Abstract
Background
Little is known about physicians’ human papillomavirus (HPV) vaccine recommendations for males while the Advisory Committee on Immunization Practices’ (ACIP) permissive guidelines for male vaccination were in effect. The purpose of this study was to examine and explore factors associated with U.S. physicians’ HPV vaccine recommendations to early (ages 11–12), middle (13–17), and late adolescent/young adult (18–26) males.
Methods
Nationally representative samples of family physicians and pediatricians were selected in 2011 (n=1,219). Physicians reported the frequency with which they recommended HPV vaccine to male patients (“always” [>75% of the time] vs. other) for each age group. Statistically significant predictors of vaccine recommendation were identified using multivariable logistic regression.
Results
The prevalence of physicians reporting they “always” recommended HPV vaccination for males was 10.8% for ages 11–12, 12.9% for ages 13–17, and 13.2% for ages 18–26. Pediatrician specialty and self-reported early adoption of new vaccines were significantly associated with recommendation for all patient age groups. Additionally, physician race and patient payment method were associated with physician recommendations to patients ages 11–12, and patient race was associated with recommendations to ages 13–17 and 18–26.
Conclusions
Less than 15% of physicians surveyed reported “always” recommending HPV vaccine to male patients following national guidelines for permissive vaccination. Vaccine financing may have affected physicians’ vaccine recommendations.
Impact
If these recommendation practices continue following the ACIP’s routine recommendation for males in October 2011, then interventions designed to increase recommendations should target family physicians and possibly utilize early adopters to encourage support of HPV vaccination guidelines.
Keywords: cancer vaccines, human papillomavirus, HPV vaccines, males, physicians
Introduction
A growing body of evidence demonstrates the role of human papillomavirus (HPV) in cancers that impact males, including anal, penile, and oropharyngeal cancer (1, 2). Quadrivalent HPV vaccine trials have demonstrated high levels of immunogenicity, reductions in genital warts, and potential reductions in precancerous anogential lesions in males (3, 4). Thus, vaccination has significant primary prevention benefits for all males (5), but particularly for those from racial/ethnic and sexual minority groups, who are disproportionately affected by HPV-related diseases (1, 6).
In the United States (U.S.), the Advisory Committee on Immunization Practices (ACIP), a national group of medical and public health experts, develops recommendations on how to use vaccines to control diseases (7). The ACIP also collaborates with the American Academy of Family Physicians and the American Academy of Pediatrics to approve an immunization schedule for persons aged 0 to 18 years (8). ACIP recommendations can be “permissive,” meaning that vaccine providers may, but are not expected to, proactively offer vaccination, or may vaccinate upon request (9). In contrast, a “routine” vaccine recommendation means that the vaccine should be proactively provided as the standard of care.
Private health insurance payers in the U.S. reference the ACIP’s recommendations to determine their immunization coverage policy, and not all payers cover immunizations with permissive recommendations (10). Therefore, physician practices may choose to purchase and stock only vaccines for which these costs are most likely to be recouped and refer those who request these vaccines to settings that are supported directly or indirectly by the federal Vaccines for Children (VFC) program. The VFC program provides vaccines for children through age 18 years who are Medicaid eligible, uninsured, American Indian or Alaska Native, or underinsured (11). Underinsured children, who have health insurance that does not cover vaccines or covers selected vaccines, can receive VFC-funded vaccines at a federally qualified health center, rural health clinic, or under an approved deputization agreement. The ACIP determines the vaccines that are available through the VFC (12) and voted for HPV vaccine coverage for males while permissive guidelines were in effect (13, 14). In addition to vaccines provided through the VFC program, state and local immunization programs may elect to use state/local funds to purchase vaccines for non-VFC eligible children (15).
In October 2009, the ACIP issued a permissive recommendation for vaccinating males ages 9–26 years against HPV (16). Although early studies suggest that pediatricians (Peds) and family physicians (FPs) support the concept of male vaccination (17–22), these studies were primarily conducted prior to the actual availability of the vaccine. As such, little is known about actual recommendation practices, particularly during the period between 2009 and 2011 when the ACIP’s permissive recommendation was in effect. Examining physicians’ HPV vaccine recommendations to males during a time of permissive recommendations from the ACIP provides the opportunity to characterize “early adopter” providers (23), establish a benchmark to which physician recommendation after the ACIP’s 2011 routine recommendation for males can be compared, and understand the potential impact on physician practices to inform the decision for permissive recommendation for future vaccines.
As part of a larger study to examine trends in physicians’ HPV vaccine recommendations to females (24, 25), we surveyed physicians after the ACIP’s permissive guidelines for male vaccination about their HPV vaccine recommendations to male patients. Based on previous research that suggests differences in physicians’ HPV vaccine recommendations by patient age (24, 26), recommendations in this study were assessed for males ages 11–12 years (the target group for HPV vaccination), ages 13–17 (a catch-up group that may still entail parental involvement in vaccine decision-making), and ages 18–26. This substudy was based on the Competing Demands Model (27), originally developed to understand delivery of clinical preventive services in the primary care setting and successfully applied by our team in prior studies of physician recommendation for HPV vaccination to females (24, 25). The Competing Demands Model proposes three domains of factors influencing physicians’ HPV vaccine recommendations. Physician factors include personal characteristics, knowledge, beliefs, attitudes, and experiences. Given the focus on adoption of a new clinical practice (i.e., HPV vaccination), we drew upon Diffusion of Innovations theory (23) to assess whether physicians who perceived themselves as early adopters of new technologies were more likely to recommend HPV vaccination. Practice factors are the immediate setting in which a physician delivers care (practice size, geographic location, single vs. multispeciality group). Finally, state/policy factors include issues outside the immediate practice that may impact HPV vaccine recommendation (e.g., physician participation in the VFC program).
