Abstract
Purpose
Immunomodulatory drugs differ in mechanism-of-action from directly cytotoxic cancer therapies. Identifying factors predicting clinical response could guide patient selection and therapeutic optimization.
Experimental Design
Patients (N=41) with melanoma, non-small cell lung carcinoma (NSCLC), renal cell carcinoma (RCC), colorectal carcinoma or castration-resistant prostate cancer were treated on an early phase trial of anti-PD-1 (nivolumab) at one institution and had evaluable pre-treatment tumor specimens. Immunoarchitectural features including PD-1, PD-L1, and PD-L2 expression, patterns of immune cell infiltration, and lymphocyte subpopulations, were assessed for interrelationships and potential correlations with clinical outcomes.
Results
Membranous (cell surface) PD-L1 expression by tumor cells and immune infiltrates varied significantly by tumor type and was most abundant in melanoma, NSCLC, and RCC. In the overall cohort, PD-L1 expression was geographically associated with infiltrating immune cells (p<0.001), although lymphocyte-rich regions were not always associated with PD-L1 expression. Expression of PD-L1 by tumor cells and immune infiltrates was significantly associated with expression of PD-1 on lymphocytes. PD-L2, the second ligand for PD-1, was associated with PD-L1 expression. Tumor cell PD-L1 expression correlated with objective response to anti-PD-1 therapy, when analyzing either the specimen obtained closest to therapy or the highest scoring sample among multiple biopsies from individual patients. These correlations were stronger than borderline associations of PD-1 expression or the presence of intratumoral immune cell infiltrates with response.
Conclusions
Tumor PD-L1 expression reflects an immune-active microenvironment and, while associated other immunosuppressive molecules including PD-1 and PD-L2, is the single factor most closely correlated with response to anti-PD-1 blockade.
Keywords: PD-1, PD-L1, PD-L2, tumor microenvironment, cancer immunotherapy
INTRODUCTION
While there is abundant in vitro evidence for human immune reactivity against solid tumors, such responses are often ineffectual in situ as the tumor exerts locally immunosuppressive effects. Current cancer immunotherapies focus on overcoming this inhibition, either by global activation of the immune system or by local manipulation of immunoregulatory molecules in the tumor microenvironment, including so-called immune checkpoints. Ipilimumab, the prototype drug directed against an immune checkpoint, is a monoclonal antibody (mAb) that blocks the co-inhibitory CTLA-4 receptor on T-cells from interacting with its ligands, B7-1 and B7-2, expressed on antigen presenting cells (APCs) but not on solid tumors. Ipilimumab was the first drug to demonstrate increased overall survival in patients with advanced melanoma(1), and there is ongoing investigation to identify biomarkers to optimize patient selection for this therapy.
Following proof-of-principle studies with anti-CTLA-4, attention has turned to the PD-1:PD-L1/PD-L2 immunologic synapse. Programmed death-1 (PD-1) is expressed on activated T- and B-cells(2). Its major ligand PD-L1 (B7-H1) is typically expressed on a subset of macrophages, but can be induced by inflammatory cytokines in a variety of tissue types(3–7). When activated T-cells expressing PD-1 encounter PD-L1, T-cell effector functions are diminished. PD-1 also binds PD-L2 (B7-DC), which is expressed selectively on macrophages and dendritic cells(7–9). These unique expression patterns suggest that PD-L1 promotes self-tolerance in peripheral tissues, while PD-L2 may function in lymphoid organs, although the role of PD-L2 in immunomodulation is not as well understood(10). Multiple tumor types have been shown to express PD-L1 and PD-L2, effectively co-opting a native tolerance mechanism(11–13). The more selective expression pattern of the ligands for PD-1, relative to those for CTLA-4, has important treatment implications. First, it suggests that more focal immune-related side effects may be encountered with PD-1 blockade compared to CTLA-4 blockade, which is also predicted by the phenotypes of murine genetic knockout models(14, 15). Second, it suggests that the local tumor microenvironment may be the key site to yield evidence of molecular markers predicting clinical response to PD-1 pathway blockade.
Clinical trials of blocking mAbs against PD-1 and PD-L1 are currently underway for patients with treatment-refractory metastatic melanoma and a variety of epithelial malignancies and have validated this pathway as a therapeutic target(16–19). We have previously reported that approximately 30% of patients with treatment-refractory advanced melanoma and renal cell carcinoma (RCC) receiving anti-PD-1(nivolumab) experienced durable objective tumor regressions(16). Objective tumor responses were also observed in 17% of patients with non-small cell lung carcinoma (NSCLC). Preliminary analysis of pre-treatment tumor specimens from a subset of 42 among 306 patients treated on this study identified tumor cell surface PD-L1 expression as one factor associated with the clinical activity of anti-PD-1 therapy(16, 17). The purpose of the current study is to expand on this initial observation by exploring additional components of the pre-treatment tumor microenvironment in patients receiving nivolumab therapy, including infiltrating immune cell subsets, the expression of PD-1, PD-L1, and PD-L2 by immune cells and by tumor cells, and the potential interrelationship of these factors to each other and to clinical response following PD-1 blockade.
METHODS
Case selection
Tumor specimens were derived from 41 patients with advanced, treatment-refractory solid tumors including non-small cell lung cancer, melanoma, kidney, castration-resistant prostate (CRPC), and colorectal cancer (CRC) who were treated on a clinical trial of nivolumab (anti-PD-1; BMS-936558, MDX-1106, ONO-4538) at the Johns Hopkins Kimmel Cancer Center (NCT00730639)(16). All patients signed informed consent. Patients received at least 3 of 4 planned biweekly doses of anti-PD1 in the first treatment cycle, had treatment responses that were evaluable, and had formalin-fixed paraffin-embedded (FFPE) archival or newly obtained pre-treatment tumor specimens available for study. Responses were classified by the investigators according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 with modifications(20). Tumor specimen characteristics including primary or metastatic lesion, anatomic site (lymph node, lung, or other), specimen size and the time interval from specimen acquisition to treatment initiation were noted.
Histopathologic analysis
The pathologic diagnosis was confirmed by one of two board-certified pathologists (JMT or RAA) who reviewed FFPE tissue sections stained with hematoxylin & eosin (H&E), and a representative paraffin block from each specimen was chosen for immunohistochemical (IHC) analysis. The size of the specimen and the presence of necrosis and lymphoid aggregates (collections of approximately 100 lymphocytes without germinal center formation, Supplementary Figure 1) were noted. Specimens were considered “small” if they were core needle biopsies or tissue blocks made from fine needle aspirate specimens containing tissue fragments. Cytology specimens where tissue architecture was not represented were not included in this study. IHC for PD-L1 and PD-1 was performed using the mAbs 5H1 and M3, respectively, as previously described(12, 21). Methods for PD-L2 IHC with the mAb MIH18 (BioLegend, San Diego, CA) are described in the Supplementary Materials. Positive control specimens for PD-L1 IHC were created by transfecting cultured human melanoma 624-mel cells with a recombinant plasmid encoding full length human PD-L1(12). PD-L1 expression was sometimes observed in the background native tissue, e.g., alveolar macrophages in lung specimens and dendritic cells in non-neoplastic lymph node parenchyma from lymph node dissections, providing an internal positive staining control. An isotype control was used as a negative control for each case stained for PD-L1, to control for potential false positive staining. Tonsil tissue served as both a positive and negative control for PD-L2, and PD-1 staining, due to cell type specific endogenous expression of these molecules (dendritic cells and activated lymphocytes, respectively). IHC for CD3, CD4, CD8, CD20, and CD68 was performed according to standard automated protocols.
