Abstract
Glioblastoma (GBM) is the most common and aggressive primary CNS malignancy with a median survival of 15 months. The average incidence rate (IR) of GBM is 3.19/100,000 population and the median age of diagnosis is 64 years. Incidence is higher in men and individuals of white race and non-Hispanic ethnicity. Many genetic and environmental factors have been studied in GBM but the majority are sporadic and no risk factor accounting for a large proportion of GBMs has been identified. However, several favorable clinical prognostic factors are identified including, younger age at diagnosis, cerebellar location, high performance status and maximal tumor resection. GBMs comprise of primary and secondary subtypes which evolve through different genetic pathways, affect patients at different ages and have differences in outcomes. We report the current epidemiology of GBM with new data from the Central Brain Tumor Registry of the United States (CBTRUS) 2006–2010 as well as demonstrate and discuss trends in incidence and survival. We also provide a concise review on molecular markers in GBM that have helped distinguish biologically similar subtypes of GBM and have prognostic and predictive value.
Keywords: glioblastoma, epidemiology, incidence, survival, prognosis
Introduction
GBM is the most aggressive diffuse glioma of astrocytic lineage and corresponds to grade IV based on WHO Classification (1). GBM is the most common brain and CNS malignancy, accounting for 45.2% of malignant primary brain and CNS tumors, 54% of all gliomas, and 16% of all primary brain and CNS tumors (2). GBM remains an incurable disease with a median survival of 15 months (3, 4). Treatment is complex and initially consists of maximal-safe surgical resection followed by radiation therapy (RT) with concurrent temozolomide (TMZ) chemotherapy followed by six cycles of maintenance TMZ (5).
GBMs comprise of primary and secondary subtypes which evolve through different genetic pathways, affect patients at different ages and have differences in outcomes (6). Primary (de novo) GBMs account for 80% of GBMs and occur in older patients (mean age 62 years). Secondary GBMs develop from lower-grade astrocytoma or oligodendrogliomas and occur in younger patients (mean age 45 years) (6–9). The WHO recently added a rare subtype of GBM termed ‘with oligodendroglioma component’ (GBM-O) defined as GBM having areas that resemble anaplastic oligodendroglioma with hallmark features of GBM, necrosis with or without microvascular proliferation (1).
Epidemiology
Incidence and risk factors
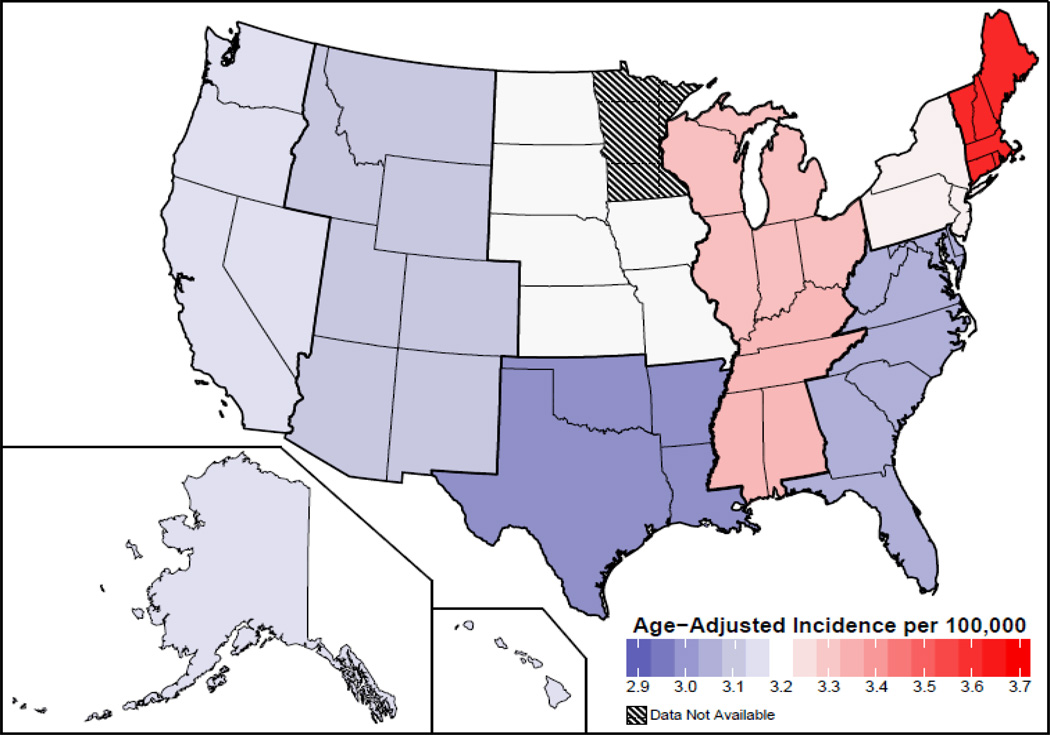
Based on the 2013 CBTRUS report, the average annual age-adjusted incidence rate (IR) of GBM is 3.19/100,000 population (2). This is the highest IR among brain and CNS tumors with malignant behavior followed by diffuse astrocytoma grade 2 (0.56/100,000), and malignant glioma not otherwise specified (0.46/100,000) (2). Incidence is highest in the northeast and lowest in the south-central region of US (Figure 1), however these differences could reflect differences in cancer reporting by region (2). Many genetic and environmental factors have been studied in GBM but no risk factor that accounts for a large proportion of GBM has been identified and like many cancers are sporadic (10).
Figure 1.
Age Adjusted Incidence Rates of Glioblastoma by Region in US, CBTRUS Statistical Report, SEER 2006–2010. Rates are per 100,000 (2). Abbreviations: CBTRUS, Central Brain Tumor Registry of United States.
Age
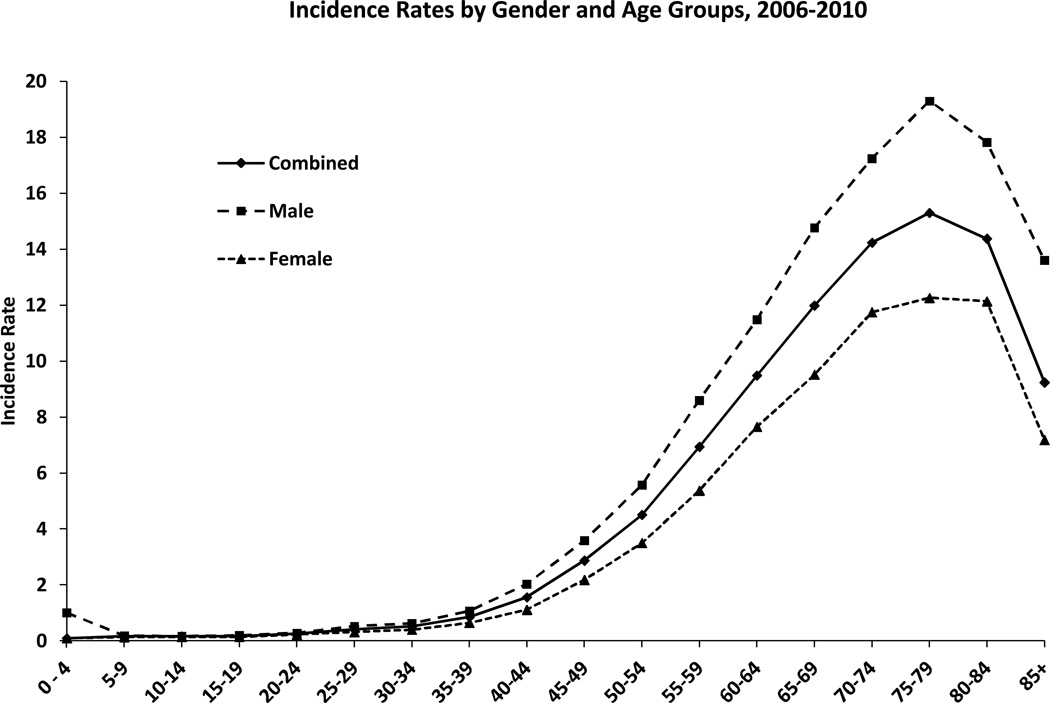
GBM is primarily diagnosed at older ages with the median age of diagnosis of 64 years (2, 11). It is uncommon in children accounting only approximately 3% of all brain and CNS tumors reported among 0–19 year olds (2). The incidence continues to rise with increasing age, peaks at 75–84 years of age and drops after 85 years (Figure 2) (2). With a growing and aging US population, the number of cases is expected to increase (12).
Figure 2.
Age-Adjusted and Age-Specific Incidence Rates for Glioblastoma by Age at Diagnosis and Gender, CBTRUS Statistical Report: NPCR and SEER, 2006–2010. X axis: age groups; Y axis: incidence rates. Rates are per 100,000 and age-adjusted to the 2000 US standard population. Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, CDC's National Program of Cancer Registries; SEER, NCI's Surveillance, Epidemiology and End Results program (2).
Gender
Differences in incidence and death rates for specific cancers based on race and ethnic groups as well as gender, suggest potential identifiable biologic and environment based variables (13, 14). A higher incidence of GBM has been reported in men as compared to women (Figure 2) (2, 11, 15–17). The IR of GBM is 1.6 times higher in males as compared to females [3.97 versus 2.53] (2) with a higher frequency of primary GBMs men (male to female ratio, 1:33) and secondary GBMs in women (male to female ratio, 0:65) (7).