The primary aims of the current study were to: (1) examine the prevalence of physicians’ HPV vaccine recommendations to early (ages 11–12), middle (13–17), and late adolescent/young adult (18–26) males; and (2) explore physician factors associated with recommendation to each patient age group after the ACIP issued permissive recommendations for HPV vaccination.
Materials and Methods
Sample and Recruitment
Study methods are described in greater detail elsewhere (25). Briefly, a serial cross-sectional study was conducted at three (2009) and five (2011) years post-HPV vaccine licensure for females. Each year, nationally representative samples of FPs, Peds, and obstetricians/gynecologists (OBGYNs) were randomly selected from the American Medical Association (AMA) Physician Masterfile based on their proportional representation in the U.S. primary care physician workforce. OBGYNs were excluded from the current study given that their practice focuses on female patients. After obtaining Institutional Review Board approval, a recruitment approach based on Dillman’s (28) method was used and included six varied contacts: pre-notice postcard, survey packet mailed via FedEx, reminder postcard, reminder survey packet mailed via the U.S. Postal Service, another reminder postcard, and a final survey packet mailed via the U.S. Postal Service. Data used in the current study were collected between April 2011 and February 2012. Three surveys were received after the ACIP guidelines for routine vaccination in October 2011, including one that the participant dated May 2011. Recommendation practices reported on these surveys were variable, ranging from “rarely” to “always,” and thus these responses were included in the current analyses.
The survey was mailed to 746 FPs and 473 Peds in 2011. Completed surveys were received from 406 FPs and 322 Peds. After accounting for undeliverable surveys and ineligible participants (e.g., non-patient care), the specialty-specific response rate was 56.7% for FPs and 70.2% for Peds. To evaluate the national representativeness of the sample, we used the AMA’s Physician Characteristics and Distribution in the U.S. (29) to compare our study samples to national data on demographic and clinical practice characteristics. Our samples were similar to the national population with respect to region for both specialties and sex for FPs; however, a higher percentage of FPs and Peds in our samples were in the older age groups, and a higher percentage of Peds in our sample were female.
Measures
Survey items and development are described elsewhere (24). The Competing Demands Model (27) and Diffusion of Innovations (23) guided survey development. Predictor and outcome variables included in the current analyses are briefly described in the following paragraphs.
Predictor variables
Physicians were surveyed about HPV information sources, HPV-related knowledge, vaccination barriers, vaccine practices, and vaccine recommendation and administration practices. Vaccine practices included the number of strategies used to get patients in for the first dose (e.g., send patient reminder regarding need for preventive visit/checkup) and number of strategies used to ensure three-dose series completion (e.g., send reminder/recall letter or call patient). Physicians were also asked whether they referred uninsured and underinsured patients elsewhere for HPV vaccination. Given that HPV vaccination for males was new at the time of the study, two questions assessed whether physicians considered themselves early adopters of medical innovations (23). These questions asked physicians about being among the first to use a newly recommended vaccine compared to clinical peers (five-point Likert-type scale ranging from strongly disagree to strongly agree) and waiting to adopt new medications, vaccines, or procedures until hearing about them from several trusted colleagues (same scale as previous item). Physician demographic, practice, and patient data also were collected.
The survey contained 10 items to measure participants’ HPV-related knowledge, including items specific to U.S. Food and Drug Administration approval of HPV vaccine for males and non-cervical cancers caused by HPV infection. Responses were summed to create a composite knowledge score and dichotomized based on a median split: lower knowledge (0–7 correct responses) and higher knowledge (8–10 correct responses). Additionally, physicians were asked to report their agreement that each of 14 items served as a barrier to immunizing their patients against HPV; a mean barrier score was obtained and a tertile split classified physicians as experiencing low, medium, and high barriers.
Outcome variable
Physicians were asked to report their HPV vaccine recommendation practices: “In the past 12 months, how often did you recommend the HPV vaccine to your male patients?” Physicians provided separate responses for each patient age group. Response options included a qualitative descriptor and quantitative estimate: “never” (0%), “rarely” (1–25%), “sometimes” (26–50%), “often” (51–75%), “always” (>75%), or “do not see patients in this age group.”
Data Analysis
Simple logistic regression models were used to determine the demographic and practice characteristics associated with “always” recommending HPV vaccine for each patient age group. The final multivariable logistic regression model for each age group was selected using the backward elimination approach (significance level of stay = 0.05). The models were assessed for multicollinearity among the predictor variables; variance inflation factors suggest multicollinearity was not problematic (most values ≤1.5; all ≤3.5). Analyses were conducted using SAS® 9.3 (SAS Institute Inc., Cary, North Carolina).
Results
As shown in Table 1, the largest proportions of physicians were aged ≥50 years (42.5%), White/Caucasian (71.2%), and not Hispanic or Latino (91.6%). Most physicians worked in a private practice setting (69.0%) and reported being a VFC provider (58.2%). About 43% did not use any strategies to get patients in for the first dose of HPV vaccine, whereas a higher percentage (57.1%) reported using ≥2 strategies to ensure vaccine completion. More than a third of physicians (38.9%) reported being among the first to use a newly recommended vaccine compared to their clinical peers.
Table 1.