Cell surface (“membranous”) PD-L1 and PD-L2 expression by tumor cells and tumor infiltrating immune cells including lymphocytes (TILs) and histiocytes was independently scored by two pathologists blinded to clinical outcomes, as previously described(12). Specifically, cases were scored at 5% intervals. Specimens with ≥ 5% membranous expression were considered “positive”. CD3 and CD68 immunostains were performed on each case and were used to interpret which cell type demonstrated expression of a given ligand. The intensity of immune infiltrates was assigned a semi-quantitative score from 0–3: 0 = “none” (no immune infiltrates), 1 = “focal” (mostly perivascular in tumor with some intratumoral extension), 2 = “moderate” (prominent extension of immune infiltrates away from perivascular areas and amongst tumor cells), or 3 = “severe” (immune infiltrates obscuring tumor). The presence of CD20+ TILs (B-cells) was scored according to the same criteria.
Intratumoral CD4:CD8 T cell subset ratios were determined as 1:1, 1:2, 1:3, or 1:4. The geographic association of immune infiltrates with tumor PD-L1 expression was noted. The proportion of TILs expressing PD-1 was scored as “focal” (isolated, <5% of lymphocytes), “moderate” (5–50% of TILs expressing PD-1), or “severe” (>50% of TILs PD-1+).
Statistical analysis
The geographic associations of immune cell infiltrates and tumor characteristics were evaluated using the Fisher’s exact test and χ2 test. We examined the correlation between proportion of tumor cells expressing PD-L1 and the intensity of immune cell infiltration using the Kruskal-Wallis rank test. For individual patients with multiple tumor specimens, each specimen was treated as an independent variable. When analyzing the association of pathologic features with treatment outcomes in patients with multiple specimens, the analysis was conducted in two different ways: first, by considering only the specimen that was procured closest to the date of treatment initiation, and secondly, by selecting the highest expression of each variable across all specimens from that patient. Statistical analyses were performed with the STATA V11 software package. All tests were two-sided except as indicated, and P values <0.05 were considered significant.
RESULTS
Patient characteristics
Among 306 response-evaluable patients on this multi-institutional trial, 60 were treated in the Kimmel Cancer Center at Johns Hopkins. From this group of 60 patients, 98 potential pre-treatment tumor specimens were identified for study. However, 14 specimens were not available (outside hospitals did not participate in research or did not send sufficient material for study, or material could not be located), 14 were exhausted (no definitive invasive tumor remaining, not diagnostic without tumor-specific IHC, or tissue architecture not preserved), and 2 had technical difficulties (material not suitable for IHC, or excessive isotype control background staining). The final study cohort consisted of 68 pre-treatment tumor specimens that were archival or newly-obtained from 41 different patients, as summarized in Table 1. Specimen acquisition dates ranged from December 1997–June 2011, with a mean time from acquisition to IHC staining of 3.1 years, and a median of 2 years (range 0–13 years). The time interval between biopsy acquisition and first dose of anti-PD-1 ranged from 1 day to 12 years. Sixteen of 41 patients (39%) had multiple specimens available for analysis.
Table 1.
Characteristics of 68 pre-treatment tumor specimens derived from 41 patients receiving anti-PD-1 therapy.
| Characteristic | No. of Patients (N=41) |
|---|---|
| Patient response to treatment | |
| Complete | 2 |
| Partial | 8 |
| Stable disease ≥6 months | 2 |
| No response | 29 |
| Patient diagnosis | |
| Melanoma | 16 |
| Non-small cell lung cancera | 12 |
| Kidney cancer | 6 |
| Colorectal cancer | 5 |
| Castration-resistant prostate cancer | 2 |
| No. tumor specimens analyzed per patient | |
| 1 | 25 |
| 2 | 9 |
| 3 | 5 |
| 4 | 0 |
| 5 | 2 |
| No. of Specimens (N=68) | |
| Primary vs. metastatic tumor | |
| Primary | 29 |
| Metastasis | 39 |
| Anatomic location | |
| lymph node (metastasis) | 17 |
| lung (primary or metastasis) | 15 |
| other | 36 |
Twelve cases include 9 adenocarcinomas and 3 squamous cell carcinomas of the lung.
PD-L1 expression by tumor and infiltrating immune cells
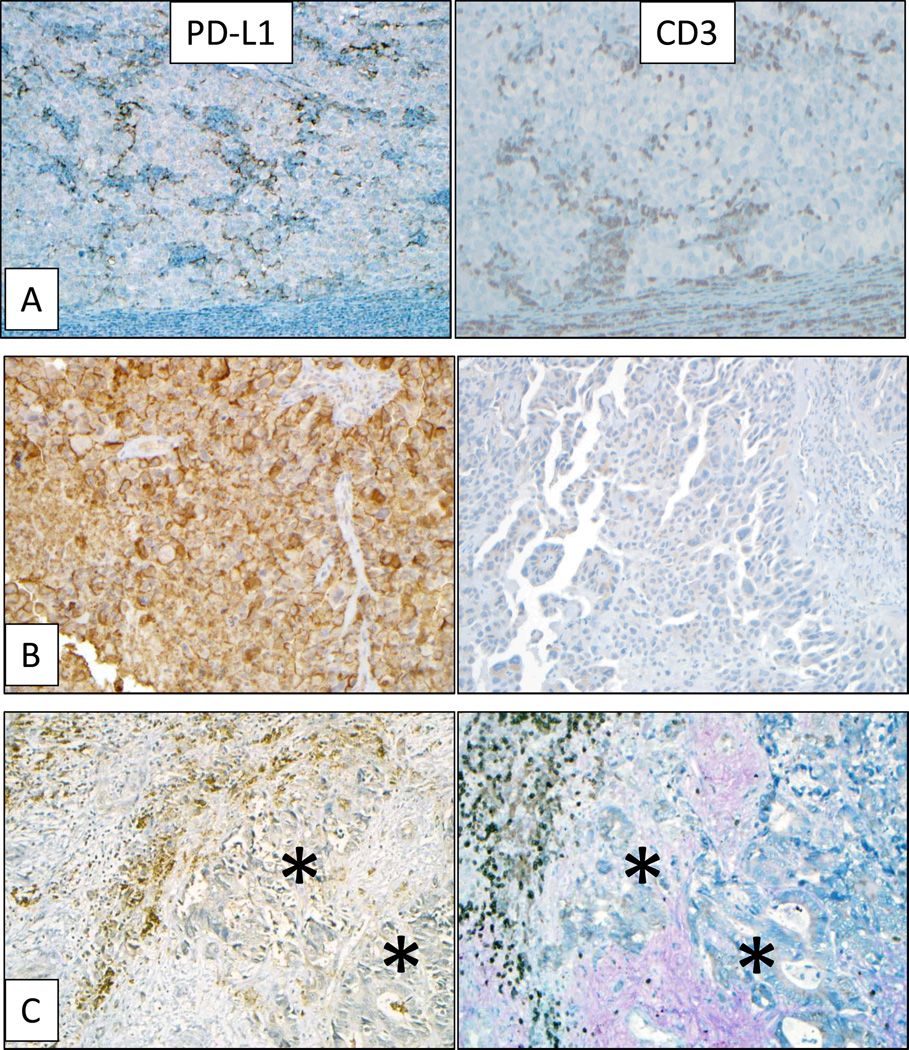
Tumor PD-L1 expression varied significantly by tumor type, with the majority of 56 melanoma, NSCLC, and RCC specimens demonstrating expression, in contrast to only one among a total of 12 CRC and CRPC specimens (p=0.005; Table 2). When tumor cell PD-L1 expression was observed, it was associated with infiltrating immune cells including lymphocytes and histiocytes in 33 of 34 cases (p=0.001; Table 2, Figure 1A). The proportion of tumor cells expressing PD-L1 correlated with the intensity of immune cell infiltration (Kruskal-Wallis test, p=0.003). A single case of NSCLC with broad tumor PD-L1 expression and no immune infiltrates was also observed (Figure 1B).
