parity with cardiovascular events later in life.
Keywords: common carotid artery, arterial stiffness, carotid intima-media thickness, women, pregnancy
Introduction
Parity has a nonlinear association with cardiovascular events;1, 2 with the minimum incidence in women with 2 births, a slightly higher incidence in nulliparas and primiparas, and a sharply higher incidence for women with higher parity. Parity is also associated with greater LV mass.3 Thus childbearing may have long lasting effects on the cardiovascular system, but the mechanism is unknown. There is a 40% increase in blood volume in pregnancy, but no increase in systolic blood pressure because of a simultaneous reduction in peripheral vascular resistance.4 Pregnancy is also associated with systemic arterial remodeling, presumably mediated by the peptide hormone relaxin.5 Because remodeled arteries may have thicker walls especially in hypertension,6 be stiffer, and stiffer arteries are associated with CVD events,7, 8 we hypothesized that pregnancies in the past would be associated with remodeled systemic arteries that had larger lumens, relatively thicker walls and lower distensibility. In this study, we investigated whether the parity and gravidity were associated with carotid artery diameter, intima-media thickness and distensibility in middle-aged and older women.
Methods
Study population
Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter multiethnic population-based study consisting of Caucasian-American, Chinese-American, African-American and Hispanic-American race/ethnicities, aged 45-84 years and free of clinical cardiovascular disease at baseline (2000-2002). The study was approved by the institutional review boards of all participating centers and participants gave written informed consent. Of 3601 enrolled women, we excluded 300 because of unavailable imaging data and 18 because of missing parity data, resulting in a sample of 3283 women for this cross sectional analysis.
Assessment of Gravidity and Parity
Gravidity and parity were self-reported. Gravidity, defined as the total number of pregnancies, and parity, defined as the total number of live births, were treated as ordinal variables (0, 1, 2, 3, 4, 5+). If women reported a higher number of live births than pregnancies (n = 30), the parity was assumed to be the number of pregnancies rather that the number of live births, presuming that this difference was because of multiple births. The validity of self-report for parity vs. chart review, is very high (kappa 0.93-0.98) in prior studies.9
Clinical examination for covariates
Participants answered questionnaires including self-reported age, race/ethnicity, educational attainment, current antihypertensive medication use, present or past use of birth control pills or hormone replacement therapy and smoking. Height, and weight were measured. Seated blood pressure was measured as the average of the second and third readings taken using Dinamap® automated blood pressure device (Dinamap Monitor Pro 100®) using appropriate Critikon® cuff sizes as per the Critikon® sizing chart. Total cholesterol categories (<200, 200-239, 240+), HDL-cholesterol categories (<40, 40-59, 60+) were defined from fasting lipid profiles. Diabetes was defined as fasting blood glucose ≥ 7 mmol/L or antidiabetic medication use.
Carotid artery imaging for measurement of distensibility
A 20-second B-mode ultrasound recording of a longitudinal section of the right distal common carotid artery was made using a Logiq 700 machine (General Electric Medical Systems). The brachial blood pressure was simultaneously measured during the recording (DINAMAPP System, General Electric Medical Systems). The Pearson correlations of this BP measurement with average seated blood pressure were 0.78, 0.74 and 0.78 for systolic, diastolic and pulse pressure, respectively. The pulse rate was also measured in the recording. Image analysis was performed centrally at Tufts Medical Center, Boston, MA. Automated edge detection software was used to determine the interadventitial carotid artery diameter during systole and diastole. For two blinded replicate images taken on the same day (n=89) the correlations between systolic and diastolic diameters and diameter change were 0.93, 0.94 and 0.66, respectively.
Common carotid intima-media thickness (cIMT) was measured as mean of the site-specific maximum measurements of all scans and near and far walls of the left and right common carotid arteries.10
Statistical methods
We tabulated the distribution of demographic and cardiovascular risk factors by nulliparity vs. parity. Group differences were assessed using χ2 tests for categorical variables and ANOVA for continuous variables.