Demographic, practice, and patient characteristics; knowledge; perceived barriers; and vaccine practices in a representative sample of U.S. physicians, 2011 (n = 728)a
| n (%) | |
|---|---|
| Demographic characteristics | |
| Age (yr) | |
| 30–39 | 137 (18.8) |
| 40–49 | 260 (35.7) |
| 50+ | 309 (42.5) |
| Gender | |
| Male | 360 (49.5) |
| Female | 361 (49.6) |
| Race | |
| White/Caucasian | 518 (71.2) |
| Other | 197 (27.1) |
| Ethnicity | |
| Hispanic or Latino | 39 (5.4) |
| Not Hispanic or Latino | 667 (91.6) |
| Practice characteristics | |
| Provider specialty | |
| Family Medicine | 406 (55.8) |
| Pediatrics | 322 (44.2) |
| No. of physicians | |
| 1 | 137 (18.8) |
| 2–15 | 503 (69.1) |
| 16+ | 79 (10.9) |
| No. of specialties | |
| Single | 517 (71.0) |
| Multiple | 178 (24.5) |
| Other | 20 (2.8) |
| Type | |
| Private practice | 502 (69.0) |
| Other | 216 (29.7) |
| Arrangement | |
| Full/part-owner physician practice | 337 (46.3) |
| Other | 379 (52.1) |
| No. of patients/day | |
| 0–19 | 222 (30.5) |
| 20–29 | 375 (51.5) |
| 30+ | 121 (16.6) |
| Location | |
| Urban | 193 (26.5) |
| Suburban | 384 (52.8) |
| Rural | 124 (17.0) |
| Other | 8 (1.1) |
| Region | |
| Northeast | 151 (20.7) |
| Midwest | 172 (23.6) |
| South | 241 (33.1) |
| West | 149 (20.5) |
| VFC provider | |
| Yes | 424 (58.2) |
| No | 213 (29.3) |
| Don’t know | 76 (10.4) |
| Refer uninsured patients for vaccine | |
| No or N/A | 428 (58.8) |
| Yes, to federally qualified health center/health department/other | 300 (41.2) |
| Refer underinsured patients for vaccine | |
| No or N/A | 427 (58.7) |
| Yes, to federally qualified health center/health department/other | 301 (41.3) |
| Patient characteristics | |
| Patient payment method | |
| Private insurance/HMO | |
| 0–50% of patients | 343 (47.1) |
| 51–100% of patients | 351 (48.2) |
| Patient race (majority) | |
| Non-Hispanic White | 502 (69.0) |
| Other | 217 (29.8) |
| HPV knowledge | |
| Lower (0–7 correct) | 313 (43.0) |
| Higher (8–10 correct) | 410 (56.3) |
| Perceived barriers related to HPV vaccination | |
| Low | 218 (30.0) |
| Medium | 236 (32.4) |
| High | 263 (36.1) |
| Vaccine practices | |
| Strategies for HPV vaccine initiation | |
| 0 | 314 (43.1) |
| 1 | 168 (23.1) |
| 2+ | 212 (29.1) |
| Strategies to ensure HPV vaccine completion | |
| 0 | 118 (16.2) |
| 1 | 160 (22.0) |
| 2+ | 416 (57.1) |
| Early vs. late adopter | |
| First to use a new vaccine | |
| Agree | 283 (38.9) |
| Neutral | 263 (36.1) |
| Disagree | 170 (23.4) |
| Wait to adopt | |
| Agree | 304 (41.8) |
| Neutral | 195 (26.8) |
| Disagree | 219 (30.1) |
Abbreviations: VFC, Vaccines for Children; HMO, health maintenance organization; HPV, human papillomavirus; STIs, sexually transmitted infections.
Percentages may not add up to 100% due to missing data.
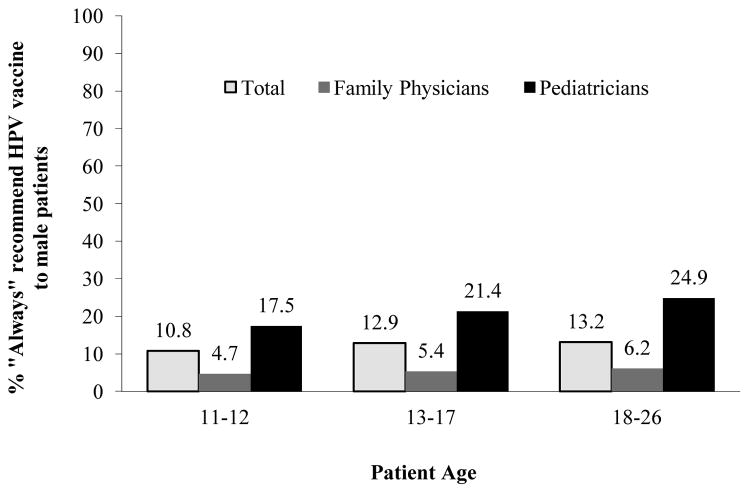
The percentage of physicians reporting they “always” recommended HPV vaccination to males when permissive guidelines were in effect was 10.8% for ages 11–12, 12.9% for ages 13–17, and 13.2% for ages 18–26. Compared to FPs, a higher percentage of Peds reported consistent recommendation for all age groups (Figure 1). In bivariate analyses, the following variables were associated with “always” recommending HPV vaccine across all age groups: Ped specialty; VFC program participation; seeing primarily patients of a race other than White; number of strategies used to get patients in for the first dose and to ensure vaccine completion; being among the first to use a new vaccine; and not waiting to adopt new medications, vaccines, or procedures (Table 2). Physicians of a race other than White, who practiced in a multispecialty or “other” practice environment, who did not refer underinsured patients for HPV vaccination, and with higher HPV-related knowledge also were more likely to consistently recommend HPV vaccination to ages 11–12 and 13–17. Variables associated with vaccine recommendation to only one age group included northeast practice region (11–12), patient payment method (11–12), perceived barriers to HPV vaccination (11–12), and not referring uninsured patients for HPV vaccination (13–17).