Table 2.
Association of tumor microenvironmental features with PD-L1 expression by tumor cellsa or infiltrating immune cells
| Parameter (no. specimens examined) | Tumor cells | Infiltrating immune cells | |||||
|---|---|---|---|---|---|---|---|
| Specimens n (%) |
PD-L1(−) n (%) |
PD-L1(+)b n (%) |
p-valuec | PD-L1(−) n (%) |
PD-L1(+)b n (%) |
p-valuec | |
| Tumor type (n=68) | |||||||
| melanoma | 30 (44) | 14 (47) | 16 (53) | 0.005 | 15 (50) | 15 (50) | 0.007 |
| non-small cell lung cancer | 17 (25) | 8 (47) | 9 (53) | 8 (47) | 9 (53) | ||
| kidney cancer | 9 (13) | 1 (11) | 8 (89) | 0 (0) | 9 (100) | ||
| colorectal cancer | 8 (12) | 7 (87) | 1 (13) | 4 (50) | 4 (50) | ||
| castration-resistant prostate ca. | 4 (6) | 4 (100) | 0 (0) | 4 (100) | 0 (0) | ||
| Tumor site (n=68) | |||||||
| lymph node | 17 (25) | 11 (65) | 6 (35) | 0.219 | 10 (59) | 7 (41) | 0.339 |
| lung | 15 (22) | 5 (33) | 10 (67) | 5 (33) | 10 (67) | ||
| other | 36 (53) | 18 (50) | 18 (50) | 16 (44) | 20 (56) | ||
| Primary vs. metastatic tumor (n=68) | |||||||
| primary | 29 (43) | 17 (59) | 12 (41) | 0.327 | 15 (52) | 14 (48) | 0.463 |
| metastasis | 39 (57) | 17 (44) | 22 (56) | 16 (41) | 23 (59) | ||
| Immune infiltrate scored (n=68) | |||||||
| 0 | 14 (21) | 13 (93) | 1 (7) | 0.001 | N/A | N/A | 0.69 |
| 1 | 35 (51) | 16 (46) | 19 (54) | 12 (34) | 23 (66) | ||
| 2 | 16 (24) | 5 (31) | 11 (69) | 5 (31) | 11 (69) | ||
| 3 | 3 (4) | 0 (0) | 3 (100) | 0 (0) | 3 (100) | ||
| Proportion of TILs expressing PD-1e(n=63) | |||||||
| 0 | 38 (60) | 25 (66) | 13 (34) | 0.001 | 22 (58) | 16 (42) | 0.005 |
| 1 | 13 (21) | 5 (38) | 8 (62) | 4 (31) | 9 (69) | ||
| 2 | 12 (19) | 1 (8) | 11 (92) | 1 (8) | 11 (92) | ||
| 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| CD4:CD8 ratio (n=50) | |||||||
| CD4≥CD8 | 22 (44) | 9 (41) | 13 (59) | 0.565 | 7 (32) | 15 (68) | 1.000 |
| CD4<CD8 | 28 (56) | 9 (32) | 19 (68) | 10 (36) | 18 (64) | ||
| CD20+ B-cells (n=51) | |||||||
| absent | 34 (67) | 23 (68) | 11 (32) | 0.006 | 21 (62) | 13 (38) | 0.017 |
| presentf | 17 (33) | 4 (24) | 13 (76) | 4 (24) | 13 (76) | ||
| Lymphoid aggregates (n=68) | |||||||
| absent | 58 (85) | 32 (55) | 26 (45) | 0.083 | 30 (52) | 28 (48) | 0.017 |
| present | 10 (15) | 2 (20) | 8 (80) | 1 (10) | 9 (90) | ||
| Necrosis (n=68) | |||||||
| absent | 50 (74) | 27 (54) | 23 (46) | 0.41 | 25 (50) | 25 (50) | 0.276 |
| present | 18 (26) | 7 (39) | 11 (61) | 6 (33) | 12 (67) | ||
| Small sample (n=68) | |||||||
| no | 44 (65) | 19 (43) | 25 (57) | 0.204 | 17 (39) | 27 (61) | 0.135 |
| yes | 24 (35) | 15 (62) | 9 (38) | 14 (58) | 10 (42) | ||
N/A, Not applicable; TIL, tumor infiltrating lymphocytes
Tumor cell PD-L1 expression was previously reported for 53 of these 68 specimens.(16)
Tumor or infiltrating immune cells were considered PD-L1(+) if ≥5% of cells had membranous (cell surface) PD-L1 expression.
Fisher’s exact test, two-sided, comparing PD-L1(+) vs. (−) specimens for the indicated parameter.
Immune infiltrates include tumor infiltrating lymphocytes (TILs) and accompanying histiocytes. Infiltrates were scored as “none” (0), “focal” (1), “moderate” (2), or “severe” (3) (See Methods).
Proportion of TILs expressing PD-1 was graded as ”none” (0), ”focal” (1), ”moderate” (2), or “severe” (3). A score of “0” includes cases where there were no TIL, or where TIL were present but did not express PD-1.
