Abstract
Background
The N-methyl-d-aspartate glutamate receptor antagonist ketamine, delivered via an intravenous route, has shown rapid antidepressant effects in patients with treatment-resistant depression. The current study was designed to test the safety, tolerability and efficacy of intranasal ketamine in patients with depression who had failed at least one prior antidepressant trial.
Methods
Twenty patients with major depression were randomized and 18 completed two treatment days with intranasal ketamine hydrochloride (50 mg) or saline solution in a randomized, double-blind, crossover study. The primary efficacy outcome measure was change in depression severity 24 hours following ketamine or placebo, measured using the Montgomery-Asberg Depression Rating Scale. Secondary outcomes included persistence of benefit, changes in self-reports of depression, changes in anxiety, and proportion of responders. Potential psychotomimetic, dissociative, hemodynamic, and general adverse effects associated with ketamine were also measured.
Results
Patients showed significant improvement in depressive symptoms at 24 hours following ketamine compared to placebo [t=4.39, p<0.001; estimated mean MADRS score difference of 7.6 ± 3.7 (95% CI: 3.9 – 11.3)]. Eight of 18 patients (44%) met response criteria 24 hours following ketamine administration, compared to 1 of 18 (6%) following placebo (p=0.033). Intranasal ketamine was well tolerated with minimal psychotomimetic or dissociative effects and was not associated with clinically significant changes in hemodynamic parameters.
Conclusions
This study provides the first controlled evidence for the rapid antidepressant effects of intranasal ketamine. Treatment was associated with minimal adverse effects. If replicated, these findings may lead to novel approaches to the pharmacologic treatment of patients with major depression.
Trial Registration
clinicaltrials.gov identifier NCT01304147
Keywords: depression, treatment resistant, ketamine, antidepressant, intranasal, glutamate
Major depressive disorder (MDD) is common and a leading worldwide cause of disability (1). Treatment-resistant depression (TRD), characterized by non-response to at least one antidepressant, is associated with a high degree of morbidity and functional disability, and is estimated to occur in up to one third of patients with MDD (2). Even for patients who do respond to standard antidepressant treatments, there is a significant delay in the onset of therapeutic benefit, which further increases illness burden and risks of associated morbidity and suicidality (3). Although neuroscience research has elucidated some of the basic mechanisms of depression and antidepressant action (4), current antidepressant drugs primarily target the monoaminergic system identified decades ago. More recently, the glutamate system has emerged as a critical focus of novel therapeutic development for MDD and particularly TRD (5, 6).
Evidence from post-mortem and in vivo brain imaging studies implicate amino acid neurotransmitter systems, particularly glutamate, and NMDA receptors in the pathophysiology of MDD (7–10). Several studies have demonstrated rapid antidepressant effects of intravenous (IV) ketamine, a high affinity N-methyl-d-aspartate (NMDA) receptor antagonist, in patients with TRD (11–15). However, there may be limitations to a therapy requiring IV administration, including lack of availability in common mental health practices, requirement for support services and monitoring, or patient discomfort. Ketamine administered via an intranasal route, in contrast, may provide a feasible and safer alternative.
Intranasal ketamine has shown safety and efficacy as an anesthetic and analgesic agent (16–20). In particular, intranasal ketamine has been successfully used in the treatment of headache and pain in ambulatory patients (21–23). In one study, 50 mg of ketamine administered intranasally was well tolerated and led to symptomatic improvement in chronic pain (23). The objective of the current proof of concept clinical trial was to test the rapid antidepressant effect of a single 50 mg administration of ketamine via an intranasal route in patients with major depression who had failed to respond to at least one prior antidepressant trial. Based on accumulating evidence supporting the efficacy and tolerability of ketamine administered IV in depression, and prior research examining intranasal ketamine in pain, we hypothesized that a dose of 50 mg, administered via an intranasal route, would be safe, well tolerated and lead to a rapid reduction in depressive symptoms.