Figure 1.
Percentage of physicians “always”a recommended HPV vaccine to males, by physician specialty and patient age groupb
a Defined as >75% of the time.
b Note: In 2011, physicians seeing 11–12 year olds n = 648; 13–17 year olds n = 658; 18–26 year olds n = 597.
Table 2.
Physician demographic, practice, and patient characteristics; knowledge; perceived barriers; and vaccine practices associated with HPV vaccine recommendation (“always” vs. other) by patient age group, 2011
| Ages 11–12 (n = 648)a | Ages 13–17 (n = 658)a | Ages 18–26 (n = 597)a | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | OR (95% CI) | n | OR (95% CI) | n | OR (95% CI) | |
| Demographic characteristics | ||||||
| Provider specialty | ||||||
| Family Medicine | 340 | Reference | 350 | Reference | 372 | Reference |
| Pediatrics | 308 | 4.3 (2.41–7.70)* | 308 | 4.8 (2.78–8.13)* | 225 | 5.0 (2.99–8.45)* |
| Age (yr) | ||||||
| 50+ | 269 | Reference | 274 | Reference | 257 | Reference |
| 40–49 | 237 | 0.6 (0.36–1.14) | 240 | 0.8 (0.45–1.29) | 214 | 0.8 (0.47–1.36) |
| 30–39 | 125 | 0.9 (0.45–1.69) | 126 | 0.9 (0.51–1.74) | 112 | 0.8 (0.43–1.59) |
| Gender | ||||||
| Male | 319 | Reference | 324 | Reference | 314 | Reference |
| Female | 325 | 1.3 (0.77–2.09) | 330 | 1.5 (0.93–2.34) | 279 | 1.4 (0.88–2.26) |
| Race | ||||||
| White/Caucasian | 463 | Reference | 469 | Reference | 440 | Reference |
| Other | 176 | 2.0 (1.19–3.36)* | 180 | 1.9 (1.17–3.03)* | 150 | 1.3 (0.75–2.15) |
| Ethnicity | ||||||
| Not Hispanic or Latino | 598 | Reference | 606 | Reference | 552 | Reference |
| Hispanic or Latino | 32 | 1.2 (0.40–3.45) | 34 | 0.9 (0.30–2.55) | 27 | 0.8 (0.23–2.71) |
| Practice characteristics | ||||||
| No. of physicians | ||||||
| 2–15 | 461 | Reference | 466 | Reference | 417 | Reference |
| 1 | 115 | 1.1 (0.59–2.15) | 118 | 0.8 (0.43–1.52) | 112 | 0.7 (0.34–1.31) |
| 16+ | 65 | 1.4 (0.66–3.05) | 67 | 1.0 (0.48–2.14) | 62 | 1.0 (0.48–2.20) |
| No. of specialties | ||||||
| Single | 470 | Reference | 476 | Reference | 437 | Reference |
| Multiple | 155 | 1.9 (1.08–3.24)* | 159 | 1.7 (1.03–2.83)* | 140 | 1.0 (0.56–1.74) |
| Other | 17 | 5.9 (2.06–16.70)* | 17 | 4.5 (1.61–12.81)* | 13 | 3.0 (0.88–9.94) |
| Type | ||||||
| Other | 175 | Reference | 178 | Reference | 162 | Reference |
| Private practice | 466 | 0.7 (0.40–1.14) | 473 | 0.8 (0.47–1.26) | 428 | 1.0 (0.58–1.66) |
| Arrangement | ||||||
| Other | 330 | Reference | 337 | Reference | 304 | Reference |
| Full/part-owner physician practice | 309 | 1.0 (0.63–1.72) | 312 | 1.1 (0.69–1.73) | 286 | 1.3 (0.82–2.12) |
| No. of patients/day | ||||||
| 30+ | 114 | Reference | 115 | Reference | 103 | Reference |
| 20–29 | 351 | 0.7 (0.36–1.31) | 357 | 0.8 (0.42–1.41) | 315 | 0.9 (0.49–1.75) |
| 0–19 | 178 | 0.9 (0.44–1.79) | 181 | 0.9 (0.45–1.72) | 175 | 0.8 (0.37–1.55) |
| Location | ||||||
| Suburban | 347 | Reference | 353 | Reference | 324 | Reference |
| Urban | 164 | 1.6 (0.88–2.82) | 167 | 1.4 (0.81–2.36) | 149 | 1.2 (0.70–2.16) |
| Rural | 115 | 1.6 (0.87–3.14) | 116 | 1.4 (0.79–2.62) | 104 | 1.2 (0.63–2.27) |
| Other | 8 | 1.5 (0.17–12.22) | 8 | 1.1 (0.13–9.32) | 6 | 0.0 (0.00-∞) |
| Region | ||||||
| West | 131 | Reference | 133 | Reference | 118 | Reference |
| Northeast | 136 | 0.4 (0.16–0.94)* | 141 | 0.7 (0.32–1.38) | 132 | 0.8 (0.37–1.58) |
| Midwest | 159 | 0.8 (0.37–1.53) | 159 | 0.9 (0.44–1.70) | 149 | 1.0 (0.49–1.89) |
| South | 210 | 0.9 (0.49–1.76) | 213 | 1.1 (0.57–1.96) | 186 | 0.8 (0.40–1.53) |
| VFC provider | ||||||
| No | 162 | Reference | 167 | Reference | 170 | Reference |
| Yes | 416 | 2.1 (1.07–4.11)* | 417 | 2.1 (1.14–3.84)* | 350 | 2.4 (1.28–4.34)* |
| Don’t know | 60 | 0.7 (0.20–2.69) | 64 | 0.5 (0.15–1.94) | 67 | 0.7 (0.22–2.23) |
| Refer uninsured patients for vaccine | ||||||