CD20 “present” refers to any grade (“focal”, “moderate”, or “severe”).
Figure 1. Association of tumor PD-L1 expression with immune infiltrates.
Left column: IHC for PD-L1. Right column: IHC for CD3+ TILs. Panel (A), representative specimen from a subcutaneous melanoma metastasis demonstrating focal PD-L1 expression by tumor cells geographically associated with TILs. Panel (B), diffuse membranous tumor cell PD-L1 expression in a NSCLC brain metastasis, not associated with TILs. Panels (C), colorectal carcinoma metastasis to liver with membranous PD-L1 expression on infiltrating immune cells (brown stain) but not on tumor cells (asterisks). Original magnification 200×, all panels. Higher power images and additional immunohistochemical studies of the representative melanoma and colorectal carcinoma case are shown in Supplementary Figures 2 and 3.
We found that PD-L1 was expressed not only on tumor cells, but also on immune infiltrating cells including TILs and associated histiocytes/macrophages. PD-L1 expression by infiltrating immune cells varied by tumor type (p=0.007), as shown in Table 2. Of interest, while only one among 8 cases of CRC displayed PD-L1+ tumor cells, 4 of 8 cases demonstrated PD-L1+ immune infiltrates (Figure 1C, Supplementary Figure 3). This was in contrast to findings in melanoma, NSCLC, and RCC specimens, where PD-L1 was often expressed coordinately on tumor cells and associated immune infiltrates. PD-L1 was also seen in the native tissue stroma, e.g., alveolar macrophages in lung specimens and dendritic cells in non-neoplastic lymph node parenchyma from lymph node dissections. Neither tumor cell nor infiltrating immune cell PD-L1 expression correlated with the anatomic site of the tumor specimen (lymph node vs. lung vs. elsewhere), or with specimens derived from primary versus metastatic lesions.
Association of PD-L1 in the tumor microenvironment with characteristics of immune cell infiltrates and other histologic features
In order to better characterize the microenvironment of PD-L1+ cancers, TILs were assessed for the presence and proportion of CD4+ and CD8+ T cells, and for the presence of CD20+ B-cells (Supplementary Figure 4). PD-1 receptor expression by these cells was also assessed. We found that TIL PD-1 expression was significantly associated with PD-L1 expression by tumor cells and by immune cell infiltrates (Table 2, p=0.001 and p=0.005, respectively), reflecting a potentially immunosuppressive environment. PD-1 expression was also associated with increasing intensity of immune infiltrates and the presence of lymphoid aggregates (Kruskal-Wallis test, p=0.002 and Fisher’s Exact test p= 0.010, respectively), but did not vary significantly by CD4:CD8 ratio. Both the presence of B-cells and lymphoid aggregates were associated with PD-L1 expression on tumor and infiltrating immune cells, but neither the CD4:CD8 ratio nor the presence of tumor necrosis correlated with PD-L1 expression in the tumor microenvironment (Table 2).
PD-L2 expression
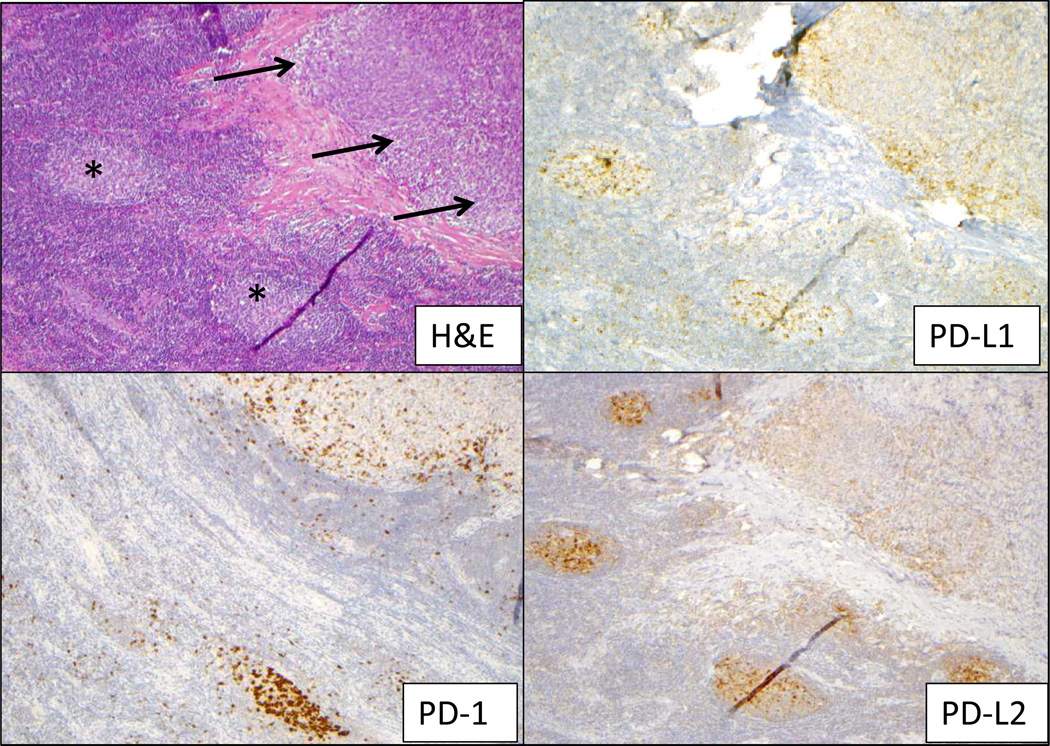
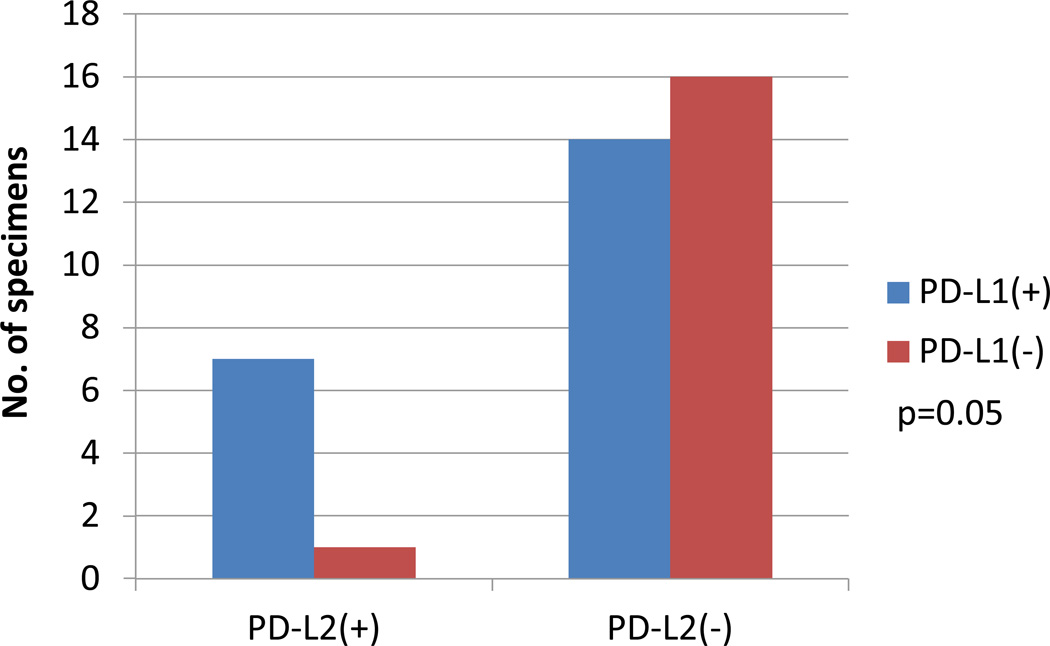
PD-L2 is the second known ligand for the PD-1 T cell co-receptor. Its binding to PD-1 is blocked by nivolumab, but its potential role in mediating immunosuppression in the human tumor microenvironment, and as a marker associated with clinical outcomes to anti-PD-1 therapy, has not been established. To explore this, 38 tumor specimens from 27 patients were examined for PD-L2 protein expression using IHC. PD-L2 expression by either tumor or infiltrating immune cells was observed in only 8 of 38 specimens (21%), including one RCC, 5 melanoma, and 2 NSCLC specimens. Four specimens demonstrated tumor cell expression of PD-L2, and in all of these cases, areas of tumor cell PD-L2 expression were adjacent to immune infiltrates (Figure 2). In 3 of these four cases, PD-L2 expression by immune cell infiltrates was also observed. The four other cases demonstrated focal membranous PD-L2 expression by immune infiltrating cells but not by tumor cells. A diffuse pattern of PD-L2 expression was not observed. PD-L2 expression was geographically associated with PD-L1 expression (p=0.053), and there was only one case in which PD-L2 expression was observed at the interface of tumor and immune cells in a PD-L1 “negative” tumor (Figure 3). In this instance, PD-L1 expression was present on singular infiltrating immune cells but not on tumor cells, and thus failed to reach the 5% threshold to be scored “positive” in either cell population.