Race/Ethnicity
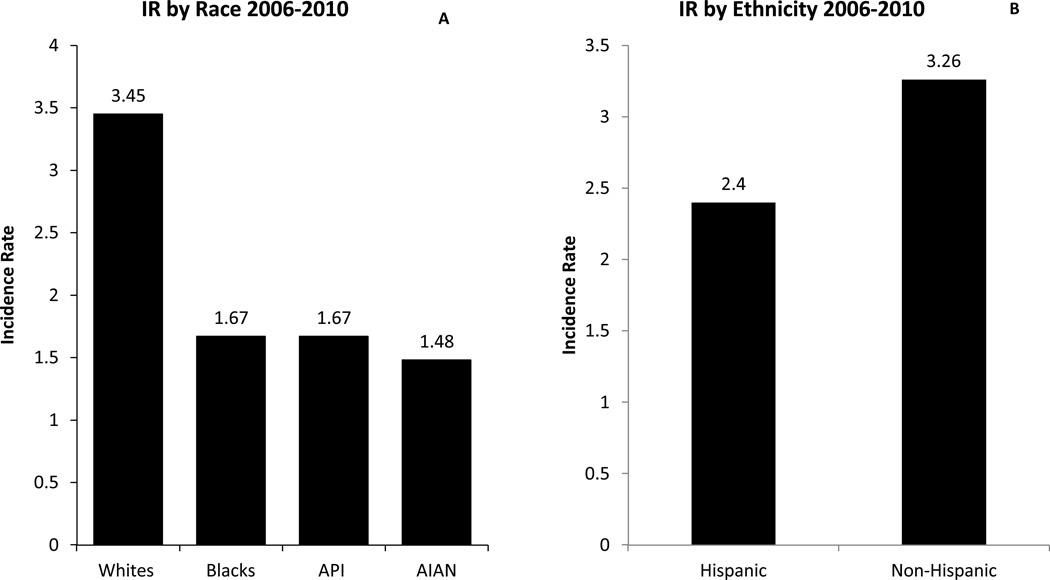
Whites have the highest incidence rates for GBM, followed by blacks, Asian/Pacific Islanders (API) and American Indian/Alaska Native (AI/ANs) (Figure 3A) (2). From 2006 to 2010, whites had two times higher IR as compared to blacks [3.45 versus 1.67] (Figure 3A) and non-Hispanics had higher IR as compared to Hispanics [3.26 versus 2.45] (Figure 3B) (2, 11).
Figure 3.
3A: Average Annual Age Adjusted Incidence Rates of Glioblastoma by Race, CBTRUS Statistical Report: NPCR and SEER, 2006–2010. X axis: race; Y axis: incidence rates.
3B: Average Annual Age Adjusted Incidence Rates of Glioblastoma by Ethnicity, CBTRUS Statistical Report: NPCR and SEER, 2006–2010. X axis: ethnicity; Y axis: incidence rates.
Rates are per 100,000. Abbreviations: IR: Incidence Rate; AIAN, Asian Indian Alaskan Native; API, Asian Pacific Islander; CBTRUS, Central Brain Tumor Registry of United States; NCPR, National Program of Cancer Registries (2).
Site
GBMs are more commonly located in the supratentorial region (frontal, temporal, parietal and occipital lobes), are rarely seen in the cerebellum and are very rare in the spinal cord (18, 19). A SEER population-based study (1973 to 2009) did find a difference in the behavior of GBM at these two locations (19). When compared to supratentorial location, cerebellar GBMs occurred in younger patients, occurred less commonly in whites and were smaller in size (19–21). Cerebellar GBM occurs rarely in adults, accounting for 0.4–3.4% of all GBMs (21). Patients with cerebellar GBM are significantly younger than those with supratentorial tumors (median age of 50–56 years in contrast to 62–64 years for patients with supratentorial GBM) (21). A population based study of the Los Angeles County reported that, GBM had the highest incidence for frontal lobe tumors and for tumors that involved two or more lobes (overlapping tumors), followed by tumors in the temporal and parietal lobes (11). The male to female ratio was elevated for each brain sub-site except the posterior fossa; occipital lobe amongst these sites had the greatest ratio (11).
Summary of Risk
Factors associated with GBM risk are prior therapeutic radiation, decreased susceptibility to allergy, immune factors and immune genes, as well as some single nucleotide polymorphisms (SNPs) detected by genome wide association studies (GWAS) (22–25). A lower risk of gliomas has been associated with allergies or atopic diseases (e.g. asthma, eczema, psoriasis) and the protective effect does not vary by major histologic subtypes of glioma or by histologic grade (26–28). Additionally, short term (< 10 years) use of anti-inflammatory medications is associated with a protective effect against GBM, especially among individuals with no history of asthma or allergies (29). GWAS have detected increased risk of high grade glioma with inherited variation in a region containing cyclin-dependent kinase inhibitor 2B (CDKN2B) on Chromosome 9p21 and in two SNPs in regulator of telomere elongation helicase 1(RTEL1) (25). Other factors associated with GBM risk are high socio-economic status (SES) and taller height (11, 30). The higher SES groups were up to 70% more likely to be diagnosed with GBM in the frontal lobes and they had higher IR for all tumor sites, except for posterior fossa tumors (11).
There is no substantial evidence of GBM association with life-style characteristics such as cigarette smoking, alcohol consumption, use of drugs of any kind or dietary exposure to N-nitroso compounds (cured or smoked meat or fish) (31). Inconsistent and non-definitive results have been published regarding the risk of glioma with use of mobile phones (32–37).
Survival and Prognostic Factors
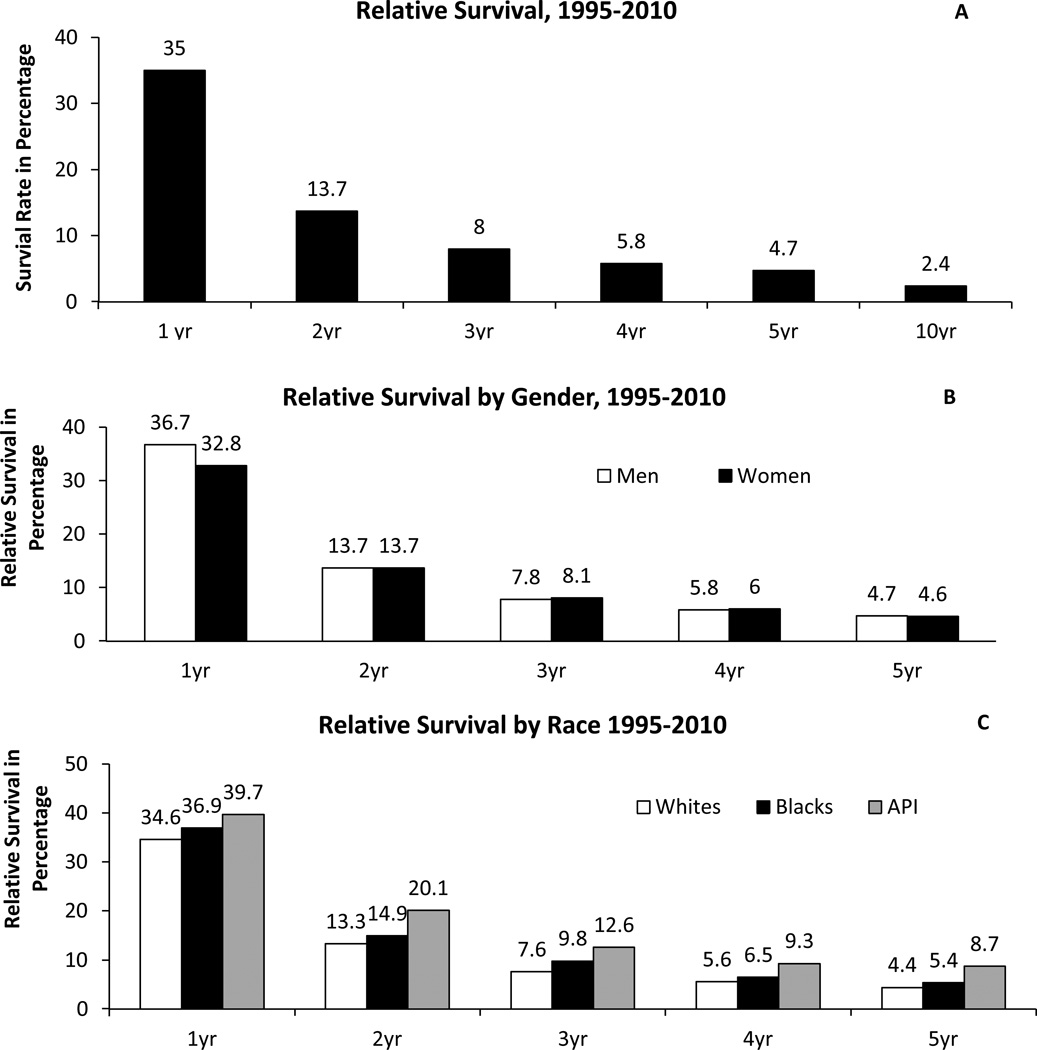
GBM has a poor prognosis with quite low relative survival estimates, only a few patients reaching long-term survival status of 2.5 years and less than 5% of patients survive 5 years post diagnosis (Figure 4A) (2, 38). The relative survival for the first year after diagnosis is 35% and it falls in the second year post diagnosis (13.7%) and thereafter (2) (Figure 4A). A population based study found that the first quarter of the second year (5th quarter) post-diagnosis is considered to be the peak incidence of mortality and the risk of death decreases to half of its rate at 2.5 years (38). Despite these unfavorable survival and mortality estimates, there exists reassuring data for conditional probability of survival in GBM (likelihood of surviving into the future based on previous survival) (39). Patients surviving past 2 years from diagnosis have a relatively favorable conditional probability of survival into the future compared to newly diagnosed patients (39, 40).