| No or N/A | 383 | Reference | 388 | Reference | 336 | Reference |
| Yes, to federally qualified health center/health department/other | 265 | 0.6 (0.34–1.00) | 270 | 0.5 (0.32–0.86)* | 261 | 0.8 (0.47–1.24) |
| Refer underinsured patients for vaccine | ||||||
| No or N/A | 381 | Reference | 386 | Reference | 336 | Reference |
| Yes, to federally qualified health center/health department/other | 267 | 0.6 (0.34–0.99)* | 272 | 0.5 (0.29–0.80)* | 261 | 0.9 (0.57–1.47) |
| Patient characteristics | ||||||
| Patient payment method | ||||||
| Private insurance | ||||||
| 51–100% of patients | 317 | Reference | 326 | Reference | 300 | Reference |
| 0–50% of patients | 309 | 1.8 (1.06–3.02)* | 309 | 1.6 (0.96–2.50) | 278 | 1.2 (0.76–2.00) |
| Patient race (majority) | ||||||
| Non-Hispanic White | 454 | Reference | 458 | Reference | 429 | Reference |
| Other | 187 | 2.6 (1.56–4.27)* | 193 | 2.3 (1.42–3.61)* | 161 | 1.9 (1.16–3.10)* |
| HPV knowledge | ||||||
| Higher (8–10 correct) | 381 | Reference | 384 | Reference | 350 | Reference |
| Lower (0–7 correct) | 264 | 0.6 (0.33–0.98)* | 271 | 0.6 (0.37–0.99)* | 244 | 0.6 (0.37–1.02) |
| Perceived barriers related to HPV vaccination | ||||||
| Overall | ||||||
| High | 225 | Reference | 231 | Reference | 211 | Reference |
| Medium | 211 | 2.0 (1.05–3.81)* | 215 | 1.7 (0.97–3.14) | 203 | 1.5 (0.83–2.74) |
| Low | 205 | 1.8 (0.94–3.50) | 206 | 1.7 (0.94–3.08) | 179 | 1.7 (0.92–3.07) |
| Vaccine practices | ||||||
| Strategies for HPV vaccine initiation | ||||||
| 2+ | 198 | Reference | 204 | Reference | 167 | Reference |
| 1 | 163 | 0.8 (0.43–1.49) | 163 | 0.8 (0.43–1.36) | 145 | 0.8 (0.43–1.38) |
| 0 | 280 | 0.5 (0.30–0.98)* | 284 | 0.5 (0.27–0.81)* | 279 | 0.4 (0.21–0.65)* |
| Strategies to ensure HPV vaccine completion | ||||||
| 2+ | 393 | Reference | 400 | Reference | 348 | Reference |
| 1 | 152 | 1.0 (0.58–1.81) | 153 | 1.0 (0.57–1.65) | 142 | 1.0 (0.58–1.75) |
| 0 | 96 | 0.2 (0.07–0.76)* | 98 | 0.2 (0.09–0.69)* | 101 | 0.4 (0.19–0.99)* |
| Early vs. late adopter | ||||||
| First to use a new vaccine | ||||||
| Disagree | 145 | Reference | 148 | Reference | 146 | Reference |
| Neutral | 231 | 2.3 (0.73–7.05) | 236 | 2.6 (0.97–7.22) | 211 | 2.7 (1.07–6.88)* |
| Agree | 267 | 8.5 (3.02–24.09)* | 269 | 8.0 (3.15–20.51)* | 235 | 6.3 (2.63–15.13)* |
| Wait to adopt | ||||||
| Agree | 268 | Reference | 272 | Reference | 253 | Reference |
| Neutral | 175 | 0.7 (0.34–1.51) | 178 | 0.9 (0.47–1.66) | 156 | 1.0 (0.50–1.84) |
| Disagree | 201 | 2.3 (1.33–4.07)* | 204 | 1.9 (1.14–3.23)* | 184 | 2.0 (1.14–3.38)* |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; OR, odds ratio; VFC, Vaccines for Children.
Number of physicians who see male patients in the age group.
Statistically significant variable included in the multivariable model.
Note: Rows containing statistically significant variables are shaded for ease of viewing patterns across age groups.
In multivariable analysis, two factors were associated with “always” recommending vaccination for all three patient age groups while adjusting for other factors: Peds (vs. FPs) and agreeing (vs. disagreeing) that one was among the first to use a newly recommended vaccine compared to clinical peers (Table 3). Two factors were independently associated with “always” recommending vaccination for males ages 11–12: physicians who reported a race other than White (vs. White) and physicians who reported that 0–50% (vs. 51–100%) of their patients used private insurance as their primary payment method. Finally, one factor was significantly associated with “always” recommending vaccination for males ages 13–17 and 18–26: physicians who reported their patients were primarily of a race other than White (vs. non-Hispanic White).
Table 3.
