Abstract
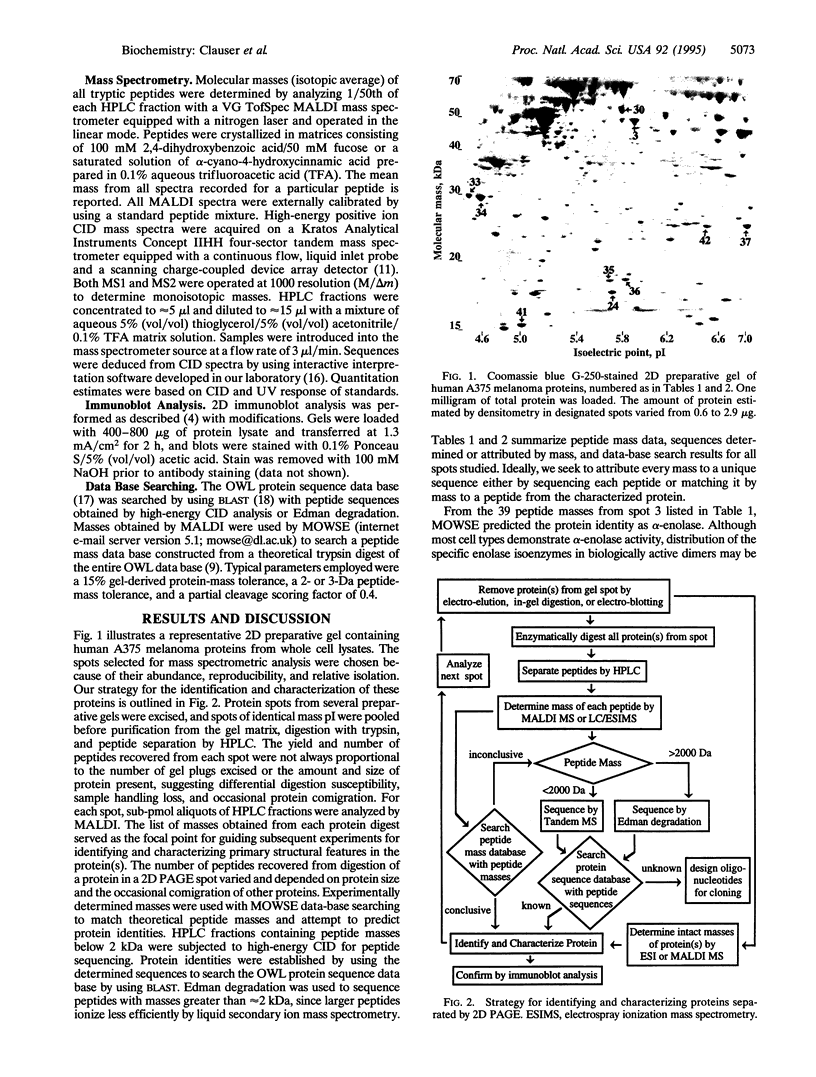
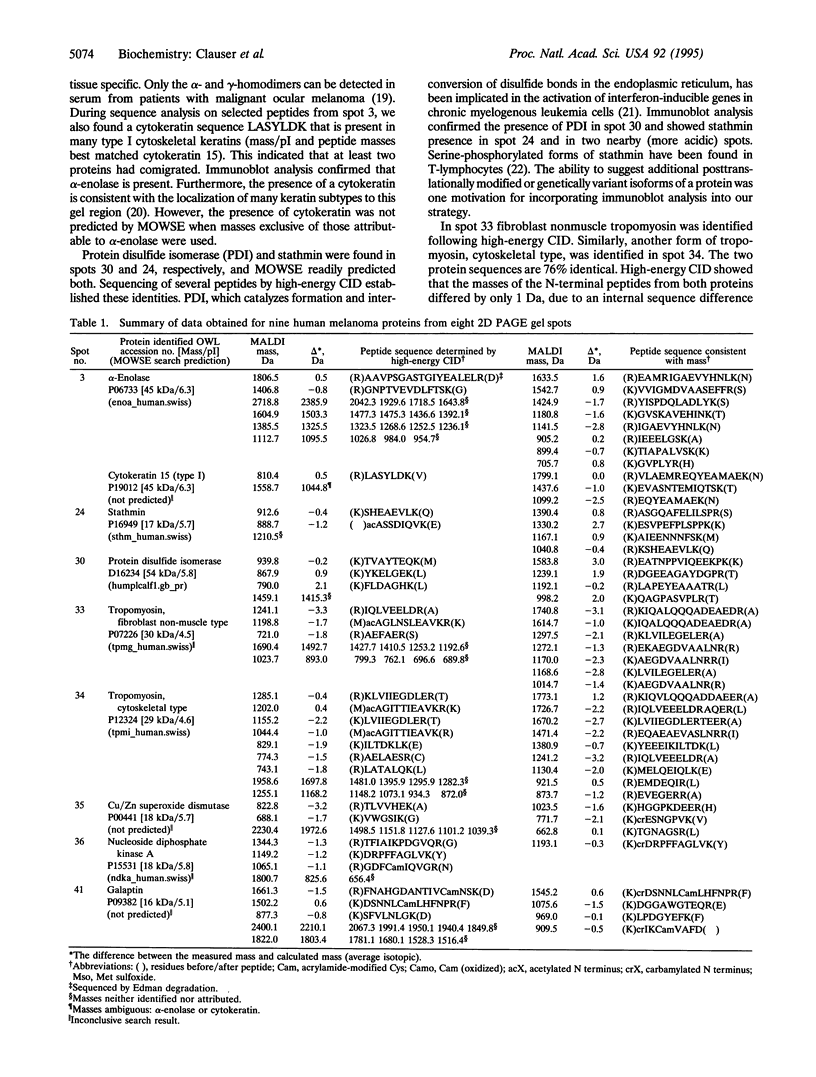
We report a general mass spectrometric approach for the rapid identification and characterization of proteins isolated by preparative two-dimensional polyacrylamide gel electrophoresis. This method possesses the inherent power to detect and structurally characterize covalent modifications. Absolute sensitivities of matrix-assisted laser desorption ionization and high-energy collision-induced dissociation tandem mass spectrometry are exploited to determine the mass and sequence of subpicomole sample quantities of tryptic peptides. These data permit mass matching and sequence homology searching of computerized peptide mass and protein sequence data bases for known proteins and design of oligonucleotide probes for cloning unknown proteins. We have identified 11 proteins in lysates of human A375 melanoma cells, including: alpha-enolase, cytokeratin, stathmin, protein disulfide isomerase, tropomyosin, Cu/Zn superoxide dismutase, nucleoside diphosphate kinase A, galaptin, and triosephosphate isomerase. We have characterized several posttranslational modifications and chemical modifications that may result from electrophoresis or subsequent sample processing steps. Detection of comigrating and covalently modified proteins illustrates the necessity of peptide sequencing and the advantages of tandem mass spectrometry to reliably and unambiguously establish the identity of each protein. This technology paves the way for studies of cell-type dependent gene expression and studies of large suites of cellular proteins with unprecedented speed and rigor to provide information complementary to the ongoing Human Genome Project.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Biemann K. Sequencing of peptides by tandem mass spectrometry and high-energy collision-induced dissociation. Methods Enzymol. 1990;193:455–479. doi: 10.1016/0076-6879(90)93433-l. [DOI] [PubMed] [Google Scholar]
- Bleasby A. J., Wootton J. C. Construction of validated, non-redundant composite protein sequence databases. Protein Eng. 1990 Jan;3(3):153–159. doi: 10.1093/protein/3.3.153. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Rasmussen H. H., Olsen E., Madsen P., Leffers H., Honoré B., Dejgaard K., Gromov P., Hoffmann H. J., Nielsen M. The human keratinocyte two-dimensional gel protein database: update 1993. Electrophoresis. 1993 Nov;14(11):1091–1198. doi: 10.1002/elps.11501401178. [DOI] [PubMed] [Google Scholar]