METHODS AND MATERIALS
Participants
Study participants were recruited from physician referrals, media advertisements, and an academic outpatient psychiatric clinic. Males and females, aged 21 to 65, with a primary diagnosis of major depressive disorder, chronic or recurrent, without psychotic features, as assessed by a trained rater with the Structured Clinical Interview for DSM-IV (24) and a diagnostic interview with a study psychiatrist were eligible to participate. All study treatments were performed at Mount Sinai Medical Center between April 2012 and June 2013. Participants were required to have failed to respond to at least one trial of adequate dose and duration of an antidepressant medication approved by the U.S. Food and Drug Administration in the current episode according to the Antidepressant Treatment History Form (25). Participants were allowed to remain on stable doses of psychotropic medication, including antidepressant treatment. Negative urine toxicology for illicit drugs was required. Participants were required to have a baseline score of ≥ 30 on the Inventory of Depressive Symptomatology—Clinician Rated (IDS-C) (26) in order to proceed in the trial. Exclusion criteria included any unstable medical or neurological condition, any Axis I disorder other than MDD that was judged to be the primary presenting problem, high risk of suicide, substance abuse or dependence in the 6 months before screen, any psychotic disorder, bipolar disorder, developmental disorder, or lifetime abuse or dependence on ketamine or phencyclidine. Physical examination, vital signs, weight, electrocardiogram, standard blood tests, and urinalysis confirmed absence of unstable medical illnesses. Women of childbearing potential were required to have a negative pregnancy test before enrollment and to maintain adequate birth control for the duration of the study. No patients were enrolled who had nasal obstructions or history of nasal surgery.
The Icahn School of Medicine at Mount Sinai Institutional Review Board approved the study, and written informed consent was obtained from all subjects before participation. The study is registered at http://clinicaltrials.gov (NCT01304147).
Study Design
Eligible participants received 50 mg of racemic ketamine hydrochloride (Bioniche/Mylan, Morgantown, WV and JHP Pharmaceuticals, Parsippany, NJ) and placebo (0.9% saline solution) at least 7 days apart in a randomized, double-bind crossover design. Each treatment period (ketamine or placebo) consisted of 7 days. In each treatment period, assessments occurred at +40 min, +120 min, +240 min, +24 h, +48 h, +72 h, and +7 days following treatment intervention. In order for subjects to proceed from the first to the second treatment period, they needed to evidence a sufficient level of depressive symptoms, defined as IDS-C ≥ 24. The order of treatment periods was randomly assigned by the research pharmacy using permuted blocks of size four. All study investigators, anesthesiologists, and raters were blind to assignment.
Study drug or placebo was provided in identical syringes, containing clear solutions of either 100mg/ml ketamine in 0.9% saline or saline alone. An LMA MADgic mucosal atomization device (LMA North America, Inc., San Diego, CA) was used to provide 5 intranasal applications of solution (volume 100 μl), separated by 5 minutes. Each of 5 ketamine applications provided 10 mg of study drug. Administrations were provided over 20 minutes by an anesthesiologist in a clinical research unit. Patients were monitored for at least 4 hours in the research unit following administration, including continuous vital signs monitoring (i.e. heart rate, blood pressure, respiration, and pulse oximetry). In the original protocol, patients remained overnight in the research unit. However, after safety was demonstrated, the protocol was modified, allowing patients to be discharged 4 hours after administration and subsequently followed as outpatients. On completion of the second treatment period, patients were seen for final safety and efficacy evaluation and were exited from the protocol. All procedures aside from administrations and post-administration monitoring occurred in an outpatient context at Mount Sinai Medical Center.
Outcome Measures
The primary study outcome was change in depression severity, measured by the Montgomery-Asberg Depression Rating Scale (MADRS) at 24 hours following intervention. Subjects were rated prior to each administration (−60 min) and +40, +120, and +240 minutes as well as 1, 2, 3, and 7 days following each administration. Secondary measures included the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR), the Hamilton Anxiety Rating Scale (HAM-A) and the proportion of individuals meeting response or remission criteria. Response was defined as a 50% or greater decrease in the MADRS score from baseline, and remission was defined as a MADRS score of ≤ 9. Safety and tolerability was assessed using the Brief Psychiatric Rating Scale – Positive sub-scale (BPRS+), the Clinician-Administered Dissociative States Scale (CADSS), the mood item of the Young Mania Rating Scale (YMRS), and the Systematic Assessment for Treatment Emergent Effects (SAFTEE) instrument. Clinically significant changes were defined in the protocol as systolic or diastolic blood pressure (BP) >180/100 mmHg or heart rate >110 beats/minute. Management and medication intervention was to be provided per protocol or as deemed necessary by the treating anesthesiologist.