Multivariable logistic regression analysis of factors associated with HPV vaccine recommendation (“always” vs. other)a by patient age group, 2011
| Predictor | Patient Age Group Adjusted OR (95% CI) |
||
|---|---|---|---|
| 11–12 (n = 581) | 13–17 (n = 619) | 18–26 (n = 571) | |
| Specialty | |||
| Family Physician | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| Pediatrician | 3.5 (1.82–6.74) | 3.5 (1.95–6.23) | 3.8 (2.23–6.50) |
| First to use a new vaccine | |||
| Disagree | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| Neutral | 2.0 (0.55–7.49) | 2.3 (0.74–6.92) | 2.1 (0.81–5.38) |
| Agree | 6.9 (2.05–23.06) | 6.4 (2.21–18.24) | 4.2 (1.72–10.37) |
| Physician race | |||
| White/Caucasian | 1.0 (Reference) | – | – |
| Other | 1.9 (1.06–3.46) | ||
| Patient payment method, private insurance | |||
| 51–100% of patients | 1.0 (Reference) | – | – |
| 0–50% of patients | 2.1 (1.18–3.81) | ||
| Patient race (majority) | |||
| Non-Hispanic White | – | 1.0 (Reference) | 1.0 (Reference) |
| Other | 2.0 (1.17–3.28) | 1.8 (1.06–3.04) | |
Abbreviations: CI, confidence interval; OR, odds ratio.
Note: Variables were selected through backward selection at the level of stay alpha = 0.05.
Discussion
Physicians’ recommendations appear to be one of the most effective methods for increasing HPV vaccine uptake (30–32), yet little research (26) has been conducted to examine primary care physicians’ HPV vaccine recommendations to male patients. The current study offers insight into physician practices while HPV vaccine was available, but not universally recommended, for males. Findings suggest that most FPs and Peds did not consistently recommend HPV vaccination to males during the ACIP’s permissive recommendation period. Results from this study highlight key variables associated with physicians’ HPV vaccine recommendation practices while permissive guidelines were in effect.
Physician clinical specialty and early adoption of new vaccines were associated with “always” recommending HPV vaccine across all three patient age groups. Consistent with prior research on HPV vaccination for females (24, 25), Peds were more likely than FPs to recommend HPV vaccination to males. If this finding persists following the ACIP’s routine recommendation for males, then this is concerning given that many adolescent males may transition to the care of FPs as they move through adolescence; therefore, FPs could be critical for providing information about and recommending vaccination (33).
In accordance with the Diffusion of Innovations theory (23), physicians who self-identified as being among the first to use a newly recommended vaccine (i.e., innovators/early adopters) were more likely to “always” recommend HPV vaccine when permissive guidelines were in effect compared to later adopters. This finding parallels previous research reporting that physicians who were late adopters of new drugs/vaccines or technologies were less likely to give HPV vaccine to all eligible patients (34) or offer HPV vaccine (35). Innovators/early adopters have more favorable attitudes toward change (23) and are likely more knowledgeable about innovations. Innovators/early adopters in our study may have been routinely offering HPV vaccine to males despite only permissive guidance from the ACIP because they may have been more knowledgeable about promising results from clinical trials of HPV vaccine in males (16) and anticipated expanded ACIP guidelines. Innovators/early adopters also may be more tolerant of and have the resources to support risk (36), such as financial resources to accept the risk of not receiving reimbursement for vaccinating male patients. Identifying and supporting innovators/early adopters may facilitate diffusion of male HPV vaccination given these physicians are watched by colleagues as they test evidence-based changes (36) (e.g., feasibility of implementing the new guidelines in clinical practice) and could influence other physicians’ support (e.g., strength of recommendation) for HPV vaccination.
In addition to physician clinical specialty and early adoption of new vaccines, patient payer status appears to be associated with physicians’ HPV vaccine recommendations to males. In this study, physicians whose patients primarily used payment methods other than private insurance (e.g., Medicaid, uninsured/self-pay) were more likely to recommend HPV vaccine to males ages 11–12 years. These patients likely were eligible for free HPV vaccine, even during the permissive recommendation period, through the federal VFC program (13, 14). Accordingly, more VFC-eligible patients may have had financial coverage for the vaccine compared to patients with private insurance and thus providers were more likely to “always” recommend vaccination to these patients. However, broader HPV vaccine coverage is necessary to help ensure that physicians’ HPV vaccine recommendations are aligned with ACIP guidelines and not contingent upon patient payer status.
Along with patient payment method, physician and patient race/ethnicity were linked to physicians’ HPV vaccine recommendations. HPV-associated anal cancer rates are higher for Black men compared to White men (37) and sexually transmitted infection rates tend to be higher in minority populations (38). Physicians’ HPV vaccine recommendation practices may have reflected their recognition of and desire to reduce these disparities, particularly among physicians seeing a patient population that is not non-Hispanic White. More research is needed to understand the role of race/ethnicity in physicians’ HPV vaccine recommendations.
Given that physicians’ perceived barriers to immunizing patients was statistically significantly associated with HPV vaccine recommendations for females (24, 25), an interesting finding was the lack of a significant association between perceived barriers and HPV vaccine recommendations for male patients. One possible explanation for this result is the absence of male-specific barrier items and the timing of survey. The assessment of physicians’ barriers related to immunizing patients against HPV was neutral with regard to patient sex and, because the ACIP guidelines for males were permissive at the time of the survey, physicians may have responded to the barrier items with their female patients in mind. More recent assessments of barriers to HPV vaccination suggest there are male-specific barriers (39), such as lack of office visits by adolescent male patients (26) and physicians’ belief that vaccinating males is not worth the cost or effort (40). Future assessments of physicians’ barriers to immunizing patients against HPV should include these barriers.
This study has notable strengths, including the use of nationally representative samples of physicians most likely to be involved in male HPV vaccination and an overall response rate exceeding 60%. Despite its strengths, there are several limitations. First, the data may be subject to recall bias; however, self-reported measures are necessary to assess physicians’ recommendation practices given that recommendation may be inconsistently documented in medical records and cannot be assessed using claims data. Similarly, physicians may have reported socially desirable responses regarding practice behaviors, although we attempted to minimize socially desirable responses through an anonymous survey. Finally, although our response rate exceeds that of other studies of U.S. physicians pertaining to HPV vaccination (22, 41, 42), the responders to our survey may have had stronger opinions about HPV vaccination or more time to devote to taking the survey than non-responders. This issue may be particularly relevant for FPs, whose response rates were lower than Peds.