Figure 4.
4A: Relative Survival Rates for Glioblastoma, SEER 18 Registries, 1995–2010.
4B: Relative Survival Rates for Glioblastoma by Gender, SEER 18 registries, 1995–2010. Number of cases in each gender: male, 16,221; female: 11,993.
4C: Relative Survival Rates for Glioblastoma by Race, SEER 18 registries, 1995–2010. Number of cases in each race: whites, 25,360; blacks, 1,547; API, 1,151. *Relative survival rates for American Indian/Alaskan native are excluded due to low numbers.
X axis: time in years; Y axis: survival in percentage. Rates are an estimate of the percentage of patients alive at one, two, three, four, five, and ten year, respectively. Estimated by CBTRUS using SEER Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2011 Sub (1973–2009 varying) - Linked To County Attributes - Total U.S., 1969–2010 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2012, based on the November 2011 submission.
Abbreviation: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, CDC's National Program of Cancer Registries; SEER, NCI's Surveillance, Epidemiology and End Results program; API: Asian Pacific Islander (2).
GBM is an aggressive neoplasm which has a median survival of 3 months if untreated (41, 42). Combined modality therapy with surgery, RT and chemotherapy has significantly improved survival of GBM patients. Surgical intervention has decompressive and cytoreductive effects and there is increasing evidence of a significant survival advantage with complete resection (43, 44). Tumor fluorescence derived from 5-aminolevulinic acid enables more complete resections of contrast-enhancing tumor, leading to improved progression-free survival in patients with malignant gliomas (45).
In 2004 the European Organization for Research and Treatment of Cancer (EORTC) group and National Cancer Institute of Canada Clinical Trials Group (NCIC) presented a phase III study demonstrating a significant improvement in 2 year overall survival from 10.4% with post-operative radiotherapy alone to 26.5% with post-operative combined radiotherapy plus TMZ and an improvement in median overall survival (OS) from 12.1 to 14.6 months (5). These results also translated into a survival benefit in a population-based cohort after introduction of TMZ in 2005 (3). Additionally, a survival benefit was seen in each recursive partitioning analysis (RPA) class with combined modality therapy as compared to RT alone (46). The RPA classification is based on pre-therapeutic prognostic factors that have a more powerful impact on survival than any adjuvant treatment (47). It helps determine a particular category of patients who will benefit most from newer therapeutic approaches. The RTOG RPA classification is based on age, Karnofsky Performance Status (KPS) and neurologic function; classes III and IV include anaplastic astrocytomas as well as GBM. The EORTC RPA classification is based on age, WHO performance status and neurologic function determined by mini mental status exam; classes III and IV include only GBM patients (46). The overall median survival and 2 year survival was highly statistically different after combined modality treatment (RT + TMZ) among the three prognostic EORTC RPA classes (class III, IV, V) (46). The survival benefit of combined treatment as compared to RT alone was highest in RPA class III, advantage in class IV remained highly significant and there was small difference of borderline significance in class V(46).
Several variables affect the prognosis of patients with GBM, including age, preoperative performance status, tumor location, preoperative imaging characteristics of the tumor and extent of resection (7, 44, 47).
Site
The prognosis of cerebellar GBMs with respect to their supratentorial counterparts has been unclear (19–21, 48–50). However, age associated differences in survival are apparent at a younger age in cerebellar as compared to supratentorial GBMs (40 years versus 60 years) (19–21). Among supratentorial GBMs, frontal lobe tumors have better survival as compared to other sites (51, 52).
Gender
The relative survival in both men and women is the highest at 1 year (36.7% and 32.8% respectively), it declines steeply in the second year (13.7% in both genders) and gradually thereafter, with a very low 5 year survival rate (4.7% and 4.6% respectively) (Figure 4B) (2). Men have a significant survival advantage than women in the first year post-diagnosis but the difference is not significant thereafter (2).
Race
Population-based studies do not demonstrate a race based disparity in GBM survival (53–57). No significant differences in GBM survival have been observed among whites and blacks; however, Asian Pacific Islanders have a significantly better survival rates than both whites and blacks at all time points (Figure 4C) (2). In our recent population-based study, we studied interrelations between race, surgery received, and survival of GBM patients who were not treated with initial RT (57). We did not find race-based differences in outcomes among these GBM patients receiving different surgical interventions (57). The limited influence of therapy on GBM may be responsible in part for this result (55). There is also a possibility that biologic factors influencing GBM outcomes could be similar across races (57).
Age
Age of 50 years has been identified as an appropriate cut-off age for the clinical subdivision of patients with GBM into prognostically relevant subsets (7). In multivariate analysis of overall survival risk factors increasing age is associated with shortened survival (Figure 5) (2). Patients aged 70–74 and those >75 have a significantly higher risk of death than those 65–69 (58). Poorer survival in the older age group has been attributed to co-morbidities as well as decreased ability to withstand neurological insults caused by the tumor itself, surgery and/or adjuvant therapy (47, 59, 60). Additionally, aggressive tumors in older patients have been attributed to resistance genes and tumors with different molecular profiles (61–63).
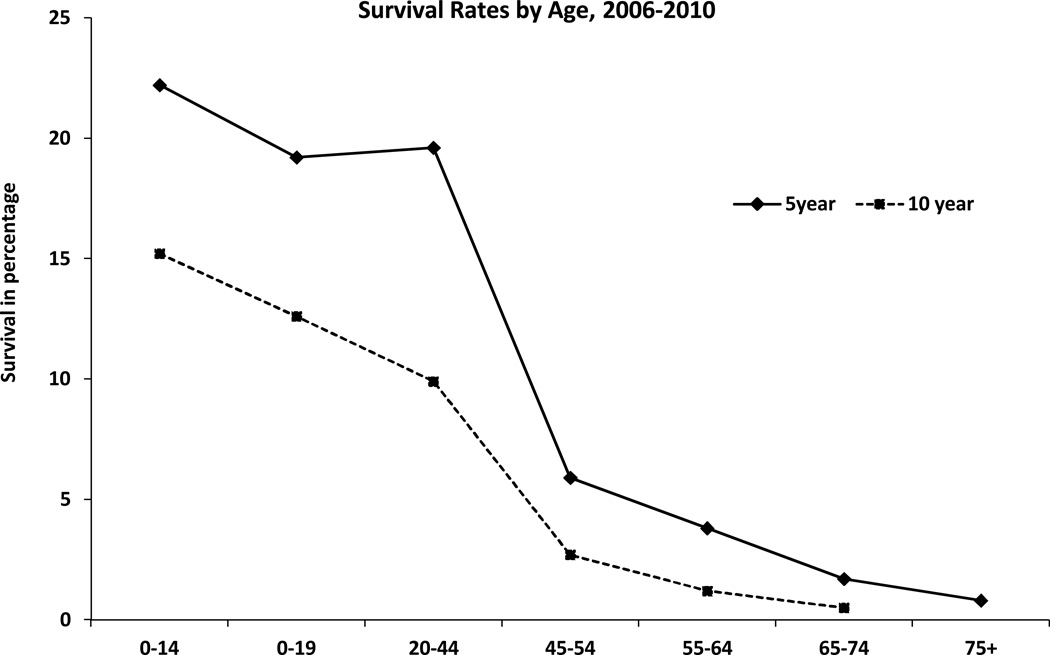
Figure 5.
Five and Ten-Year Relative Survival Rate for Glioblastoma by Age, SEER 18 Registries, 1995–2010. X axis: age groups; Y axis: survival in percentage. Rates are in percentage (%). Estimated by CBTRUS using SEER Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2011 Sub (1973–2009 varying) - Linked To County Attributes - Total U.S., 1969–2010 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2012, based on the November 2011 submission. Abbreviation: CBTRUS, Central Brain Tumor Registry of the United States; SEER, NCI's Surveillance, Epidemiology and End Results program (2).
Miscellaneous Factors
Various other factors including socioeconomic status (SES) and marital status could be associated with GBM survival differences. Marital status had a beneficial effect on survival in a SEER based population study, where unmarried patients with supratentorial GBM presented with larger tumors, were less likely to undergo both surgical resection and postoperative RT, and had a shorter survival after diagnosis when compared with married patients (64).
Effects of SES on survival have been examined by studying the primary payer for care (Medicare, Medicaid, self-pay, or private insurance) as predictor of in-hospital mortality (65). Compared with Medicare patients, Medicaid patients had higher mortality (58). For GBM patients with dual eligibility in Medicare and Medicaid were much less likely to report radiation claims than those with Medicare alone (58). Higher chemotherapy claims were reported in patients with a median annual income >35,000 than those with <25,000 (58). Those who reported radiation or chemotherapy claims had a significantly lower risk for death than those who did not (58).
Disparities in healthcare
In our recent SEER population-based study we did not find an obvious racial difference in receipt of RT for GBM patients (57). However, another SEER population-based study investigating the influence of regional health system resources on the surgical management of GBM and receipt of post-operative RT, found that younger, married patients in health service areas (HSAs) with higher median incomes were significantly more likely to receive both gross total resection and post-operative RT (66). For every $10,000 increase in the median income of a HSA, a patient’s likelihood of receiving gross resection increased by 7 % and post-operative RT receipt by 6.3 % (66). Their findings indicated that it may not be the density of individual radiation oncologists, but rather the prevalence of radiation oncology centers that influences post-operative RT receipt, suggesting a dominant role of hospital-level infrastructure over individual providers for addressing disparities in GBM management (66). It is possible that the large variations in treatment of GBM may be related less to access to neuro-oncology services, but a larger apprehension of physicians to attempt aggressive surgery and RT for patients with less favorable prognosis (66).