Figure 2. Immunoarchitechture within a melanoma lymph nodal metastasis.
On a section stained with H&E (upper left), a tumor deposit is indicated by arrows and lymph node germinal centers by asterisks. Expression of PD-1, PD-L1 and PD-L2 was observed in the lymph node germinal centers, providing an internal positive control for staining. Within the tumor deposit, PD-L1 and PD-L2 were expressed by both tumor and infiltrating immune cells, associated geographically with PD-1 expression. Additional characterization of the immune infiltrate is provided in Supplementary Figure 4. Original magnification 100×, all panels.
Figure 3. Association of PD-L2 expression in the tumor microenvironment with PD-L1 expression by tumor cells.
PD-L2 expression as assessed by IHC was positive in 8 of 38 tumor specimens, and was expressed on tumor cells and/or immune infiltrating cells. Although PD-L2 was observed less frequently than PD-L1 expression, when present, it was almost always geographically associated with tumor cell PD-L1 expression.
Additional immune microenvironmental features
Potential interrelationships of several tumor microenvironmental parameters listed in Table 2 were examined independently from PD-L1 expression. Among 51 tumor specimens assessed for the presence of CD20+ B-cells, there was a borderline association with tumor type (p=0.072), with B-cells most likely to be seen in RCC (3/4, 75%), followed by melanoma (9/21, 42%), CRPC (1/3, 33%), and NSCLC (4/16, 25%); B-cells were not found in 7 CRC specimens examined. The presence of B-cells correlated with both increasing immune infiltrate grade and PD-1 expression (p=0.017 and 0.001, respectively). A borderline association between TIL PD-1 expression and tumor location was observed (p=0.067): among 63 specimens examined for PD-1 expression, 46% (6/13) of lung lesions (primary or metastatic), 20% (3/15) of lymph node metastases, and 9% (3/35) of tumors located elsewhere had ≥5% of TILs expressing PD-1, suggesting that the native tissue stroma may influence the expression of PD-1.
Biopsy characteristics
Due to the focal, geographic expression of many of the immunological markers studied here, we queried whether the size of the biopsy sample, i.e., core needle biopsy or fine needle aspirate containing tissue fragments (n=24), versus larger excisional specimen (n=44), impacted our assessment; dispersed single-cell cytology specimens were not included in this analysis. There was no significant relationship between biopsy size and PD-L1 expression (Table 2), PD-1 expression by TIL, or intensity score of immune infiltrates. In 30 melanoma specimens ranging in age from 2 months to 13 years, we also queried whether our ability to detect PD-L1 expression by IHC was related to the age of the specimen, defined as the time from specimen acquisition to staining for PD-L1, which might indicate epitope degradation over time. There was no significant relationship between specimen age and tumor PD-L1 expression based on the IHC technique used in this analysis.
Pathologic parameters associated with clinical response to anti-PD-1 therapy
We previously reported a preliminary correlation between tumor cell surface PD-L1 expression in pre-treatment biopsies, and objective response to nivolumab therapy.(16) In that study, if multiple specimens were assessed from individual patients, a patient was defined as “PD-L1 positive” if any specimen contained ≥5% PD-L1+ tumor cells. In the current study, among 41 patients, 16 had multiple pre-treatment tumor specimens for study (2–5 specimens per patient, Table 1). We assessed a panel of pathological parameters in the single tumor specimen obtained closest to the initiation of anti-PD-1 therapy (Table 3), reflecting anticipated clinical practice, or in the specimen with maximum expression in the case of multiple samples from a given patient (Supplementary Table 1). Pathological findings were correlated with objective tumor regression (complete or partial response, RECIST 1.0 with modification), as well as with “clinical benefit” (objective response, or stable disease lasting at least 6 months), versus no response to therapy.
Table 3.