Conclusions and Future Directions
This paper provides a unique glimpse into physicians’ HPV vaccine recommendations early in the dissemination of the vaccine for male patients. Overall rates of recommendation were low, and possibly influenced by the permissive nature of the ACIP recommendation. However, the study results largely mirror previous studies of recommendations to females (24, 25) demonstrating the strong association between physician recommendation and specialty. In addition, recommendation to males was associated with self-identification as an innovator/early adopter of new vaccines. An important next step is to examine whether physicians’ recommendation rates have improved now that the vaccine is fully recommended by the ACIP and thus is more likely to be covered by private insurance. If these same factors continue to be influential in predicting recommendation practices, then public health interventions designed to increase HPV vaccine recommendations for males should focus on increasing recommendations by FPs, who likely assume care of adolescent males as they transition from pediatric settings. Additionally, identifying and supporting innovators/early adopters may help diffuse HPV vaccination guidelines and encourage later adopters to support guidelines.
Acknowledgments
Financial support: This research was supported by a grant from the National Institutes of Health (R01AI076440-01; awarded to S. T. Vadaparampil).
The work contained within this publication was supported in part by the Survey Methods Core Facility at the H. Lee Moffitt Cancer Center & Research Institute.
Footnotes
Conflict of Interest: Regarding potential conflicts of interest, Dr. Giuliano has received funding from Merck for consultancy and lectures and receives grant funding from Merck and GlaxoSmithKline. Dr. Kahn is co-chair of two NIH-funded HPV vaccine trials in HIV-infected youth for which she does not receive salary support, but Merck provides vaccine and immunogenicity testing for both trials. Dr. Zimet has received investigator initiated grants from Merck, has served as a consultant to Sanofi Pasteur, and has received unrestricted program development funding from GlaxoSmithKline. All other authors report no conflicts.
References
- 1.Li D, Li H, Wan H, Chen J, Gong G, Wang H, et al. Multiscale registration of medical images based on edge preserving scale space with application in image-guided radiation therapy. Phys Med Biol. 2012;57:5187–204. doi: 10.1088/0031-9155/57/16/5187. [DOI] [PubMed] [Google Scholar]
- 2.Hartwig S, Syrjanen S, Dominiak-Felden G, Brotons M, Castellsague X. Estimation of the epidemiological burden of human papillomavirus-related cancers and non-malignant diseases in men in Europe: a review. BMC Cancer. 2012;12:30. doi: 10.1186/1471-2407-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Jr, Aranda C, Jessen H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. New Engl J Med. 2011;365:1576–85. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 4.Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Jr, Penny ME, Aranda C, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. New Engl J Med. 2011;364:401–11. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113:3036–46. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seto K, Marra F, Raymakers A, Marra CA. The cost effectiveness of human papillomavirus vaccines: a systematic review. Drugs. 2012;72:715–43. doi: 10.2165/11599470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed F, Temte JL, Campos-Outcalt D, Schunemann HJ. Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC) Vaccine. 2011;29:9171–6. doi: 10.1016/j.vaccine.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Akinsanya-Beysolow I Advisory Committee on Immunization Practices (ACIP), ACIP Child/Adolescent Immunization Work Group, Centers for Disease Control and Prevention (CDC). . Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 0 through 18 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:108–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Iowa Department of Public Health. Immunization Program, Vaccines for Children Program, Human Papillomavirus (HPV) Vaccine. [cited 2014 Mar 28]. Available from: http://www.idph.state.ia.us/adper/common/pdf/immunization/vfc_hpv_jan2010.pdf.
- 10.American Academy of Pediatrics. [accessed 2014 Jun 10];AAP Private Payer Advocacy Update. Available from: http://www.aapca3.org/law/ppa/ppa1010.pdf.
- 11.Centers for Disease Control and Prevention. [accessed 2014 Jun 10];VFC Eligibility Criteria. Available from: http://www.cdc.gov/vaccines/programs/vfc/providers/eligibility.html.
- 12.Centers for Disease Control and Prevention. [accessed 2014 Jun 10];VFC Detailed Questions and Answers for Parents. Available from: http://www.cdc.gov/vaccines/programs/vfc/parents/qa-detailed.html#f1.
- 13.Centers for Disease Control and Prevention. [accessed 2014 Jun 10];ACIP recommends all 11–12 year-old males get vaccinated against HPV. Available from: http://www.cdc.gov/media/releases/2011/t1025_hpv_12yroldvaccine.html.
- 14.Wells K, Zucker JR, Hannan C. The Impact of ACIP HPV Vaccine Permissive Recommendation on State, Local and Territorial Immunization Programs. 45th National Immunization Conference; Washington, D.C. 2011. [Google Scholar]
- 15.Centers for Disease Control and Prevention. [accessed 2014 Jun 10];Immunization Program Operations Manual (IPOM) Available from: http://www.cdc.gov/vaccines/imz-managers/guides-pubs/ipom/basics.html.