A significant percentage of GBM patients (27.3%) do not receive RT in the initial round of therapy and majority of these are elderly patients (65 years and older) (57, 67). Similar trends are seen in other SEER based population studies for breast and lung cancer where lower rates of RT with increased age were observed (68, 69). Under-use of RT in a large number of elderly GBM patients could be attributed increased risk of cognitive side-effects associated with RT (67, 70). However, recent data does show benefit of RT in the elderly population (71).
Prognostic Molecular Markers in GBM
Various molecular markers are associated with varying grades of glioma (Figure 6). All GBMs are WHO grade IV but exhibit significant genetic heterogeneity and tumor subtypes with genetic alterations exist within this larger homogeneous histologic category that carry prognostic significance (6, 72). Various prognostic markers have been identified in GBM, including methylation status of the gene promoter for O6-methylguanine-DNA methyltransferase (MGMT), isocitrate dehydrogenase enzyme 1/2 (IDH1/2) mutation, epidermal growth factor receptor (EGFR) overexpression and amplification, glioma-CpG island methylator phenotype (G-CIMP), tumor protein (TP53) mutation and genetic losses of chromosomes.
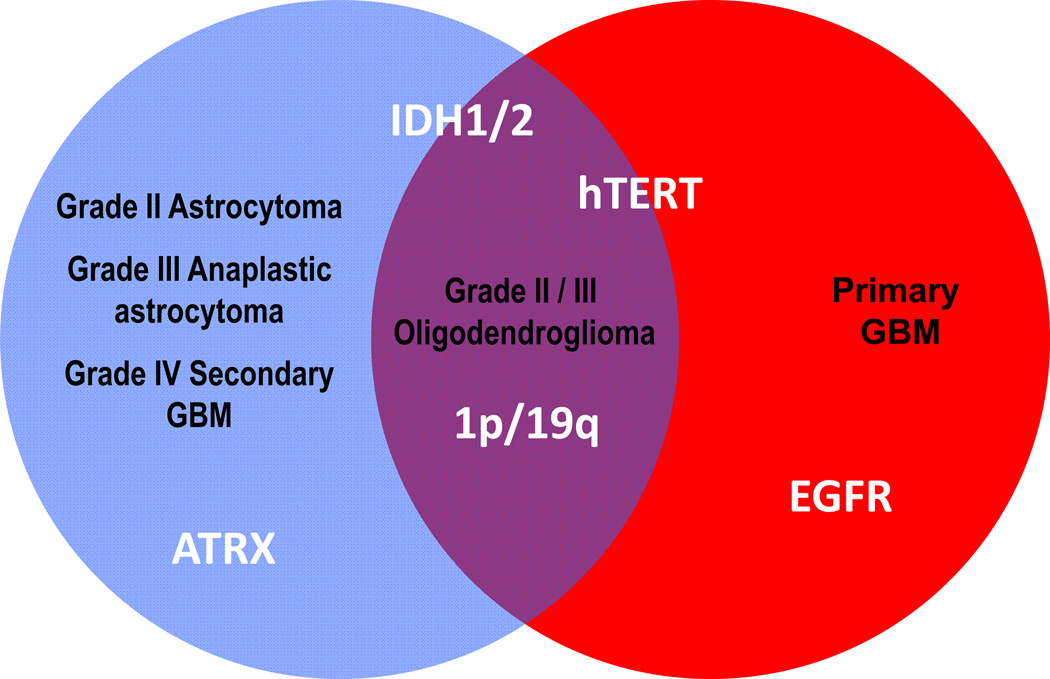
Figure 6.
Relationship between molecular markers and the different grades of glioma. Red = primary glioblastoma, blue = grades II, III astrocytomas and secondary glioblastoma, purple = grades II and III oligodendrogliomas. Abbreviations: ATRX, alpha thalassemia/mental retardation syndrome X-linked; IDH1/2, isocitrate dehydrogenase enzyme 1/2 mutation; EGFR, epidermal growth factor receptor, hTERT, high frequency of telomerase reverse transcriptase (hTERT) promoter mutation.
Primary GBMs show EGFR overexpression, phosphatase and tensin homolog gene (PTEN) mutations, loss of heterozygosity (LOH) 10q, p16 deletions, less frequently mouse double-minute 2 (MDM2) amplification, high frequency of telomerase reverse transcriptase (hTERT) promoter mutations and absence of IDH1 mutation (9, 73). The hallmark of secondary GBMs is TP53, alpha thalassemia/mental retardation syndrome X-linked (ATRX) and IDH1 mutations; additionally, they show LOH 10q (7–9). GBM-O occurs in younger patients and often contains TP53 mutations, IDH1 mutation and lack of EGFR amplification (6, 74). They have been reported to have longer survival as compared to other GBMs, have a lower frequency of PTEN deletions, and genetic heterogeneity (75–79).
There is a complex interaction between age and genetic alterations that result in variation of clinical outcomes in different age groups (72, 80, 81). GBM patients age < 50 years have molecularly and clinically distinct disease and age 40 years appears to be a more appropriate cut-off point for their further prognostic subdivision (82). Patient age < 40 years is strongly associated with a favorable prognosis while ≥ 40 years shows markers associated with shorter survival (wild type IDH1/2, EGFR amplification, loss of 9p, loss of 10q, and gain of chromosome 19) (82).
MGMT Status
TMZ is an alkylating agent that functions by transferring alkyl groups to guanine bases causing DNA damage and cellular death. Failure to repair alkylation results in apoptosis. MGMT is a DNA repair protein that removes alkyl groups from the O6 position of guanine in DNA, making cells resistant to the alkylating agent TMZ (83, 84). Methylation causes MGMT silencing that interferes with DNA repair and increases TMZ sensitivity while an unmethylated promoter for MGMT, results in active gene expression and high levels of the repair enzyme that results in chemotherapy resistance (85, 86).
The MGMT promoter is methylated in approximately 50% of newly diagnosed GBMs (87–89). MGMT methylation is associated with IDH1/2 mutant tumors due to which it is more common in secondary as compared to primary GBM (75% versus 36% respectively) (86, 90, 91). MGMT promoter methylation has prognostic and predictive significance in patients with GBM with better overall survival (OS) irrespective of treatment choices (84, 92, 93). Additionally, it is associated with better response to TMZ as well as RT and improves progression free survival (PFS) and OS with combined treatment approach (TMZ + RT) than either treatment modality alone (77, 84, 92, 94, 95).
IDH Mutation
IDH1/2 mutations are far more common in grades II and III astrocytomas and oligodendrogliomas compared to GBMs and over 90 % of the mutations involve IDH1(74, 96–99). The effects of IDH1/2 mutations on gliomagenesis are greater than inhibition of their wild-type counterparts, and more likely represent a true gain-of-function genetic change (100). IDH1-R132H point mutated enzyme (mutation in IDH1 at R132) prefers binding to alpha-ketoglutarate and reduces it to D-2-hydroxyglutarate (100). The levels of this oncometabolite are 10–100-fold higher in mutant gliomas than their wild-type counterparts (100).
IDH mutations tend to occur in younger adults (20–60-year range) (101–104). The relative frequency of IDH1 tumors rises sharply in the third decade of life and decreases in the fifth decade (105). IDH1 mutated high grade gliomas arise by transformation from lower-grade gliomas and have distinguishing radiographic, histologic and transcriptional features (frontal location and lesser extent of contrast enhancement and necrosis) that are consistent with a less aggressive clinical course (105). They are a selective molecular marker of secondary GBMs and distinguish them from primary GBMs (86, 97, 101). IDH1 mutated high grade gliomas have a more favorable prognosis than the ones without IDH1 mutation and the sequence from more favorable to poorer outcome is: anaplastic astrocytoma (AA) with IDH1 mutation, GBM with IDH1mutation, AA without IDH1 mutation and GBM without IDH1 mutation (97, 106).
G-CIMP
Analysis of the GBM DNA methylation array data generated by The Cancer Genome Atlas Research Network (TCGA) identified G-CIMP, a DNA methylation phenotype present in ~10% of GBM (107). This phenotype is strongly associated with IDH1 mutation and proneural tumor subtype, and is rare in primary GBM (~5–8%). There is a significant overall survival advantage of patients with G-CIMP, proneural tumor subtype and IDH1 mutation (107–109).
EGFR
EGFR is a transmembrane tyrosine kinase on chromosome 7p12 whose downstream signaling pathways modulate a wide range of cellular activities, including growth, migration, and survival (110). In GBMs, EGFR signaling promotes cell division, tumor invasiveness, and resistance to RT and chemotherapy (111–113). EGFR activity is enhanced by upregulation of EGFR protein expression, inhibition/deletion of downstream pathway inhibitors, constitutively active EGFR (EGFRvIII), and EGFR amplification (114, 115). EGFR amplification results in over-expression of EGFR (116–118). Alteration of the EGFR gene, results in over-expression of varied mutations, including the most common mutation, EGFRvIII (variant III), as well as wild-type EGFR (EGFRwt) (119–121). EGFRvIII (variant III) is the most common mutation among EGFR amplified GBMs and has been described in approximately 60–70% of these tumors (120, 122). EGFRvIII over-expression was found to be a strong predictor of poor prognosis in presence of EGFR amplification (123).