- Chait B. T., Kent S. B. Weighing naked proteins: practical, high-accuracy mass measurement of peptides and proteins. Science. 1992 Sep 25;257(5078):1885–1894. doi: 10.1126/science.1411504. [DOI] [PubMed] [Google Scholar]
- Gracy R. W., Yüksel K. U., Chapman M. L., Dimitrijevich S. D. Isoprotein changes in aging: biochemical basis and physiological consequences. Prog Clin Biol Res. 1990;344:787–817. [PubMed] [Google Scholar]
- Hall S. C., Smith D. M., Masiarz F. R., Soo V. W., Tran H. M., Epstein L. B., Burlingame A. L. Mass spectrometric and Edman sequencing of lipocortin I isolated by two-dimensional SDS/PAGE of human melanoma lysates. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1927–1931. doi: 10.1073/pnas.90.5.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzel W. J., Billeci T. M., Stults J. T., Wong S. C., Grimley C., Watanabe C. Identifying proteins from two-dimensional gels by molecular mass searching of peptide fragments in protein sequence databases. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5011–5015. doi: 10.1073/pnas.90.11.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H., Whitehead R. H., Reid G. E., Moritz R. L., Ward L. D., Simpson R. J. Two-dimensional electrophoretic analysis of proteins expressed by normal and cancerous human crypts: application of mass spectrometry to peptide-mass fingerprinting. Electrophoresis. 1994 Mar-Apr;15(3-4):391–405. doi: 10.1002/elps.1150150158. [DOI] [PubMed] [Google Scholar]
- Johnson E., Henzel W., Deisseroth A. An isoform of protein disulfide isomerase isolated from chronic myelogenous leukemia cells alters complex formation between nuclear proteins and regulatory regions of interferon-inducible genes. J Biol Chem. 1992 Jul 15;267(20):14412–14417. [PubMed] [Google Scholar]
- Lees-Miller J. P., Helfman D. M. The molecular basis for tropomyosin isoform diversity. Bioessays. 1991 Sep;13(9):429–437. doi: 10.1002/bies.950130902. [DOI] [PubMed] [Google Scholar]
- Leone A., Flatow U., King C. R., Sandeen M. A., Margulies I. M., Liotta L. A., Steeg P. S. Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell. 1991 Apr 5;65(1):25–35. doi: 10.1016/0092-8674(91)90404-m. [DOI] [PubMed] [Google Scholar]
- Mann M., Højrup P., Roepstorff P. Use of mass spectrometric molecular weight information to identify proteins in sequence databases. Biol Mass Spectrom. 1993 Jun;22(6):338–345. doi: 10.1002/bms.1200220605. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pappin D. J., Hojrup P., Bleasby A. J. Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol. 1993 Jun 1;3(6):327–332. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