Arterial distensibility coefficient (DC) is defined as:
where A is the arterial cross sectional area, P is the arterial pressure and Δ represents the change in diameter and pressure from diastole to systole.13 The slope Δ(logA)/ΔP was calculated directly within regression models adjusting for the confounders of both A and P in a single step.14 The excursions from the mean of the systolic and diastolic pressures, together constituting pulse pressure as separate observations for each individual were added to the mixed regression model, while the mean of systolic and diastolic pressures was added as a covariate. Individual-level pulse-cycle mean log(area), as well as the individual level slope of log(area) vs. pressure between systole and diastole were estimated as random effects, and group-level differences in diameter and slopes are estimated as fixed effects. An illustrative example of this mixed model is shown in the appendix (Figure S1).
Models were fit to estimate the association of parity and gravidity with carotid diameter and DC. Additional covariates included hemodynamic and physical characteristics (height, weight, and heart rate measured from the ultrasound recording), demographic characteristics (age and race/ethnicity), and cardiovascular risk factors (total cholesterol categories, HDL-cholesterol categories, current smoking, diabetes and BP medication use).
We examined the association of carotid DC with the number of live births and pregnancies in different models. First we analyzed these variables dichotomously (gravidas vs. nulligravidas, and parous vs. nulliparous, respectively). Then we categorized as 0, 1, 2, 3, 4, 5+. We tested whether any of the groups 1-5+ differed from each other using an omnibus χ2 test. If the omnibus test was positive, we also fit a spline model with a different linear association of carotid diameter DC with nulliparity, 1-2 live births and with 3+ live births.
Similar analyses were performed secondarily to determine the association of parity with cIMT and W/L ratio.
Sensitivity analysis
We examined if the association of carotid DC with parity or gravidity was robust to the addition further covariates, parity redefinition, exclusion of 68 women with self-reported kidney disease and age stratification, as described in the online appendix.
Results
Table 1 shows the sample characteristics by parity groups. At baseline, 13% of women were nulliparous and 30% of nulliparous women reported one or more past pregnancies. Nulliparous women had a lower mean age, were more likely to be Caucasian-American and had a more favorable cardiovascular risk profile than parous women. In these unadjusted tabulations, there were statically significant trends for higher cIMT and larger lumen diameters with higher parity. DC was also statistically significantly different by parity, but the differences were not monotonic – higher parity was associated with lower DC with the exception of parity 2, which did not follow the trend. A slightly higher level W/L ratio is with higher parity (table S3).
Table 1.