Relationship between pre-treatment microenvironmental parameters in the tumor specimen obtained closest to treatment initiation, and clinical response to anti-PD-1a
| Pathologic parameter (no. patients analyzed) |
All patients n (%) |
Objective Responseb | Clinical Benefitc | |||||
|---|---|---|---|---|---|---|---|---|
| No n (%) |
Yes n (%) |
p-valued | No n (%) |
Yes n (%) |
p-valued | |||
| Tumor PD-L1 expression (n=41)e | ||||||||
| absent | 18 (44) | 17 (94) | 1 (6) | 0.025 | 17 (94) | 1 (6) | 0.005 | |
| present | 23 (56) | 14 (61) | 9 (39) | 12 (52) | 11 (48) | |||
| Immune cell infiltrate PD-L1 expression (n=41)e | ||||||||
| absent | 18 (44) | 16 (89) | 2 (11) | 0.142 | 16 (89) | 2 (11) | 0.038 | |
| present | 23 (56) | 15 (65) | 8 (35) | 13 (57) | 10 (43) | |||
| Immune infiltrate score (n=41)f | ||||||||
| absent | 9 (22) | 8 (89) | 1 (11) | 0.410 | 8 (89) | 1 (11) | 0.240 | |
| present | 32 (78) | 23 (72) | 9 (28) | 21 (66) | 11 (34) | |||
| TIL PD-1 expression (n=37)g | ||||||||
| absent | 21 (57) | 18 (86) | 3 (14) | 0.067 | 17 (81) | 4 (19) | 0.077 | |
| present | 16 (43) | 9 (56) | 7 (44) | 8 (50) | 8 (50) | |||
| Immune cell or tumor cell PD-L2 expression (n=13)e | ||||||||
| absent | 9 (69) | 7 (78) | 2 (22) | 1.000 | 6 (67) | 3 (33) | 0.497 | |
| present | 4 (31) | 4 (100) | 0 (0) | 4 (100) | 0 (0) | |||
| CD4:CD8 (n=29) | ||||||||
| CD4≥CD8 | 13 (45) | 10 (77) | 3 (23) | 0.454 | 9 (69) | 4 (31) | 0.702 | |
| CD4<CD8 | 16 (55) | 10 (63) | 6 (37) | 9 (56) | 7 (44) | |||
| CD20+ B-cells (n=30) | ||||||||
| absent | 19 (63) | 16 (84) | 3 (16) | 1.000 | 16 (84) | 3 (16) | 0.372 | |
| presentf | 11 (37) | 9 (82) | 2 (18) | 7 (64) | 4 (36) | |||
| Lymphoid aggregates (n=41) | ||||||||
| absent | 35 (85) | 26 (74) | 9 (26) | 1.000 | 25 (71) | 10 (29) | 1.000 | |
| present | 6 (15) | 5 (83) | 1 (17) | 4 (67) | 2 (33) | |||
| Necrosis (n=41) | ||||||||
| absent | 27 (66) | 19 (70) | 8 (30) | 0.447 | 19 (70) | 8 (30) | 1.000 | |
| present | 14 (34) | 12 (86) | 2 (14) | 10 (71) | 4 (29) | |||
| Small sample (n=41) | ||||||||
| no | 27 (66) | 20 (74) | 7 (26) | 1.000 | 18 (67) | 9 (33) | 0.494 | |
| yes | 14 (34) | 11 (79) | 3 (21) | 11 (79) | 3 (21) | |||
| Interval between specimen procurement and treatment initiation (n=41) | ||||||||
| < 1 year | 16 (39) | 10 (63) | 6 (37) | 0.15 | 9 (56) | 7 (44) | 0.16 | |
| ≥1 year | 25 (61) | 21 (84) | 4 (16) | 20 (80) | 5 (20) | |||
Correlation of tumor cell PD-L1 expression with objective response was previously reported for 34 of 41 patients included in this series, using the “highest ever” value in the case of multiple specimens from individual patients.(16)
Objective response is defined as complete or partial tumor regression, RECIST 1.0 with modifications.
Clinical benefit is defined as objective response or stable disease lasting ≥6 months.
Fisher’s Exact Test, comparing responders to non-responders for the pathologic parameter analyzed.
Tumor or infiltrating immune cells were considered PD-L1 or PD-L2 (+) if ≥ 5% of cells had membranous (cell surface) expression detected by IHC.
Immune infiltrates (lymphocytes and histiocytes) and CD20+TIL were graded as ‘none’, ‘focal’, ‘moderate’, or ‘severe’ (see Methods). TIL or CD20 “present” indicates grades ‘focal’, ‘moderate’, and ‘severe’.
Absent TIL PD-1 expression includes both cases where there were no TILs, and cases where TILs were present but did not express PD-1.
Similar to our previous analysis based on the “highest ever” expression in individual patients with multiple tumor specimens(16), PD-L1 expression by tumor cells correlated significantly with objective response and with clinical benefit, when analyzed in the single specimen procured closest to the start of therapy (p=0.025 and 0.005, respectively, Table 3). Of interest, correlation of PD-L1 expression by infiltrating immune cells with objective clinical response did not reach statistical significance (p=0.14), although there was a significant correlation with clinical benefit (p=0.038). Furthermore, expression of the PD-1 receptor on TILs had only a borderline association with clinical response, even though PD-1 is the immediate target of nivolumab. Importantly, the presence of TILs when analyzed as an independent factor did not correlate with clinical outcomes. Additional microenvironmental factors including PD-L2 expression by tumor cells or TILs, the CD4:CD8 ratio, the presence of CD20+ B-cells, tumor necrosis, and lymphoid aggregates, did not correlate with treatment response when either the sample closest to therapy or the specimen with the “highest ever” value were assessed. However, because some of these factors were analyzed in fewer than 30 patients, more extensive study is needed to confirm these observations.
The range of time from tumor specimen procurement to treatment initiation was broad in our study (1 day to 12 years). The vast majority of specimens analyzed were archival, and obtaining a new tumor biopsy prior to treatment initiation was not a requirement of this clinical trial. Given the known spatial and temporal heterogeneity of PD-L1 expression(12,16), we queried whether the time between tumor biopsy and the initiation of anti-PD-1 therapy independently correlated with clinical outcomes. Despite the wide range of specimen age, we did not observe such an association. As shown in Table 3, responding patients were evenly distributed between <1year versus ≥1year biopsy interval groups, and 9 of the 10 responders demonstrated PD-L1 tumor expression, suggesting that the correlation of PD-L1 expression with clinical outcomes is not related to the timing of tissue acquisition. While consideration of sampling interval warrants additional investigation, our results do not provide direct support for the idea that tumor PD-L1 expression must be determined immediately prior to anti-PD-1 treatment initiation, in order to be predictive of clinical outcome. Realistically, such an approach will not always be possible, and is not uniformly practiced in current clinical trials of drugs blocking the PD-1pathway.
DISCUSSION
Cancer immunotherapy, which targets and modulates anti-tumor immune cells, differs mechanistically from cytotoxic therapies and kinase inhibitors, which directly mediate tumor cell death. Accordingly, these treatment approaches differ in their profiles of clinical activity as well as safety(22–24). The identification of factors predicting response to immunotherapy is highly desirable, in order to pre-select patients most likely to benefit and spare others from unnecessary exposure to potential side effects. However, this is challenging due to the dynamic nature of the anti-tumor immune response and its heterogeneity across space (anatomic location) and time (progression from primary to metastatic cancer). We previously reported a correlation between pre-treatment tumoral PD-L1 expression and response to anti-PD-1 therapy (nivolumab) in a subset of patients on an expanded phase 1 trial(16). In the current study, we have re-examined PD-L1 as a marker associated with anti-PD-1 response, and have extended our investigations to evaluate other factors in the tumor microenvironment potentially associated with the clinical activity of anti-PD-1.