Bioanalytical Methods
Plasma samples were collected at 20 min (end of administration) and 40 min following start of administration. Samples were frozen at −80°C until analysis. Plasma concentrations of ketamine and norketamine (ketamine’s primary active metabolite) were determined using Gas Chromatography/Mass Spectrometry at NMS Labs (Willow Grove, PA) as previously described (27). The lower limit of quantification was 40 ng/mL.
Statistical Analyses
The primary analysis adhered to a modified intent to treat principle, including all eighteen patients with outcome assessment from both periods. A mixed model approach was employed to test effects of treatment (ketamine versus placebo), period, and carryover; both for differences in the MADRS score and for rates of clinical response. Changes over time up to 7 days were also assessed using mixed models. The mixed models approach accounts for the fact that each patient is assessed under both treatments per the crossover design. Secondary analyses of additional endpoints, notably the QIDS-SR and HAM-A, followed the same analytic approach as for the primary endpoint. Treatment effects are quantified as mean differences between groups and associated 95% confidence intervals for continuous outcomes, and by odds ratios and associated 95% confidence intervals for categorical outcomes.
All statistical tests were two-sided 0.05 level tests. No adjustment for multiple tests was employed; all p-values are reported at their nominal level. Patient characteristics, as well as safety and tolerability, are summarized using descriptive statistics. All analyses were performed using SAS version 9.2 (Cary NC).
RESULTS
Patient Characteristics
Thirty-six individuals signed consent and underwent screening. Twenty individuals met all eligibility requirements and were randomized to one of two treatment orders: ketamine-placebo or placebo-ketamine. Two subjects withdrew consent following randomization and did not complete both treatment periods (one following ketamine administration and the other following placebo). Thus, 18 patients completed both treatment periods and constituted the modified intention to treat (mITT) sample. (See supplementary Figure S1).
Demographic and clinical characteristics of study participants are summarized in Table 1. Participants had failed on average 4.1±3.9 adequate antidepressant treatment trials and had been ill for an average of 27 years. Twenty percent of the sample had a history of treatment with electroconvulsive therapy (ECT). Patients who met inclusion criteria and were on a stable dose of antidepressant medication were allowed to continue their concomitant treatment (see supplementary Table S1). No patients had previously been exposed to ketamine as per protocol.
Table 1.
Characteristics of Study Sample
| Characteristic | Value |
|---|---|
| Participants treated, n (%) | 20 (100) |
| Gender (M/F) | 10/10 |
| Age at enrollment (yrs) | 48.0±12.8 |
| Race (%): | |
| Caucasian | 18/20 (90) |
| Asian | 1/20 (5) |
| Black | 0/20 (0) |
| Other | 1/20 (5) |
| Hispanic (%) | 3/20 (15) |
| Married (%) | 6/20 (30) |
| Employed (%) | 10/20 (50) |
| Age of Onset | 21.4±12.0 |
| Illness Duration in Years | 27.4±13.7 |
| Chronic (%) | 9/20 (45) |
| Recurrent (%) | 17/20 (85) |
| Length of Current Episode in Years | 15.2±17.4 |