Demographic, Cardiovascular Risk Factor, and Carotid Measurement Distributions by Parity in the Multiethnic Study of Atherosclerosis (MESA) Baseline Examination
| Sample Characteristic | Nulliparous | 1 birth | 2 births | 3 births | 4 births | 5+ births | p |
|---|---|---|---|---|---|---|---|
| N | 588 | 492 | 801 | 606 | 564 | 232 | |
| Number of live births, median[IQR] | 0 | 1 | 2 | 3 | 4 | 7 [6 to 8] | |
| Any history of pregnancy, | 177 (30%) | − | − | − | − | − | |
| Age (years) | 60.7 ± 11.0 | 60.6 ± 10.1 | 61.2 ± 9.9 | 62.7 ± 9.7 | 65.9 ± 9.8 | 68.4 ± 8.2 | <0.001 |
| Race (column adds to 100%) | |||||||
| Caucasian-American | 290 (49%) | 162 (33%) | 347 (43%) | 220 (36%) | 172 (30%) | 43 (19%) | <0.001 |
| Chinese-American | 35(6%) | 54(11%) | 112(14%) | 82(14%) | 80(14%) | 19(8%) | |
| African-American | 186(32%) | 192(39%) | 212(26%) | 160(26%) | 154(27%) | 46(20%) | |
| Hispanic-American | 77(13%) | 84(17%) | 130(16%) | 144(24%) | 158(28%) | 124(53%) | |
| Diabetes* | 43(7%) | 60(12%) | 82(10%) | 71(12%) | 70(12%) | 45(19%) | 0.001 |
| Current Smoking | 74(13%) | 83(17%) | 91(11%) | 68(11%) | 48(9%) | 21(9%) | <0.001 |
| Hypertension† | 231(39%) | 212(43%) | 348(43%) | 298(49%) | 288(51%) | 135(58%) | <0.001 |
| Use of Blood Pressure Medication | 169(29%) | 165(34%) | 263(33%) | 223(37%) | 210(37%) | 108(47%) | <0.001 |
| Past or Present Birth Control Pill Use | 292(50%) | 256(52%) | 432(54%) | 315(52%) | 234(41%) | 67(29%) | <0.001 |
| Past or Present Hormone | 257(44%) | 230(47%) | 404(50%) | 293(48%) | 246(44%) | 84(36%) | <0.001 |
| Recent Therapy BMI (kg/m2) | 28.2 ± 6.3 | 28.7 ± 6.6 | 28.1 ± 6.0 | 28.9 ± 6.1 | 29.0 ± 6.1 | 29.4 ± 4.9 | 0.011 |
| Total Cholesterol (mmol/L ) | 5.2( 0.9) | 5.1( 0.9) | 5.2( 0.9) | 5.2( 0.9) | 5.2( 0.9) | 5.1( 1.0) | 0.46 |
| HDL cholesterol (mmol/L) | 1.5( 0.4) | 1.5( 0.4) | 1.5( 0.4) | 1.4( 0.4) | 1.4( 0.4) | 1.3( 0.3) | <0.001 |
| Seated Systolic Blood Pressure (mmHg) | 124.3 ±23.1 | 125.1 ±23.2 | 125.0 ±22.9 | 127.2 ±22.7 | 130.5 ±23.2 | 135.0 ±25.4 | <0.001 |
| Seated Diastolic Blood Pressure (mmHg) | 69.1 ± 10.0 | 69.5 ± 10.4 | 68.7 ± 10.2 | 69.6 ± 10.4 | 68.8 ± 9.8 | 69.6 ± 10.8 | 0.55 |
| Heart Rate (bpm) | 64.9 ± 10.3 | 66.0 ± 10.1 | 64.4 ± 10.0 | 64.2 ± 10.0 | 65.0 ± 10.2 | 64.4 ±9.3 | 0.050 |
| Common Carotid (IMT mm) | 0.82 ±0.17 | 0.83 ±0.20 | 0.83 ±0.17 | 0.85 ±0.17 | 0.89 ±0.20 | 0.90 ±0.18 | <0.001 |
| Lumen Diastolic Diameter (mm) | 5.7 ± 0.7 | 5.8 ± 0.7 | 5.8 ± 0.7 | 5.8 ± 0.7 | 6.0 ± 0.7 | 6.0 ± 0.8 | <0.001 |
| Carotid Distensibility Coefficient (x 10-5 Pa-1) | 2.13 ± 1.10 | 1.97 ± 0.81 | 2.05 ± 0.94 | 1.90 ± 0.80 | 1.72 ± 0.80 | 1.57 ± 0.78 | <0.001 |
Distributions shown as median [interquartile range], number (%), or mean ± standard deviation. Differences in distributions by parity were tested using χ2 tests for categorical variables and ANOVA for continuous variables.
Diabetes was defined as fasting blood glucose ≥ 7 mmol/L or the use of antidiabetic medications
Hypertension was defined as blood pressure ≥ 140/90 or antihypertensive medication use. 1 Pa-1 = 133.32 mmHg-1 (conventional units)
The cross tabulation of the number of live births vs. the number of pregnancies is shown in appendix Table S1.