Recent studies associate an inflammatory tumor microenvironment with responsiveness to certain forms of immunotherapy such as cancer vaccines and ipilimumab,(25, 26) and our observations suggest that this may also be true for PD-1 pathway blockade. In the current study, patients whose tumors expressed PD-L1 were more likely to respond to anti-PD-1 therapy. While PD-L1 is generally regarded as an immunosuppressive molecule, its expression is not necessarily synonymous with tumor immune evasion and may reflect an ongoing anti-tumor immune response that includes the production of interferon-gamma and other inflammatory factors(12). This is consistent with retrospective studies in select tumor types, such as melanoma, Merkel cell carcinoma, mismatch repair-proficient CRC, and NSCLC where tumor PD-L1 expression has been shown to be a positive prognostic factor(12, 13, 27, 28). We observed tumor cell surface PD-L1 expression in distinct patterns, which generally correlated with tumor type. Tumor cell surface PD-L1 expression was associated with immune cell infiltrates in some cases (mainly melanoma and RCC), while in others it was constitutive or out of proportion to infiltrating immune cells (NSCLC). We also observed instances of PD-L1 membranous expression on infiltrating immune cells but not on tumor cells, particularly in CRC(21). While the biological significance of these distinct expression patterns is currently unclear, they likely reflect the combined effects of innate and adaptive cellular and soluble factors that shape the tumor microenvironment, as well as the type of malignancy and composition of other components of the tumor stroma. For example, neoantigens associated with infection by tumor-promoting viruses or somatic mutational events in malignant cells may trigger inflammatory responses leading to local PD-L1 expression(13, 21, 27), while PD-L1 expression in non-virus-associated head and neck squamous cell cancers, glioblastoma multiforme, and ALK-positive T-cell lymphomas has been associated with PTEN and ALK/STAT3 oncogenic signaling pathways(29–31).
In this study, we examined a potential relationship between TIL expression of PD-1, the direct target of nivolumab, with clinical outcomes but found only a borderline association. Because the intensity of immune cell infiltrates was significantly associated with tumor cell PD-L1 expression, we also explored the possibility that the simply the presence immune cell infiltrates might predict favorable clinical outcomes to anti-PD-1 therapy. The presence of TIL has been correlated with improved outcomes in retrospective studies of different tumor types, including melanoma and colorectal carcinoma(32–35). In addition, HER2-positive breast cancer patients with TIL in their pre-treatment specimens have shown improved benefit from certain chemotherapeutic regimens(36). Further, increased numbers of TIL in post-treatment biopsies have been shown to correlate with the activity of ipilimumab in patients with melanoma(37). However, the current study is the first to examine the relationship of the presence of TIL in pre-treatment tumor specimens to anti-PD-1 response, and a significant relationship between these factors was not observed. These findings suggest that the functional profile of TILs is a key factor determining PD-L1 expression(12). That is, TILs may be necessary to drive PD-L1 expression in some tumors, but their presence alone is not sufficient to induce PD-L1 and was not an independent factor correlating with clinical response in this relatively limited cohort. Because pre-clinical evidence suggests that anti-PD-1 can restore dampened B-cell functions(38), we also examined whether the presence and intensity of B-cell infiltrates correlated with clinical outcomes. Similar to our findings with CD3+ TILs, CD20+ B-cells were significantly associated with PD-L1 expression by tumor and infiltrating immune cells, but their presence alone did not correlate with clinical outcomes following PD-1 blockade, suggesting the importance of defining cellular functional profiles. Other immune cell types, including suppressive cells (regulatory T-cells and myeloid-derived suppressor cells), remain to be explored in the context of PD-1 pathway blockade(39, 40).
Recent work by others to analyze a potential association between pre-treatment tumor PD-L1 expression and response to PD-1 pathway blockade -- anti-PD-1(41) or anti-PD-L1(42)-- has confirmed our original observation(16) linking PD-L1+ tumors with the likelihood of treatment response. However, in these new studies, some PD-L1 negative patients also responded to treatment, raising concerns that excluding the “marker negative” patient population from treatment might exclude potential responders. It is important to note that these three studies differ in the anti-PD-L1 mAbs used for IHC, staining techniques (manual versus automated), definitions of PD-L1 “positive” tumor (cell surface versus cytoplasmic expression, by tumor cells only or by other cells in the tumor milieu, threshold of “positivity”), scoring increments, and definitions of PD-L1 “positive” patients (based on a single tumor biopsy, or on maximal expression in the case of multiple biopsies from an individual patient). Also, because of the focal nature of PD-L1 expression within many tumors and emerging information about intratumoral genetic heterogeneity(43), if very small needle biopsies or dispersed single-cell cytology specimens are evaluated, a false-negative evaluation could potentially result. Another potential explanation for PD-L1(-) responders includes yet unidentified factors contributing to response. Despite these methodological differences, the overall conclusions of these reports are remarkably similar, highlighting a robust association between the PD-L1 marker and mechanism-of-action for this class of drugs.
Although response rates are enhanced in the PD-L1+ patient population, it is currently unknown why the majority of PD-L1+ patients do not respond to PD-1 pathway blocking drugs. One possibility is that PD-L1+ tumors from non-responders express additional dominant or co-dominant immune checkpoints supporting treatment resistance. To address this, we examined PD-L2, the second known ligand for PD-1, for possible associations with PD-L1 expression and clinical outcomes. PD-L2 protein detected by IHC was found almost exclusively in geographic association with PD-L1 protein, consistent with its known up-regulation by inflammatory cytokines including interferon-gamma which also drives PD-L1 expression(44). However, PD-L2 expression was seen less frequently than PD-L1 in our series (in only 8 of 38 specimens examined), and no significant correlation with clinical outcomes was observed. Although the results of our series should be considered preliminary, similar conclusions were drawn in a recent report of PD-L2 expression detected by quantitative molecular techniques, in patients receiving anti-PD-L1 therapy(45). Studies aimed at identifying additional positive or negative predictive markers of response to anti-PD-1 treatment, and potential interactions among multiple factors in the tumor microenvironment, are currently underway in our laboratories.
In summary, this in-depth analysis of multiple factors in pre-treatment tumor specimens from patients with advanced cancers receiving anti-PD-1 therapy prioritizes tumor cell PD-L1 expression as being most closely associated with objective tumor regression. It reveals other microenvironmental features such as TIL PD-1 expression and the intensity of T-cell and B-cell infiltrates, as being associated with PD-L1 expression by tumor cells or immune infiltrating cells, but not independently associated with treatment response. Thus PD-L1 expression reflects an immune-active tumor milieu, and may illuminate additional tumor types that should be targeted for clinical testing with PD-1 pathway blockade. These results should still be considered preliminary, and ongoing Phase 2 and 3 clinical trials of PD-1 pathway blockade are broadening the assessment PD-L1 expression as it relates to clinical outcomes including survival, in larger cohorts of patients. Additional investigations will be necessary to confirm these findings and will address whether multi-component panels of pre-treatment tumor markers may have more powerful associations with clinical outcomes, compared to individual factors. Assessment of on-treatment alterations in tumor molecular profiles will also be necessary to reveal whether tumors lacking PD-L1 expression and TILs may convert to PD-L1-expressing tumors following “priming” with combinatorial treatment regimens designed to incite an immune response, followed by PD-1 pathway blockade to liberate anti-tumor immunity.