- Patton W. F., Pluskal M. G., Skea W. M., Buecker J. L., Lopez M. F., Zimmermann R., Belanger L. M., Hatch P. D. Development of a dedicated two-dimensional gel electrophoresis system that provides optimal pattern reproducibility and polypeptide resolution. Biotechniques. 1990 May;8(5):518–527. [PubMed] [Google Scholar]
- Rasmussen H. H., Mørtz E., Mann M., Roepstorff P., Celis J. E. Identification of transformation sensitive proteins recorded in human two-dimensional gel protein databases by mass spectrometric peptide mapping alone and in combination with microsequencing. Electrophoresis. 1994 Mar-Apr;15(3-4):406–416. doi: 10.1002/elps.1150150159. [DOI] [PubMed] [Google Scholar]
- Rosenfeld J., Capdevielle J., Guillemot J. C., Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem. 1992 May 15;203(1):173–179. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- Santa Lucia P., Wilson B. D., Allen H. J. Localization of endogenous beta-galactoside-binding lectin as a means to distinguish malignant from benign skin tissue. J Dermatol Surg Oncol. 1991 Aug;17(8):653–655. doi: 10.1111/j.1524-4725.1991.tb01314.x. [DOI] [PubMed] [Google Scholar]
- Shine B. S., Hungerford J., Vaghela B., Sheraidah G. A. Electrophoretic assessment of aqueous and serum neurone-specific enolase in retinoblastoma and ocular malignant melanoma. Br J Ophthalmol. 1990 Jul;74(7):427–430. doi: 10.1136/bjo.74.7.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahler J. R., Hailat N., Lamb B. J., Rogers K. P., Underhill J. A., Melhem R. F., Keim D. R., Zhu X., Kuick R. D., Fox D. A. Activation of resting peripheral blood lymphocytes through the T cell receptor induces rapid phosphorylation of Op18. J Immunol. 1992 Aug 15;149(4):1191–1198. [PubMed] [Google Scholar]
- Sun T. T., Tseng S. C., Huang A. J., Cooper D., Schermer A., Lynch M. H., Weiss R., Eichner R. Monoclonal antibody studies of mammalian epithelial keratins: a review. Ann N Y Acad Sci. 1985;455:307–329. doi: 10.1111/j.1749-6632.1985.tb50419.x. [DOI] [PubMed] [Google Scholar]
- Toussi A., Paquet N., Huber O., Frutiger S., Tissot J. D., Hughes G. J., Hochstrasser D. F. Polypeptide marker and disease patterns found while mapping proteins in ascitis. J Chromatogr. 1992 Nov 6;582(1-2):87–92. doi: 10.1016/0378-4347(92)80306-b. [DOI] [PubMed] [Google Scholar]