Association of mean carotid artery diameter and DC with parity and gravidity
In adjusted models mean carotid artery diameter (geometric mean of systolic and diastolic diameters) was not different comparing parous vs. nulliparous women (0.26% larger in parous women, 95% CI: −0.73% to 1.25%, p = 0.61), nor gravid vs. nulligravid women (0.63% larger in gravidas, 95% CI: −0.50% to 1.78%, p = 0.27). However, carotid DC was 0.09 × 10−5 Pa−1 lower (95% CI −0.16 to −0.02 × 10−5 Pa−1, p = 0.009) in parous vs. nulliparous women, and was 0.12 × 10−5 Pa−1 lower (95% CI −0.19 to −0.04× 10−5 Pa−1, p = 0.003) in gravid vs. nulligravid women. In analyses restricted to 588 nulliparous women, gravidity was associated with a similar magnitude of lower carotid DC (0.09 × 10−5 Pa−1) however, this difference did not reach statistical significance (95% CI −0.25 to 0.06 × 10−5 Pa−1, p = 0.23).
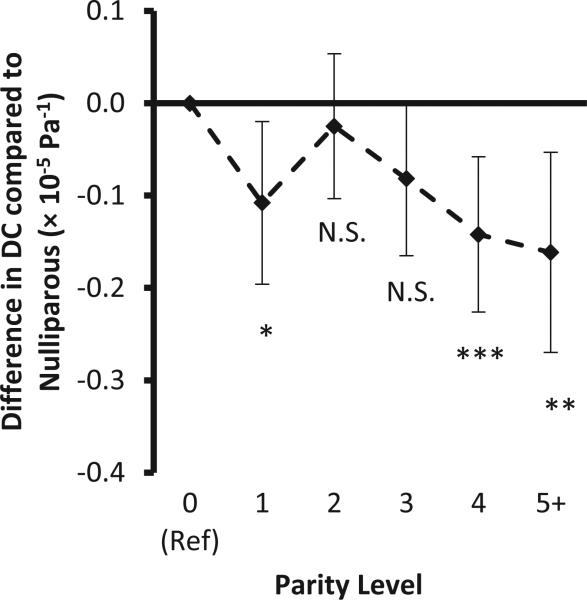
Neither the degree of parity nor gravidity had any association with carotid artery diameter in adjusted models (appendix Table S2). Figure 1 shows the association of carotid DC with parity. Non-linearity is seen in this association: In spline models primiparous women have lower DC than nulliparous women (p = 0.014), women with 2 live births have higher DC than primiparous women (p=0.050), and women with more than 2 births have a linear decline in DC as compared to women with 2 live births (p=0.018).
Figure 1.
Differences in carotid artery distensibility coefficient (×10−5Pa−1) by parity compared to nulliparas. Regression coefficients and 95% confidence intervals adjusted for systolic and diastolic blood pressure, height, weight, pulse rate, age and race/ethnicity, total cholesterol categories, HDL-cholesterol categories, current smoking, diabetes, BP medication use. Overall significance of the association of distensibility coefficient with parity, p = 0.002) Asterisks (*) mark significant differences from the nulliparous group (p<0.05). In pairwise comparisons, parity 2 differs from parity levels 1, 4 and 5+ (p<0.05), all other pairwise differences do not reach statistical significance.
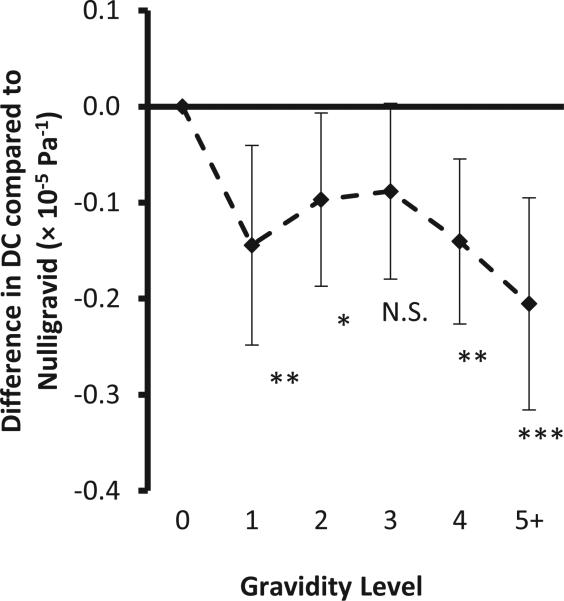
Figure 2 shows a similar analysis by increasing gravidity. However, only nulligravidas differ significantly from women with any pregnancy history, there is no difference in the distensibility coefficient by number of pregnancies among gravidas (p=0.17).