About 40% of all GBMs have EGFR amplification, and it is more common in primary as compared to secondary GBMs (7–9, 124, 125). There is experimental evidence that EGFR amplification may result in a less favorable prognosis; however, clinical studies are inconclusive (72, 117, 118). Some have shown the degree of EGFR amplification to impact survival with higher levels associated with longer median survival while others have found it to be differentially prognostic with age (115, 126). EGFR over-expression was associated with worse prognosis in younger patients and better prognosis in older patients (41, 72, 127). Data also suggest existence of a complex relationship of survival in GBM with the patient’s age, p53 and EGFR amplification. Poorer survival was noted in younger patients whose tumors over-expressed EFGR but had normal p53 immunohistochemistry (72). Additionally, lower levels of amplification correlated with worse response to TMZ-containing adjuvant therapeutic regimens compared to GBMs with high amplification or none at all (126).
TP53 mutation
Mutation of the TP53 gene has been found in 60% to 70% of secondary GBMs, 25% to 30% of primary GBMs and occurs more frequently in younger patients (9). Studies of TP53 mutations as a prognostic marker have not been definitive (72, 128, 129).
ATRX mutation
Mutations in ATRX have been identified in multiple tumor types and appear to cause alternative lengthening of telomeres (ALT), a presumed precursor to genomic instability (130, 131). ATRX alterations are present mainly in tumors of an astrocytic lineage and are specific to astrocytic tumors carrying IDH1/2 and TP53 mutations (132). They are more common in secondary as compared to primary GBMs (132, 133).
ATRX is frequently mutated in grade II-III astrocytomas (71%), oligoastrocytomas (68%), and secondary GBMs (57%), but is infrequent in primary (4%) and pediatric GBMs (20%) as well as pure oligodendroglial tumors (14%) (133). ATRX mutations are associated with alternative lengthening of telomeres phenotype among GBMs (133, 134). They cluster with IDH1 and TP53 mutations in secondary GBMs (133).
In a prospective cohort of patients with astrocytic tumors, those harboring ATRX loss had a significantly better prognosis than the ones that expressed ATRX and had IDH mutation (135). Jiao et al described the prognostic molecular classification of gliomas and based on 3 gene signatures (133). The I-A signature was defined by alterations in ATRX and IDH; with ALT and TP53 mutations. These tumors were grade II, III astrocytomas and secondary GBMs, were often diagnosed in the fourth decade of life and had a median survival of 51 months (133). I-CF signature was defined by IDH mutations and by alterations in either capicua transcriptional repressor (CIC), far upstream element (FUSE) binding protein 1 (FUBP1) and/or 1p/19q, rarely displayed ALT. These tumors typically had an oligodendroglial component and were often diagnosed in the fifth decade of life with a median survival of 96 months (133). I-X signature was defined by lack of IDH or ATRX mutations. I-X tumors are a genetically heterogeneous group, associated with poor patient survival 13 months, advanced patient age, and are similar to primary GBMs (133, 134).
TERT
TERT is involved in telomere maintenance which is essential for actively growing cells. TERT mutation is one of the most frequent genetic alterations in primary adult GBMs and is significantly higher in these tumors as compared to secondary adult or any pediatric GBMs (73, 136). In GBMs TERT mutations are significantly correlated with EGFR amplification but have an inverse correlation with IDH and TP53 mutations (73, 136). Although TERT mutations have yet to be directly compared with ATRX mutations, it is highly probable that the two are mutually exclusive. GBMs with TERT mutation have a shorter survival than those without TERT mutations (136). However, when adjusted for GBM subtype (primary and secondary), they do not have a significant impact on survival (136).
Genetic losses of chromosomes
Losses on chromosome 10
Some of the most frequent genetic alterations in GBMs are genetic losses on chromosome 10 (80–90%), occurring either as loss of the entire chromosome or as loss of only the long or short arms (9). Phosphatase and tensin (PTEN), located at 10q23.3, was the first tumor suppressor gene identified on chromosome 10 and is mutated in 20% to 40% of GBMs and almost exclusively in primary GBM (7, 137). Prognostic role of 10q deletion in GBM are controversial, with some studies suggesting 10q loss as an indicator of poor outcome whereas others did not report a significant role as prognostic factor (127, 129, 138, 139).
1p/19q status
1p/19q deletions (loss of the short arm of chromosome 1 and the long arm of chromosome 19) predict response to chemotherapy and better prognosis in anaplastic oligodendrogliomas (140). However, it has no utility in histologically unequivocal GBMs (141).
Conclusion
While many studies have investigated the basis of incidence differences by gender, age, race, and risk factors for GBM, many of these studies had inconclusive findings. Although ionizing RT increases risk and allergies decrease risk, these factors do not account for a large proportion of GBMs. Hence, further studies are warranted to untangle the complexities of GBM etiology.
Advances have been made in development of prognostic tools and identifying molecular markers that help predict prognosis and response to therapy. Progress in investigating the molecular biology has led to identification of GBM subsets that are biologically similar, are more susceptible to standard therapy and have a better prognosis. Efforts to establish the role of IDH1 and MGMT in predicting therapeutic response is ongoing. Understanding the role of IDH1/2 mutations in promoting gliomagenesis, its effect on prognosis, and targeting IDH1/2 mutations in novel therapies holds promise to make advances in preventive and treatment strategies (86).
In summary, GBM represents a molecularly heterogeneous disease with numerous sub-classifications. The field has invested significant resources on this characterization and is now poised to advance therapies specific to the genetic abnormalities of each subtype. The success of m-TOR pathway inhibition for subependymal giant-cell astrocytomas and the possibility of identifying a subtype of GBM sensitive to up-front treatment with bevacizumab are examples, but we need much more (142, 143). The complex molecular changes associated with GBM will likely make personalized therapy challenging and although clinical advances in GBM are rare, we are in a new era in cancer biology. Whether an immune based therapy or treating multiple targets will provide the breakthroughs is yet unknown, but we expect meaningful clinical advances to occur—and soon.
Acknowledgments
Financial disclosure: J. Thakkar, T. Dolecek, and J. Villano were supported by the National Cancer Institute (R03CA156561).
Q. Ostorm and J. Barnholtz-Sloan were supported in part by the Case Comprehensive Cancer Center Support Grant (NIH/NCI P30 CA043703), and provided in part by the Central Brain Tumor Registry of the United States (CBTRUS), which received support from the National Brain Tumor Society, the Pediatric Brain Tumor Foundation, Novocure Inc., private donations, and from the Cooperative Agreement 5U58DP003831 from the Centers for Disease Control and Prevention.
C. Horbinski was supported by the National Cancer Institute (K08CA155764) and a 2P20 RR020171 COBRE pilot grant (National Institute of General Medical Sciences).