Supplementary Material
Statement of Translational Relevance.
One of the most intriguing findings from early clinical trials of PD-1 pathway blockade for advanced solid tumors has been the correlation between pre-treatment tumor PD-L1 expression and treatment response. The current study expands on this observation by exploring PD-1, PD-L1, and PD-L2 expression by tumor cells and infiltrating immune cell subsets, and their relationships to each other and to clinical response to anti-PD-1 (nivolumab). Significant associations were found among tumor cell PD-L1 expression, the presence of intratumoral immune cell infiltrates, and PD-1 receptor expression by tumor infiltrating lymphocytes, suggesting that PD-L1 reflects an immune-reactive milieu. However, among these parameters, tumor cell PD-L1 expression was most closely associated with response to anti-PD-1 therapy. PD-L1 expression was also significantly associated with tumor types responding to anti-PD-1 (melanoma, lung and kidney cancer), suggesting that it could provide a means for identifying additional tumor types which may respond to PD-1 pathway blockade.
ACKNOWLEDGEMENTS
We would like to thank Evan Lipson, William Sharfman, and Charles Drake (Johns Hopkins University School of Medicine) for providing outstanding clinical care to the patients on this trial. We acknowledge Gulsun Erdag for helpful discussions on PD-L2 assay development, and Cherylann Carr-Cozier, Marina Laiko, and Deborah Roberts for regulatory and administrative assistance (all from Johns Hopkins University School of Medicine). We are grateful to Mark Salvati, Joseph Grosso and John Cogswell (Bristol-Myers Squibb) for helpful comments regarding this manuscript. Research support for this study was provided by Bristol-Myers Squibb (JMT, SLT, DMP, JRB, RAA), National Institutes of Health (R01 CA142779; JMT, SLT, DMP, LC), the Melanoma Research Alliance (SLT, DMP, LC, JMT), the Barney Family Foundation (JMT, SLT), the Laverna Hahn Charitable Trust (SLT, JMT), the Dermatology Foundation (JMT), the Commonwealth Foundation (JMT, DMP), and Moving for Melanoma of Delaware (JMT, DMP, SLT). JMT, SLT, DMP, JRB, RAA were also supported by a Stand Up To Cancer—Cancer Research Institute Cancer Immunology Translational Cancer Research Grant (SU2C-AACR-DT1012). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Conflicts of Interest: Dr. Taube has received research grants from Bristol-Myers Squibb and has served as an advisory board member (compensated) for Bristol-Myers Squibb. Dr. Brahmer has received grants from MedImmune, Regeneron, Merck, Bristol-Myers Squibb, and Synta; has served as a consultant to Bristol-Myers Squibb (uncompensated), Genentech, Merck and Lilly; and has received travel/meeting support from Bristol-Myers Squibb and Genentech. Dr. Chen is a co-founder, board observer and consultant for Amplimmune; patent royalties from Amplimmune, Bristol-Myers Squibb, ImmuneNext; and received sponsored research funds from Amplimmune, AbbVie and Eli Lily. Dr. Pardoll has served as a consultant for Bristol-Myers Squibb, Aduro, NextImmune, ImmuneExcite, Jounce, GlaxoSmithKline (all uncompensated); and receives patent royalties from Bristol-Myers Squibb, Amplimmune and Aduro. Dr. Topalian has received research grants from Bristol-Myers Squibb, is a consultant (uncompensated) and has received travel/meeting support from Bristol-Myers Squibb, and is a consultant for Sanofi and Jounce Therapeutics. Dr. Anders has received research grants from Bristol-Myers Squibb and has received travel/meeting support from Bristol-Myers Squibb.
REFERENCES
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;19(363):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 3.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 4.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 5.Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169:3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 6.Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol Reprod. 2003;68:1496–1504. doi: 10.1095/biolreprod.102.010058. [DOI] [PubMed] [Google Scholar]
- 7.Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N, et al. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett. 2002;84:57–62. doi: 10.1016/s0165-2478(02)00142-6. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 9.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flies DB, Chen L. The new B7s: Playing a pivotal role in tumor immunity. J Immunother. 2007;30:251–260. doi: 10.1097/CJI.0b013e31802e085a. [DOI] [PubMed] [Google Scholar]
- 11.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 12.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, et al. PD-L1 expression in the merkel cell carcinoma microenvironment: Association with inflammation, merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1:54–63. doi: 10.1158/2326-6066.CIR-13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 16.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. european organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–468. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blansfield JA, Beck KE, Tran K, Yang JC, Hughes MS, Kammula US, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593–598. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gajewski TF, Fuertes M, Spaapen R, Zheng Y, Kline J. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr Opin Immunol. 2011;23:286–292. doi: 10.1016/j.coi.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:1019–1031. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49:2233–2242. doi: 10.1016/j.ejca.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 31.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci U S A. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30:2678–2683. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 33.Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, Shima K, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412–6420. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72:1070–1080. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas NE, Busam KJ, From L, Kricker A, Armstrong BK, Anton-Culver H, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol. 2013;31:4252–4259. doi: 10.1200/JCO.2013.51.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 37.Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204–219. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohos A, Sebestyen T, Liszkay G, Plotar V, Horvath S, Gaudi I, et al. Immune cell profile of sentinel lymph nodes in patients with malignant melanoma - FOXP3+ cell density in cases with positive sentinel node status is associated with unfavorable clinical outcome. J Transl Med. 2013;11:43–53. doi: 10.1186/1479-5876-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taube JM. Emerging immunologic biomarkers: Setting the [TNM-immune] stage. Clin Cancer Res. 2014 Mar 14; doi: 10.1158/1078-0432.CCR-14-0328. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grosso J, Horak C, Inzunza D, Cardona D, Simon J, Gupta A, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients with advanced solid tumors treated with nivolumab. ASCO meeting. 2013 Abstract #3016. [Google Scholar]
- 42.Herbst R, Gordon M, Fine G, Sosman J, Charles J, Hamid O, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. ASCO meeting. 2013 Abstract #3000. [Google Scholar]
- 43.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 45.Hodi FS, Powles T, Cassier P, Kowanetz M, Herbst RS, Soria JC, Mokatrin A, Stroh M, Chen DS, Tabernero J. MPDL3280A (anti-PDL1): Clinical activity, safety and biomarkers of an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. European Cancer Congress. 2013 Abstract #879. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.