Figure 2.
Differences in carotid artery distensibility coefficient (×10−5Pa−1) by gravidity compared to nulligravidas. Regression coefficients and 95% confidence intervals adjusted for systolic and diastolic blood pressure, height, weight, pulse rate, age and race/ethnicity, total cholesterol categories, HDL-cholesterol categories, current smoking, diabetes, BP medication use. Asterisks (*) mark significant differences from the nulliparous group. In pairwise comparisons, parity 2 and 3 differ from parity 5+ (p<0.05), all other pairwise differences do not reach statistical significance.
Secondary analyses showed that there was no overall adjusted association between parity groups and either W/L ratio (appendix figure S2, p = 0.50) or for cIMT (appendix figure S3, p = 0.32). There associations are also tabulated for adjacent comparison in appendix table S4.
Sensitivity analyses showed that the association of carotid artery DC with parity and gravidity was robust to further adjustment for birth control pill use, hormone replacement therapy, serum creatinine as a proxy for renal function, and education, the use of as-reported number of live births not harmonized with number of pregnancies, exclusion of women with self-reported kidney disease and age stratified analysis as detailed in the online appendix.
Discussion
We have shown a strong association of the history of parity and gravidity with carotid artery distensibility, but not to lumen diameter of intima-media thickness, in women from a diverse multi-ethnic population with a wide age range. To our knowledge, this is the first demonstration of this association. We have shown a non-linear and non-monotonic association of parity with DC, with relatively protected carotid artery diameters in women with 2 live births. However, this non-monotonic association is not apparent in the analysis of gravidity. This suggests that live births, possibly leading to a lifetime of exposure to child rearing may have different implications for this risk factor as compared to the hemodynamic and cardiometabolic consequences of pregnancy per se. Because lower arterial distensibility, i.e., greater arterial stiffness is associated with cardiovascular events,7, 8 our results suggest that the vascular effects of pregnancy and childbirth may contribute to cardiovascular risk in women. Our results after demographic, cardiovascular risk factor and blood pressure adjustment show that the parity-related changes are not primarily due to thicker wall (cIMT), or external remodeling leading to changes in W/L ratio, but rather, they are due to the hemodynamics of distensibility.
Two studies have shown a J-shaped relationship of parity with cardiovascular events.1, 2 Parikh et al1 showed in a Swedish population that the nadir of CVD risk was found in women with 2 children, while women with fewer or a larger number of children had a greater CVD risk. Parikh et al1 adjusted for the effects of pregnancy related complications including gestational diabetes and pregnancy induced hypertension, which may be in the causal pathway for cardiovascular risk, and which did not have information about in our population. Lawlor et al2 also showed a similar J-shaped relationship with CHD in a British population. However, this study also showed that men with 2 children had a nadir of CHD risk, suggesting that child rearing, rather than pregnancy and birth may be partially responsible for this relative protection that extends to both men and women. Parikh et al showed an increase in LV mass with larger LV volume in parous vs nulliparous women from MESA.3
We show that even a single pregnancy without live birth may have the same magnitude of association with lower carotid DC, even though this association did not reach statistical significance due to the smaller sample size. The contrasting association of gravidity and parity with carotid DC in our study is consistent with the idea that the vascular consequences of pregnancies may not have a J-shaped relationship, and may be superimposed on the consequences of child rearing which may indeed have a J-shaped relationship.