- 16.Centers for Disease Control and Prevention. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:630–2. [PubMed] [Google Scholar]
- 17.Daley M, Liddon N, Crane L, Beaty B, Barrow J, Babbel C, et al. A national survey of pediatrician knowledge and attitudes regarding human papilloma virus vaccination. Pediatrics. 2006;118:2280–9. doi: 10.1542/peds.2006-1946. [DOI] [PubMed] [Google Scholar]
- 18.Weiss TW, Zimet GD, Rosenthal SL, Brenneman SK, Klein JD. Human papillomavirus vaccination of males: attitudes and perceptions of physicians who vaccinate females. J Adolesc Health. 2010;47:3–11. doi: 10.1016/j.jadohealth.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Kahn JA, Zimet GD, Bernstein DI, Riedesel JM, Lan D, Huang B, et al. Pediatricians’ intention to administer human papillomavirus vaccine: the role of practice characteristics, knowledge, and attitudes. J Adolesc Health. 2005;37:502–10. doi: 10.1016/j.jadohealth.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Kahn JA, Rosenthal SL, Tissot AM, Bernstein DI, Wetzel C, Zimet GD. Factors influencing pediatricians’ intention to recommend human papillomavirus vaccines. Ambul Pediatr. 2007;7:367–73. doi: 10.1016/j.ambp.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Riedesel JM, Rosenthal SL, Zimet GD, Bernstein DI, Huang B, Lan D, et al. Attitudes about human papillomavirus vaccine among family physicians. J Pediatr Adolesc Gynecol. 2005;18:391–8. doi: 10.1016/j.jpag.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Kahn JA, Cooper HP, Vadaparampil ST, Pence BC, Weinberg AD, LoCoco SJ, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: a statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev. 2009;18:2325–32. doi: 10.1158/1055-9965.EPI-09-0184. [DOI] [PubMed] [Google Scholar]
- 23.Rogers EM. Diffusion of innovations. New York: Free Press; 2003. [Google Scholar]
- 24.Vadaparampil ST, Kahn JA, Salmon D, Lee JH, Quinn GP, Roetzheim R, et al. Missed clinical opportunities: Provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine. 2011;29:8634–41. doi: 10.1016/j.vaccine.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vadaparampil ST, Malo TL, Kahn JA, Salmon DA, Lee JH, Quinn GP, et al. Physicians’ human papillomavirus vaccine recommendations, 2009 and 2011. Am J Prev Med. 2014;46:80–4. doi: 10.1016/j.amepre.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allison MA, Dunne EF, Markowitz LE, O’Leary ST, Crane LA, Hurley LP, et al. HPV vaccination of boys in primary care practices. Acad Pediatr. 2013;13:466–74. doi: 10.1016/j.acap.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaen CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166–71. [PubMed] [Google Scholar]
- 28.Dillman D. Mail and internet surveys: the tailored design method. New York: Wiley; 2000. [Google Scholar]
- 29.Smart DR American Medical Association. Physician characteristics and distribution in the US. Chicago: American Medical Association; 2009. [Google Scholar]
- 30.Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US National Immunization Survey. Am J Public Health. 2013;103:164–9. doi: 10.2105/AJPH.2011.300600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau M, Lin H, Flores G. Factors associated with human papillomavirus vaccine-series initiation and healthcare provider recommendation in US adolescent females: 2007 National Survey of Children’s Health. Vaccine. 2012;30:3112. doi: 10.1016/j.vaccine.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 32.Kramer MR, Dunlop AL. Inter-state variation in human papilloma virus vaccine coverage among adolescent girls in the 50 US states, 2007. Matern Child Health J. 2012;16 (Suppl 1):S102–10. doi: 10.1007/s10995-012-0999-6. [DOI] [PubMed] [Google Scholar]
- 33.Rand CM, Shone LP, Albertin C, Auinger P, Klein JD, Szilagyi PG. National health care visit patterns of adolescents: Implications for delivery of new adolescent vaccines. Arch Ped Adolesc Med. 2007;161:252–9. doi: 10.1001/archpedi.161.3.252. [DOI] [PubMed] [Google Scholar]
- 34.Ishibashi KL, Koopmans J, Curlin FA, Alexander KA, Ross LF. Paediatricians’ attitudes and practices towards HPV vaccination. Acta Paediatr. 2008;97:1550–6. doi: 10.1111/j.1651-2227.2008.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young JL, Bernheim RG, Korte JE, Stoler MH, Guterbock TM, Rice LW. Human papillomavirus vaccination recommendation may be linked to reimbursement: a survey of Virginia family practitioners and gynecologists. J Pediatr Adolesc Gynecol. 2011;24:380–5. doi: 10.1016/j.jpag.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Berwick DM. Disseminating innovations in health care. JAMA. 2003;289:1969–75. doi: 10.1001/jama.289.15.1969. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. [accessed 2012 Dec 21];HPV-Associated Anal Cancer Rates by Race and Ethnicity. Available from: http://www.cdc.gov/cancer/hpv/statistics/anal.htm.
- 38.Centers for Disease Control and Prevention. [accessed 2012 Dec 21];Sexually Transmitted Disease Sureveillance. 2010 Available from: http://www.cdc.gov/std/stats10/surv2010.pdf.
- 39.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168:76–82. doi: 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perkins RB, Clark JA. Providers’ attitudes toward human papillomavirus vaccination in young men: challenges for implementation of 2011 recommendations. Am J Mens Health. 2012;6:320–3. doi: 10.1177/1557988312438911. [DOI] [PubMed] [Google Scholar]
- 41.Krieger JL, Katz ML, Kam JA, Roberto A. Appalachian and non-Appalachian pediatricians’ encouragement of the human papillomavirus vaccine: implications for health disparities. Women’s Health Issues. 2012;22:e19–e26. doi: 10.1016/j.whi.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberto AJ, Krieger JL, Katz ML, Goei R, Jain P. Predicting pediatricians’ communication with parents about the human papillomavirus (hpv) vaccine: an application of the theory of reasoned action. Health Commun. 2011;26:303–12. doi: 10.1080/10410236.2010.550021. [DOI] [PMC free article] [PubMed] [Google Scholar]