D. Lightner did not receive funding.
Footnotes
Conflict of interest: None
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koshy M, Villano JL, Dolecek TA, Howard A, Mahmood U, Chmura SJ, et al. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. 2012;107:207–212. doi: 10.1007/s11060-011-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran B, Rosenthal MA. Survival comparison between glioblastoma multiforme and other incurable cancers. J Clin Neurosci. 2010;17:417–421. doi: 10.1016/j.jocn.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6.Kleihues P, Ohgaki H. Phenotype vs genotype in the evolution of astrocytic brain tumors. Toxicol Pathol. 2000;28:164–170. doi: 10.1177/019262330002800121. [DOI] [PubMed] [Google Scholar]
- 7.Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 8.Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009;100:2235–2241. doi: 10.1111/j.1349-7006.2009.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278–299. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakrabarti I, Cockburn M, Cozen W, Wang YP, Preston-Martin S. A population-based description of glioblastoma multiforme in Los Angeles County, 1974-1999. Cancer. 2005;104:2798–2806. doi: 10.1002/cncr.21539. [DOI] [PubMed] [Google Scholar]
- 12.Yabroff KR, Harlan L, Zeruto C, Abrams J, Mann B. Patterns of care and survival for patients with glioblastoma multiforme diagnosed during 2006. Neuro Oncol. 2012;14:351–359. doi: 10.1093/neuonc/nor218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Cancer Society. Cancer Facts & Figures 2010. Atlanta: American Cancer Society; 2010. [Google Scholar]
- 14.Sloane D. Cancer epidemiology in the United States: racial, social, and economic factors. Methods Mol Biol. 2009;471:65–83. doi: 10.1007/978-1-59745-416-2_4. [DOI] [PubMed] [Google Scholar]
- 15.McKinley BP, Michalek AM, Fenstermaker RA, Plunkett RJ. The impact of age and sex on the incidence of glial tumors in New York state from 1976 to 1995. J Neurosurg. 2000;93:932–939. doi: 10.3171/jns.2000.93.6.0932. [DOI] [PubMed] [Google Scholar]
- 16.Fleury A, Menegoz F, Grosclaude P, Daures J-P, Henry-Amar M, Raverdy N, et al. Descriptive epidemiology of cerebral gliomas in France. Cancer. 1997;79:1195–1202. doi: 10.1002/(sici)1097-0142(19970315)79:6<1195::aid-cncr19>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Walker AE, Robins M, Weinfeld FD. Epidemiology of brain tumors: the national survey of intracranial neoplasms. Neurology. 1985;35:219–226. doi: 10.1212/wnl.35.2.219. [DOI] [PubMed] [Google Scholar]
- 18.Engelhard HH, Villano JL, Porter KR, Stewart AK, Barua M, Barker FG, et al. Clinical presentation, histology, and treatment in 430 patients with primary tumors of the spinal cord, spinal meninges, or cauda equina. J Neurosurg Spine. 2010;13:67–77. doi: 10.3171/2010.3.SPINE09430. [DOI] [PubMed] [Google Scholar]
- 19.Adams H, Chaichana KL, Avendano J, Liu B, Raza SM, Quinones-Hinojosa A. Adult Cerebellar Glioblastoma: Understanding Survival and Prognostic Factors Using A Population-Based Database from 1973-- 2009. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeswani S, Nuno M, Folkerts V, Mukherjee D, Black KL, Patil CG. Comparison of survival between cerebellar and supratentorial glioblastoma patients: surveillance, epidemiology, and end results (SEER) analysis. Neurosurgery. 2013;73:240–246. doi: 10.1227/01.neu.0000430288.85680.37. discussion 6; quiz 6. [DOI] [PubMed] [Google Scholar]
- 21.Babu R, Sharma R, Karikari IO, Owens TR, Friedman AH, Adamson C. Outcome and prognostic factors in adult cerebellar glioblastoma. J Clin Neurosci. 2013;20:1117–1121. doi: 10.1016/j.jocn.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Hodges LC, Smith JL, Garrett A, Tate S. Prevalence of glioblastoma multiforme in subjects with prior therapeutic radiation. The Journal of neuroscience nursing : journal of the American Association of Neuroscience Nurses. 1992;24:79–83. doi: 10.1097/01376517-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Schwartzbaum JA, Ahlbom A, Lonn S, Malmer B, Wigertz A, Auvinen A, et al. An international case-control study of interleukin-4Ralpha, interleukin-13, and cyclooxygenase-2 polymorphisms and glioblastoma risk. Cancer Epidemiol Biomarkers Prev. 2007;16:2448–2454. doi: 10.1158/1055-9965.EPI-07-0480. [DOI] [PubMed] [Google Scholar]
- 24.Schwartzbaum JA, Xiao Y, Liu Y, Tsavachidis S, Berger MS, Bondy ML, et al. Inherited variation in immune genes and pathways and glioblastoma risk. Carcinogenesis. 2010;31:1770–1777. doi: 10.1093/carcin/bgq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wrensch M, Jenkins RB, Chang JS, Yeh RF, Xiao Y, Decker PA, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nature genetics. 2009;41:905–908. doi: 10.1038/ng.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner AV, Linet MS, Fine HA, Shapiro WR, Selker RG, Black PM, et al. History of allergies and autoimmune diseases and risk of brain tumors in adults. International journal of cancer Journal international du cancer. 2002;99:252–259. doi: 10.1002/ijc.10320. [DOI] [PubMed] [Google Scholar]
- 27.Schlehofer B, Blettner M, Preston-Martin S, Niehoff D, Wahrendorf J, Arslan A, et al. Role of medical history in brain tumour development. Results from the international adult brain tumour study. International journal of cancer Journal international du cancer. 1999;82:155–160. doi: 10.1002/(sici)1097-0215(19990719)82:2<155::aid-ijc1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 28.Lachance DH, Yang P, Johnson DR, Decker PA, Kollmeyer TM, McCoy LS, et al. Associations of high-grade glioma with glioma risk alleles and histories of allergy and smoking. American journal of epidemiology. 2011;174:574–581. doi: 10.1093/aje/kwr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheurer ME, Amirian ES, Davlin SL, Rice T, Wrensch M, Bondy ML. Effects of antihistamine and anti-inflammatory medication use on risk of specific glioma histologies. International journal of cancer Journal international du cancer. 2011;129:2290–2296. doi: 10.1002/ijc.25883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitahara CM, Wang SS, Melin BS, Wang Z, Braganza M, Inskip PD, et al. Association between adult height, genetic susceptibility and risk of glioma. Int J Epidemiol. 2012;41:1075–1085. doi: 10.1093/ije/dys114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hochberg F, Toniolo P, Cole P, Salcman M. Nonoccupational risk indicators of glioblastoma in adults. J Neurooncol. 1990;8:55–60. doi: 10.1007/BF00182087. [DOI] [PubMed] [Google Scholar]
- 32.Frei P, Poulsen AH, Johansen C, Olsen JH, Steding-Jessen M, Schuz J. Use of mobile phones and risk of brain tumours: update of Danish cohort study. Bmj. 2011;343:d6387. doi: 10.1136/bmj.d6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagorio S, Roosli M. Mobile phone use and risk of intracranial tumors: A consistency analysis. Bioelectromagnetics. 2014;35:79–90. doi: 10.1002/bem.21829. [DOI] [PubMed] [Google Scholar]
- 34.Hardell L, Carlberg M, Soderqvist F, Mild KH. Case-control study of the association between malignant brain tumours diagnosed between 2007 and 2009 and mobile and cordless phone use. Int J Oncol. 2013;43:1833–1845. doi: 10.3892/ijo.2013.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Group IS. Brain tumour risk in relation to mobile telephone use: results of the INTERPHONE international case-control study. Int J Epidemiol. 2010;39:675–694. doi: 10.1093/ije/dyq079. [DOI] [PubMed] [Google Scholar]
- 36.Benson VS, Pirie K, Schuz J, Reeves GK, Beral V, Green J, et al. Mobile phone use and risk of brain neoplasms and other cancers: prospective study. Int J Epidemiol. 2013;42:792–802. doi: 10.1093/ije/dyt072. [DOI] [PubMed] [Google Scholar]
- 37.Deltour I, Auvinen A, Feychting M, Johansen C, Klaeboe L, Sankila R, et al. Mobile phone use and incidence of glioma in the Nordic countries 1979-2008: consistency check. Epidemiology. 2012;23:301–307. doi: 10.1097/EDE.0b013e3182448295. [DOI] [PubMed] [Google Scholar]
- 38.Smoll NR, Schaller K, Gautschi OP. Long-term survival of patients with glioblastoma multiforme (GBM) J Clin Neurosci. 2013 doi: 10.1016/j.jocn.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 39.Johnson DR, Ma DJ, Buckner JC, Hammack JE. Conditional probability of long-term survival in glioblastoma: a population-based analysis. Cancer. 2012;118:5608–5613. doi: 10.1002/cncr.27590. [DOI] [PubMed] [Google Scholar]
- 40.Porter KR, McCarthy BJ, Berbaum ML, Davis FG. Conditional survival of all primary brain tumor patients by age, behavior, and histology. Neuroepidemiology. 2011;36:230–239. doi: 10.1159/000327752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malmstrom A, Gronberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 42.Percy AK, Elveback LR, Okazaki H, Kurland LT. Neoplasms of the central nervous system. Epidemiologic considerations. Neurology. 1972;22:40–48. doi: 10.1212/wnl.22.1.40. [DOI] [PubMed] [Google Scholar]
- 43.Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62:564–576. doi: 10.1227/01.neu.0000317304.31579.17. discussion-76. [DOI] [PubMed] [Google Scholar]
- 44.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 45.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 46.Mirimanoff RO, Gorlia T, Mason W, Van den Bent MJ, Kortmann RD, Fisher B, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol. 2006;24:2563–2569. doi: 10.1200/JCO.2005.04.5963. [DOI] [PubMed] [Google Scholar]
- 47.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6:227–235. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber DC, Miller RC, Villa S, Hanssens P, Baumert BG, Castadot P, et al. Outcome and prognostic factors in cerebellar glioblastoma multiforme in adults: a retrospective study from the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2006;66:179–186. doi: 10.1016/j.ijrobp.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 49.Djalilian HR, Hall WA. Malignant gliomas of the cerebellum: an analytic review. J Neurooncol. 1998;36:247–257. doi: 10.1023/a:1005704006244. [DOI] [PubMed] [Google Scholar]