The mechanism by which pregnancy may affect the long term vasculature modeling and changes are not well understood. During pregnancy, relaxins secreted by the corpus luteum result in vasodilatation and a reduction in peripheral vascular resistance.15 The matrix metalloproteinase (MMP) system has been a suggested mechanism for the remodeling of systemic arteries during pregnancy.16 Circulating MMP-9 levels are higher in normal pregnancy as compared non-pregnant women.17 In women with pregnancy induced hypertension, MMP-9 levels are raised, but the circulating levels of tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) are also raised.17 Thus a balance between the metalloproteinases and their inhibitors is required for appropriate remodeling of the systemic vasculature. Although pregnancy-related hemodynamic changes are largely reversed postpartum,18 our findings suggest that some of the changes remain on the long term. In addition, although gestational insulin resistance is normal, its consequences may persist beyond repeated pregnancies resulting in long lasting vascular insult.19, 20
Our study shows that nulliparous women have better levels of cardiovascular risk factors including diabetes, hypertension and lipid profile than parous women, as shown by others.21 Parous women tend to gain obesity that persists after pregnancy.21 Bennett et al showed worsening weight and weight-related health behaviors among women with and without gestational diabetes mellitus after their pregnancies.22 A persistence of these factors over a lifetime may result in vascular injury. Nicholson et al showed that grand multiparity (5+births) was associated with a higher incidence of diabetes.23 Nicholson et al's study pooled 1-2 live births into a single group, thus we cannot distinguish whether women with 2 live births were relatively protected vs. those with 1 live birth.23 We adjusted our analysis for lipid levels and diabetes as confounders. Another effect of parity, rather than gravidity relates to the socioeconomic consequences of child-rearing, especially for larger families. We have attempted to account for this by using educational attainment as a proxy for socioeconomic status in sensitivity analyses. In addition, our results were unaffected by exclusion of women with self-reported kidney disease, or adjustment by serum creatine as a proxy for imapaired renal function which may result in remodeling with thinner cIMT.24
A strength of our study is the population-based multi-ethnic sample with a wide age range, enhancing the generalizability to multiple sub-groups of women. The questionnaires and imaging measurement protocols were centrally standardized. We have also analyzed both gravidity and parity unlike prior studies. Our study also suffers from certain limitations. All covariates are measured during a cross sectional study in middle age or older women, although the mechanism we suggest is related to changes that occur during pregnancy and a lifetime of exposure. We did not have any information of pregnancy related confounders, which may be risk factors for (or indicators of) cardiovascular disease, including gestational weight gain, gestational diabetes mellitus, preeclampsia, preterm birth or small for gestational age infants.25 We have also estimated carotid DC from brachial blood pressure measurements, which may differ systematically from carotid blood pressure measurements, especially in younger people. However, we did not see significant qualitative differences in our findings in age-stratified analyses. We do not have information whether pregnancies that did not result in live births were as a result of miscarriages representing existing vascular disease or due to elective procedures. We also did not information on health behaviors associated with childrearing. We were unable to discern the reasons why women had a pregnancy but no live birth (e.g. elective abortion, miscarriage, or intrauterine fetal death), limiting our ability to characterize differential risks in the nulliparous population. Also, educational attainment, our proxy measure of socioeconomic status, may not appropriately represent the socioeconomic pressures related to child rearing. Nevertheless, the association we have found is robust correcting for the covariates that we do have, and represents the situation in a population-based sample.
Supplementary Material
Perspectives.
Nulliparas and nulligravidas have more distensible carotid arteries than parous women and gravidas. Gravidity is not further associated with carotid distensibility. However, women with a parity level of 2 are relatively protected from loss of carotid distensibility. This effect on the vasculature may partially explain the effects of gravidity and parity on cardiovascular disease events. Longitudinal vascular and biochemical studies performed through pregnancy and postpartum will be necessary to determine the mechanism through which gravidity and parity affect arterial distensibility.