- 50.Levine SA, McKeever PE, Greenberg HS. Primary cerebellar glioblastoma multiforme. J Neurooncol. 1987;5:231–236. doi: 10.1007/BF00151226. [DOI] [PubMed] [Google Scholar]
- 51.Simpson JR, Horton J, Scott C, Curran WJ, Rubin P, Fischbach J, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993;26:239–244. doi: 10.1016/0360-3016(93)90203-8. [DOI] [PubMed] [Google Scholar]
- 52.Jeremic B, Grujicic D, Antunovic V, Djuric L, Stojanovic M, Shibamoto Y. Influence of extent of surgery and tumor location on treatment outcome of patients with glioblastoma multiforme treated with combined modality approach. J Neurooncol. 1994;21:177–185. doi: 10.1007/BF01052902. [DOI] [PubMed] [Google Scholar]
- 53.Barnholtz-Sloan JS, Maldonado JL, Williams VL, Curry WT, Rodkey EA, Barker FG, 2nd, et al. Racial/ethnic differences in survival among elderly patients with a primary glioblastoma. J Neurooncol. 2007;85:171–180. doi: 10.1007/s11060-007-9405-4. [DOI] [PubMed] [Google Scholar]
- 54.McLendon RE, Robinson JS, Jr, Chambers DB, Grufferman S, Burger PC. The glioblastoma multiforme in Georgia, 1977-1981. Cancer. 1985;56:894–897. doi: 10.1002/1097-0142(19850815)56:4<894::aid-cncr2820560432>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 55.Simpson JR, Scott CB, Rotman M, Curran WJ, Constine LS, 3rd, Fischbach AJ, et al. Race and prognosis of brain tumor patients entering multicenter clinical trials. A report from the Radiation Therapy Oncology Group. Am J Clin Oncol. 1996;19:114–120. doi: 10.1097/00000421-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Robertson JT, Gunter BC, Somes GW. Racial differences in the incidence of gliomas: a retrospective study from Memphis, Tennessee. Br J Neurosurg. 2002;16:562–566. [PubMed] [Google Scholar]
- 57.Thakkar JP, Dolecek TA, Popa AM, Villano JL. Racial disparities in survival of glioblastoma patients not treated with radiation. American Society of Clinical Oncology; 2013 ASCO Annual Meeting; Chicago, IL. J Clin Oncol. 31 (suppl; abstr 2069). [Google Scholar]
- 58.Sherwood PR, Dahman BA, Donovan HS, Mintz A, Given CW, Bradley CJ. Treatment disparities following the diagnosis of an astrocytoma. J Neurooncol. 2011;101:67–74. doi: 10.1007/s11060-010-0223-8. [DOI] [PubMed] [Google Scholar]
- 59.Krex D, Klink B, Hartmann C, Von Deimling A, Pietsch T, Simon M, et al. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 60.Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–473. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 61.Alonso M, Hamelin R, Kim M, Porwancher K, Sung T, Parhar P, et al. Microsatellite instability occurs in distinct subtypes of pediatric but not adult central nervous system tumors. Cancer Res. 2001;61:2124–2128. [PubMed] [Google Scholar]
- 62.Rickert CH, Strater R, Kaatsch P, Wassmann H, Jurgens H, Dockhorn-Dworniczak B, et al. Pediatric high-grade astrocytomas show chromosomal imbalances distinct from adult cases. Am J Pathol. 2001;158:1525–1532. doi: 10.1016/S0002-9440(10)64103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sung T, Miller DC, Hayes RL, Alonso M, Yee H, Newcomb EW. Preferential inactivation of the p53 tumor suppressor pathway and lack of EGFR amplification distinguish de novo high grade pediatric astrocytomas from de novo adult astrocytomas. Brain Pathol. 2000;10:249–259. doi: 10.1111/j.1750-3639.2000.tb00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang SM, Barker FG., 2nd Marital status, treatment, and survival in patients with glioblastoma multiforme: a population based study. Cancer. 2005;104:1975–1984. doi: 10.1002/cncr.21399. [DOI] [PubMed] [Google Scholar]
- 65.Curry WT, Jr, Barker FG., 2nd Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol. 2009;93:25–39. doi: 10.1007/s11060-009-9840-5. [DOI] [PubMed] [Google Scholar]
- 66.Aneja S, Khullar D, Yu JB. The influence of regional health system characteristics on the surgical management and receipt of post operative radiation therapy for glioblastoma multiforme. J Neurooncol. 2013 doi: 10.1007/s11060-013-1068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barnholtz-Sloan JS, Williams VL, Maldonado JL, Shahani D, Stockwell HG, Chamberlain M, et al. Patterns of care and outcomes among elderly individuals with primary malignant astrocytoma. J Neurosurg. 2008;108:642–648. doi: 10.3171/JNS/2008/108/4/0642. [DOI] [PubMed] [Google Scholar]
- 68.Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, McCarthy EP. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010;28:2038–2045. doi: 10.1200/JCO.2009.25.9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Owonikoko TK, Ragin CC, Belani CP, Oton AB, Gooding WE, Taioli E, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007;25:5570–5577. doi: 10.1200/JCO.2007.12.5435. [DOI] [PubMed] [Google Scholar]
- 70.Weller M, Platten M, Roth P, Wick W. Geriatric neuro-oncology: from mythology to biology. Curr Opin Neurol. 2011;24:599–604. doi: 10.1097/WCO.0b013e32834c4967. [DOI] [PubMed] [Google Scholar]
- 71.Gzell C, Wheeler H, Guo L, Kastelan M, Back M. Elderly patients aged 65-75 years with glioblastoma multiforme may benefit from long course radiation therapy with temozolomide. J Neurooncol. 2014 doi: 10.1007/s11060-014-1472-8. [DOI] [PubMed] [Google Scholar]
- 72.Simmons ML, Lamborn KR, Takahashi M, Chen P, Israel MA, Berger MS, et al. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res. 2001;61:1122–1128. [PubMed] [Google Scholar]
- 73.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, Jr, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110:6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Appin CL, Gao J, Chisolm C, Torian M, Alexis D, Vincentelli C, et al. Glioblastoma with oligodendroglioma component (GBM-O): molecular genetic and clinical characteristics. Brain Pathol. 2013;23:454–461. doi: 10.1111/bpa.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ha SY, Kang SY, Do IG, Suh YL. Glioblastoma with oligodendroglial component represents a subgroup of glioblastoma with high prevalence of IDH1 mutation and association with younger age. J Neurooncol. 2013;112:439–448. doi: 10.1007/s11060-013-1073-y. [DOI] [PubMed] [Google Scholar]
- 77.Hegi ME, Janzer RC, Lambiv WL, Gorlia T, Kouwenhoven MC, Hartmann C, et al. Presence of an oligodendroglioma-like component in newly diagnosed glioblastoma identifies a pathogenetically heterogeneous subgroup and lacks prognostic value: central pathology review of the EORTC_26981/NCIC_CE.3 trial. Acta Neuropathol. 2012;123:841–852. doi: 10.1007/s00401-011-0938-4. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y, Li S, Chen L, You G, Bao Z, Yan W, et al. Glioblastoma with an oligodendroglioma component: distinct clinical behavior, genetic alterations, and outcome. Neuro Oncol. 2012;14:518–525. doi: 10.1093/neuonc/nor232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franco-Hernandez C, Martinez-Glez V, de Campos JM, Isla A, Vaquero J, Gutierrez M, et al. Allelic status of 1p and 19q in oligodendrogliomas and glioblastomas: multiplex ligation-dependent probe amplification versus loss of heterozygosity. Cancer genetics and cytogenetics. 2009;190:93–96. doi: 10.1016/j.cancergencyto.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 80.Ciammella P, Podgornii A, Galeandro M, N DA, Pisanello A, Botti A, et al. Hypofractionated stereotactic radiation therapy for recurrent glioblastoma: single institutional experience. Radiation oncology. 2013;8:222. doi: 10.1186/1748-717X-8-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kleinberg L. Polifeprosan 20, 3.85% carmustine slow-release wafer in malignant glioma: evidence for role in era of standard adjuvant temozolomide. Core evidence. 2012;7:115–130. doi: 10.2147/CE.S23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Korshunov A, Sycheva R, Golanov A. The prognostic relevance of molecular alterations in glioblastomas for patients age < 50 years. Cancer. 2005;104:825–832. doi: 10.1002/cncr.21221. [DOI] [PubMed] [Google Scholar]
- 83.Tabatabai G, Hegi M, Stupp R, Weller M. Clinical implications of molecular neuropathology and biomarkers for malignant glioma. Curr Neurol Neurosci Rep. 2012;12:302–307. doi: 10.1007/s11910-012-0263-x. [DOI] [PubMed] [Google Scholar]
- 84.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 85.Nakagawachi T, Soejima H, Urano T, Zhao W, Higashimoto K, Satoh Y, et al. Silencing effect of CpG island hypermethylation and histone modifications on O6-methylguanine-DNA methyltransferase (MGMT) gene expression in human cancer. Oncogene. 2003;22:8835–8844. doi: 10.1038/sj.onc.1207183. [DOI] [PubMed] [Google Scholar]
- 86.Horbinski C. What do we know about IDH1/2 mutations so far, and how do we use it? Acta Neuropathol. 2013;125:621–636. doi: 10.1007/s00401-013-1106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mellai M, Monzeglio O, Piazzi A, Caldera V, Annovazzi L, Cassoni P, et al. MGMT promoter hypermethylation and its associations with genetic alterations in a series of 350 brain tumors. J Neurooncol. 2012;107:617–631. doi: 10.1007/s11060-011-0787-y. [DOI] [PubMed] [Google Scholar]
- 88.Ohka F, Natsume A, Motomura K, Kishida Y, Kondo Y, Abe T, et al. The global DNA methylation surrogate LINE-1 methylation is correlated with MGMT promoter methylation and is a better prognostic factor for glioma. PLoS One. 2011;6:e23332. doi: 10.1371/journal.pone.0023332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Preusser M, Charles Janzer R, Felsberg J, Reifenberger G, Hamou MF, Diserens AC, et al. Anti-O6-methylguanine-methyltransferase (MGMT) immunohistochemistry in glioblastoma multiforme: observer variability and lack of association with patient survival impede its use as clinical biomarker. Brain Pathol. 2008;18:520–532. doi: 10.1111/j.1750-3639.2008.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakamura M, Watanabe T, Yonekawa Y, Kleihues P, Ohgaki H. Promoter methylation of the DNA repair gene MGMT in astrocytomas is frequently associated with G:C -->A:T mutations of the TP53 tumor suppressor gene. Carcinogenesis. 2001;22:1715–1719. doi: 10.1093/carcin/22.10.1715. [DOI] [PubMed] [Google Scholar]
- 91.Eoli M, Menghi F, Bruzzone MG, De Simone T, Valletta L, Pollo B, et al. Methylation of O6-methylguanine DNA methyltransferase and loss of heterozygosity on 19q and/or 17p are overlapping features of secondary glioblastomas with prolonged survival. Clin Cancer Res. 2007;13:2606–2613. doi: 10.1158/1078-0432.CCR-06-2184. [DOI] [PubMed] [Google Scholar]
- 92.Olson RA, Brastianos PK, Palma DA. Prognostic and predictive value of epigenetic silencing of MGMT in patients with high grade gliomas: a systematic review and meta-analysis. J Neurooncol. 2011;105:325–335. doi: 10.1007/s11060-011-0594-5. [DOI] [PubMed] [Google Scholar]
- 93.Hegi ME, Diserens AC, Godard S, Dietrich PY, Regli L, Ostermann S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10:1871–1874. doi: 10.1158/1078-0432.ccr-03-0384. [DOI] [PubMed] [Google Scholar]
- 94.Rivera AL, Pelloski CE, Gilbert MR, Colman H, De La Cruz C, Sulman EP, et al. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 2010;12:116–121. doi: 10.1093/neuonc/nop020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 96.Bleeker FE, Lamba S, Leenstra S, Troost D, Hulsebos T, Vandertop WP, et al. IDH1 mutations at residue p.R132 (IDH1(R132)) occur frequently in high-grade gliomas but not in other solid tumors. Human mutation. 2009;30:7–11. doi: 10.1002/humu.20937. [DOI] [PubMed] [Google Scholar]
- 97.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68:1319–1325. doi: 10.1097/NEN.0b013e3181c391be. [DOI] [PubMed] [Google Scholar]
- 99.Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–474. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 100.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15:6002–6007. doi: 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- 102.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pollack IF, Hamilton RL, Sobol RW, Nikiforova MN, Lyons-Weiler MA, LaFramboise WA, et al. IDH1 mutations are common in malignant gliomas arising in adolescents: a report from the Children's Oncology Group. Childs Nerv Syst. 2011;27:87–94. doi: 10.1007/s00381-010-1264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.SongTao Q, Lei Y, Si G, YanQing D, HuiXia H, XueLin Z, et al. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci. 2012;103:269–273. doi: 10.1111/j.1349-7006.2011.02134.x. [DOI] [PubMed] [Google Scholar]
- 105.Lai A, Kharbanda S, Pope WB, Tran A, Solis OE, Peale F, et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29:4482–4490. doi: 10.1200/JCO.2010.33.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120:707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 107.Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nagane M, Coufal F, Lin H, Bogler O, Cavenee WK, Huang HJ. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 111.Chakravarti A, Chakladar A, Delaney MA, Latham DE, Loeffler JS. The epidermal growth factor receptor pathway mediates resistance to sequential administration of radiation and chemotherapy in primary human glioblastoma cells in a RAS-dependent manner. Cancer Res. 2002;62:4307–4315. [PubMed] [Google Scholar]
- 112.Mazzoleni S, Politi LS, Pala M, Cominelli M, Franzin A, Sergi Sergi L, et al. Epidermal growth factor receptor expression identifies functionally and molecularly distinct tumor-initiating cells in human glioblastoma multiforme and is required for gliomagenesis. Cancer Res. 2010;70:7500–7513. doi: 10.1158/0008-5472.CAN-10-2353. [DOI] [PubMed] [Google Scholar]
- 113.Hatanpaa KJ, Burma S, Zhao D, Habib AA. Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia. 2010;12:675–684. doi: 10.1593/neo.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aldape KD, Ballman K, Furth A, Buckner JC, Giannini C, Burger PC, et al. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol. 2004;63:700–707. doi: 10.1093/jnen/63.7.700. [DOI] [PubMed] [Google Scholar]
- 115.Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N, et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93:1246–1256. doi: 10.1093/jnci/93.16.1246. [DOI] [PubMed] [Google Scholar]
- 116.von Deimling A, Louis DN, von Ammon K, Petersen I, Hoell T, Chung RY, et al. Association of epidermal growth factor receptor gene amplification with loss of chromosome 10 in human glioblastoma multiforme. J Neurosurg. 1992;77:295–301. doi: 10.3171/jns.1992.77.2.0295. [DOI] [PubMed] [Google Scholar]
- 117.Waha A, Baumann A, Wolf HK, Fimmers R, Neumann J, Kindermann D, et al. Lack of prognostic relevance of alterations in the epidermal growth factor receptor-transforming growth factor-alpha pathway in human astrocytic gliomas. J Neurosurg. 1996;85:634–641. doi: 10.3171/jns.1996.85.4.0634. [DOI] [PubMed] [Google Scholar]
- 118.Galanis E, Buckner J, Kimmel D, Jenkins R, Alderete B, O'Fallon J, et al. Gene amplification as a prognostic factor in primary and secondary high-grade malignant gliomas. Int J Oncol. 1998;13:717–724. [PubMed] [Google Scholar]
- 119.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A. 1987;84:6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. [PubMed] [Google Scholar]
- 121.Ekstrand AJ, Sugawa N, James CD, Collins VP. Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tails. Proc Natl Acad Sci U S A. 1992;89:4309–4313. doi: 10.1073/pnas.89.10.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schwechheimer K, Huang S, Cavenee WK. EGFR gene amplification--rearrangement in human glioblastomas. International journal of cancer Journal international du cancer. 1995;62:145–148. doi: 10.1002/ijc.2910620206. [DOI] [PubMed] [Google Scholar]
- 123.Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–6970. [PubMed] [Google Scholar]
- 124.Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–2172. [PubMed] [Google Scholar]
- 125.Benito R, Gil-Benso R, Quilis V, Perez M, Gregori-Romero M, Roldan P, et al. Primary glioblastomas with and without EGFR amplification: relationship to genetic alterations and clinicopathological features. Neuropathology : official journal of the Japanese Society of Neuropathology. 2010;30:392–400. doi: 10.1111/j.1440-1789.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- 126.Hobbs J, Nikiforova MN, Fardo DW, Bortoluzzi S, Cieply K, Hamilton RL, et al. Paradoxical relationship between the degree of EGFR amplification and outcome in glioblastomas. The American journal of surgical pathology. 2012;36:1186–1193. doi: 10.1097/PAS.0b013e3182518e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Batchelor TT, Betensky RA, Esposito JM, Pham LD, Dorfman MV, Piscatelli N, et al. Age-dependent prognostic effects of genetic alterations in glioblastoma. Clin Cancer Res. 2004;10:228–233. doi: 10.1158/1078-0432.ccr-0841-3. [DOI] [PubMed] [Google Scholar]
- 128.Leenstra S, Oskam NT, Bijleveld EH, Bosch DA, Troost D, Hulsebos TJ. Genetic sub-types of human malignant astrocytoma correlate with survival. International journal of cancer Journal international du cancer. 1998;79:159–165. doi: 10.1002/(sici)1097-0215(19980417)79:2<159::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 129.Hill C, Hunter SB, Brat DJ. Genetic markers in glioblastoma: prognostic significance and future therapeutic implications. Advances in anatomic pathology. 2003;10:212–217. doi: 10.1097/00125480-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 130.Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 131.Lovejoy CA, Li W, Reisenweber S, Thongthip S, Bruno J, de Lange T, et al. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS genetics. 2012;8:e1002772. doi: 10.1371/journal.pgen.1002772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu XY, Gerges N, Korshunov A, Sabha N, Khuong-Quang DA, Fontebasso AM, et al. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012;124:615–625. doi: 10.1007/s00401-012-1031-3. [DOI] [PubMed] [Google Scholar]
- 133.Jiao Y, Killela PJ, Reitman ZJ, Rasheed AB, Heaphy CM, de Wilde RF, et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3:709–722. doi: 10.18632/oncotarget.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333:425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wiestler B, Capper D, Holland-Letz T, Korshunov A, von Deimling A, Pfister SM, et al. ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol. 2013;126:443–451. doi: 10.1007/s00401-013-1156-z. [DOI] [PubMed] [Google Scholar]
- 136.Nonoguchi N, Ohta T, Oh JE, Kim YH, Kleihues P, Ohgaki H. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol. 2013;126:931–937. doi: 10.1007/s00401-013-1163-0. [DOI] [PubMed] [Google Scholar]
- 137.Knobbe CB, Merlo A, Reifenberger G. Pten signaling in gliomas. Neuro Oncol. 2002;4:196–211. [PMC free article] [PubMed] [Google Scholar]
- 138.Houillier C, Lejeune J, Benouaich-Amiel A, Laigle-Donadey F, Criniere E, Mokhtari K, et al. Prognostic impact of molecular markers in a series of 220 primary glioblastomas. Cancer. 2006;106:2218–2223. doi: 10.1002/cncr.21819. [DOI] [PubMed] [Google Scholar]
- 139.Felsberg J, Rapp M, Loeser S, Fimmers R, Stummer W, Goeppert M, et al. Prognostic significance of molecular markers and extent of resection in primary glioblastoma patients. Clin Cancer Res. 2009;15:6683–6693. doi: 10.1158/1078-0432.CCR-08-2801. [DOI] [PubMed] [Google Scholar]
- 140.Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 141.Clark KH, Villano JL, Nikiforova MN, Hamilton RL, Horbinski C. 1p/19q testing has no significance in the workup of glioblastomas. Neuropathol Appl Neurobiol. 2013;39:706–717. doi: 10.1111/nan.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, et al. Everolimus for Subependymal Giant-Cell Astrocytomas in Tuberous Sclerosis. New England Journal of Medicine. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 143.Aldape KD. American Society of Clinical Oncology (ASCO) 50th Annual Meeting May 30-June 3, 2014. Chicago, IL: Impact of Molecular Signatures on Clinical Outcome. [Google Scholar]