In conclusion, we have shown that gravidity and parity are associated with lower carotid artery distensibility, suggesting arterial remodeling that lasts beyond childbirth.
Novelty and Significance.
1) What Is New
We show the association between pregnancy and live birth history with arterial distensibility in later life.
2) What Is Relevant?
The association of gravidity and parity with lower carotid artery distensibility suggests arterial remodeling that lasts beyond childbirth.
3) Summary:
Our manuscript examines a large multi-ethnic US population sample and shows that pregnancy and childbearing have long term implications on vascular properties. Our study provides a possible explanation of the previously reported association of parity with cardiovascular events.
Acknowledgments
Sources of Funding: This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesanhlbi.org. DV was supported by Grant Number UL1 TR 001079 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
Footnotes
Supplementary imaging data:
Because a study of the uterine artery11 has found that pregnancy affects outward remodeling, we also examined data for carotid wall-to-lumen (W/L) ratio measured using magnetic resonance imaging,12 briefly described in the supplementary material.
Access of protocols and data:
The full protocols and data access procedures for the MESA study can be found at http://www.mesa-nhlbi.org.
Conflict of Interest/Disclosures: DV is a consultant for MBC, Inc. No other conflicts.
References
- 1.Parikh NI, Cnattingius S, Dickman PW, Mittleman MA, Ludvigsson JF, Ingelsson E. Parity and risk of later-life maternal cardiovascular disease. Am Heart J. 2010;159:215–221. e216. doi: 10.1016/j.ahj.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Lawlor DA, Emberson JR, Ebrahim S, Whincup PH, Wannamethee SG, Walker M, Smith GD. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Findings from the british women's heart and health study and the british regional heart study. Circulation. 2003;107:1260–1264. doi: 10.1161/01.cir.0000053441.43495.1a. [DOI] [PubMed] [Google Scholar]
- 3.Parikh NI, Lloyd-Jones DM, Ning H, Ouyang P, Polak JF, Lima JA, Bluemke D, Mittleman MA. Association of number of live births with left ventricular structure and function. The multi-ethnic study of atherosclerosis (mesa). Am Heart J. 2012;163:470–476. doi: 10.1016/j.ahj.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Semin Perinatol. 2000;24:11–14. doi: 10.1016/s0146-0005(00)80047-6. [DOI] [PubMed] [Google Scholar]
- 5.Cernaro V, Lacquaniti A, Lupica R, Buemi A, Trimboli D, Giorgianni G, Bolignano D, Buemi M. Relaxin: New pathophysiological aspects and pharmacological perspectives for an old protein. Med Res Rev. 2014;34:77–105. doi: 10.1002/med.21277. [DOI] [PubMed] [Google Scholar]
- 6.Roman MJ, Pickering TG, Schwartz JE, Pini R, Devereux RB. Relation of arterial structure and function to left ventricular geometric patterns in hypertensive adults. J Am Coll Cardiol. 1996;28:751–756. doi: 10.1016/0735-1097(96)00225-2. [DOI] [PubMed] [Google Scholar]
- 7.Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- 8.Yang EY, Chambless L, Sharrett AR, Virani SS, Liu X, Tang Z, Boerwinkle E, Ballantyne CM, Nambi V. Carotid arterial wall characteristics are associated with incident ischemic stroke but not coronary heart disease in the atherosclerosis risk in communities (aric) study. Stroke. 2012;43:103–108. doi: 10.1161/STROKEAHA.111.626200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buka SL, Goldstein JM, Spartos E, Tsuang MT. The retrospective measurement of prenatal and perinatal events: Accuracy of maternal recall. Schizophr Res. 2004;71:417–426. doi: 10.1016/j.schres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr., Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Hale SA, Weger L, Mandala M, Osol G. Reduced no signaling during pregnancy attenuates outward uterine artery remodeling by altering mmp expression and collagen and elastin deposition. Am J Physiol Heart Circ Physiol. 2011;301:H1266–1275. doi: 10.1152/ajpheart.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wasserman BA, Sharrett AR, Lai S, Gomes AS, Cushman M, Folsom AR, Bild DE, Kronmal RA, Sinha S, Bluemke DA. Risk factor associations with the presence of a lipid core in carotid plaque of asymptomatic individuals using high-resolution mri: The multi-ethnic study of atherosclerosis (mesa). Stroke. 2008;39:329–335. doi: 10.1161/STROKEAHA.107.498634. [DOI] [PubMed] [Google Scholar]
- 13.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 14.Vaidya D, Heckbert SR, Wasserman BA, Ouyang P. Sex-specific association of age with carotid artery distensibility: Multi-ethnic study of atherosclerosis. J Womens Health (Larchmt) 2012;21:516–520. doi: 10.1089/jwh.2011.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cernaro V, Lacquaniti A, Lupica R, Buemi A, Trimboli D, Giorgianni G, Bolignano D, Buemi M. Relaxin: New pathophysiological aspects and pharmacological perspectives for an old protein. Med Res Rev. 2013 doi: 10.1002/med.21277. [DOI] [PubMed] [Google Scholar]
- 16.Karthikeyan VJ, Lane DA, Beevers DG, Lip GY, Blann AD. Matrix metalloproteinases and their tissue inhibitors in hypertension-related pregnancy complications. J Hum Hypertens. 2013;27:72–78. doi: 10.1038/jhh.2012.8. [DOI] [PubMed] [Google Scholar]
- 17.Tayebjee MH, Karalis I, Nadar SK, Beevers DG, MacFadyen RJ, Lip GY. Circulating matrix metalloproteinase-9 and tissue inhibitors of metalloproteinases-1 and -2 levels in gestational hypertension. Am J Hypertens. 2005;18:325–329. doi: 10.1016/j.amjhyper.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Melchiorre K, Sharma R, Thilaganathan B. Cardiac structure and function in normal pregnancy. Curr Opin Obstet Gynecol. 2012;24:413–421. doi: 10.1097/GCO.0b013e328359826f. [DOI] [PubMed] [Google Scholar]
- 19.Martin U, Davies C, Hayavi S, Hartland A, Dunne F. Is normal pregnancy atherogenic? Clin Sci (Lond) 1999;96:421–425. doi: 10.1042/cs0960421. [DOI] [PubMed] [Google Scholar]
- 20.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: Opportunities for intervention and screening? BMJ. 2002;325:157–160. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humphries KH, Westendorp IC, Bots ML, Spinelli JJ, Carere RG, Hofman A, Witteman JC. Parity and carotid artery atherosclerosis in elderly women: The rotterdam study. Stroke. 2001;32:2259–2264. doi: 10.1161/hs1001.097224. [DOI] [PubMed] [Google Scholar]
- 22.Bennett WL, Liu SH, Yeh HC, Nicholson WK, Gunderson EP, Lewis CE, Clark JM. Changes in weight and health behaviors after pregnancies complicated by gestational diabetes mellitus: The cardia study. Obesity (Silver Spring) 2013;21:1269–1275. doi: 10.1002/oby.20133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholson WK, Asao K, Brancati F, Coresh J, Pankow JS, Powe NR. Parity and risk of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes Care. 2006;29:2349–2354. doi: 10.2337/dc06-0825. [DOI] [PubMed] [Google Scholar]
- 24.Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, Stengel B, Houillier P, Froissart M, Boutouyrie P. Arterial remodeling associates with ckd progression. J Am Soc Nephrol. 2011;22:967–974. doi: 10.1681/ASN.2010080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catov JM, Newman AB, Sutton-Tyrrell K, Harris TB, Tylavsky F, Visser M, Ayonayon HN, Ness RB. Parity and cardiovascular disease risk among older women: How do pregnancy complications mediate the association? Ann Epidemiol. 2008;18:873–879. doi: 10.1016/j.annepidem.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.