| Failed Antidepressants Medications* | 4.1±3.9 |
| History of ECT (%) | 4/20 (20) |
| History of Psychotherapy* (%) | 17/19 (89) |
| History of Suicide Attempts (%) | 2/20 (10) |
| Past Substance Use Disorder (%) | 3/20 (15) |
| Current Anxiety Disorder (%) | 4/20 (20) |
| Melancholic (%) | 9/20 (45) |
| Atypical (%) | 2/20 (10) |
| Baseline IDS-C (Screen) | 42.7±8.5 |
ECT – Electroconvulsive Therapy, IDS-C – Inventory of Depressive Symptoms – Clinician Rated;
n=19
Efficacy
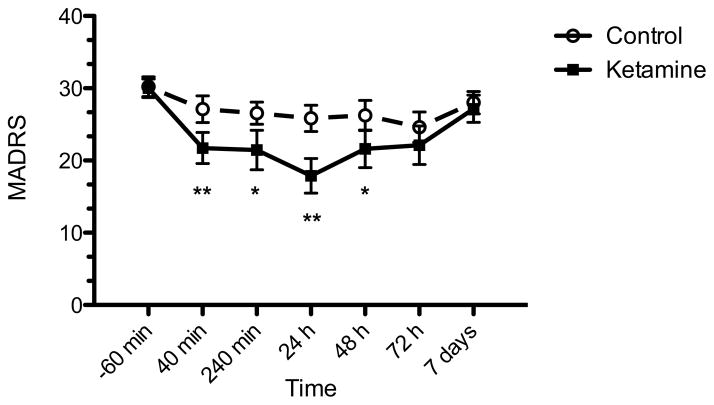
Depressive symptoms 24 hours post treatment were significantly improved in the ketamine condition compared to placebo (t=4.39, p<0.001). The estimated mean difference in MARDS score between ketamine and placebo was 7.6 ± 3.7 (95% CI: 3.9 – 11.3) (Figure 1). There was no evident residual effect of treatment. Treatment with ketamine was associated with a greater likelihood of response at 24 h compared to placebo (p=0.033). Eight of 18 subjects (44%) met response criteria at 24 h after ketamine administration, compared to 1 of 18 (6%) following placebo (number needed to treat = 2.6) (Figure 2). Based on a repeated measures mixed linear models analysis, ketamine exhibited greater improvement compared to placebo over the full seven day follow-up period (F1,18=28.10, p<0.001). A significant time effect was also noted (F5,95=10.65, p<0.001). In addition to the primary 24 h outcome, ketamine was significantly different from placebo at 40 min (p<0.001), 240 min (p=0.026) and 48 h (p=0.048). There was no significant separation at 72 h or 7 days.
Figure 1. Change in depression severity in patients with treatment-resistant depression following intranasal ketamine or placebo.
Change in MADRS depression severity 24 hours following administration was the primary outcome measure and was significantly greater following intranasal ketamine than placebo in the modified intention-to-treat group (n=18; p<0.001). MADRS, Montgomery-Asberg Depression Rating Scale; range is 0–60 with higher scores indicating greater severity of depressive symptoms. *p<0.05, **p<0.01.
Figure 2. Response rates in patients with treatment-resistant depression following intranasal ketamine or placebo.
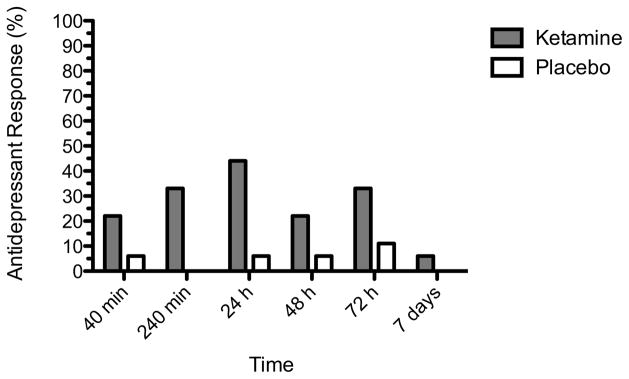
Response was defined as a decrease from baseline of at least 50% on MADRS. Modified intention-to-treat group (n=18).
Ketamine was associated with significant improvement on self-reports of depression as measured by the QIDS-SR at 24 hours [t17=3.30, p=0.004; mean difference of 3.0 ± 2.4 (95% CI: 1.1 – 4.9)]. Ketamine was superior to placebo in improving anxiety symptoms at 24 hours as measured by HAM-A scores [t17=3.06, p=0.007; mean benefit of 4.5 ± 3.2 (95% CI: 1.4–7.6)].
Acute Psychotomimetic and Dissociative Effects
Intranasal ketamine was associated with small increases on measures of psychosis and dissociation (Table 2). No relationship between ketamine associated changes in dissociative or psychotomimetic symptoms and antidepressant response was found (p<0.05 for CADSS and BPRS). Among ketamine responders, the increase in CADSS score at +40 min was 1.75 ± 4.17 compared to 1.09 ± 1.76 in ketamine nonresponders.
Table 2.
Acute Dissociative, Psychotomimetic and Hemodynamic Effects of Intranasal Ketamine and Placebo in Treatment-Resistant Depression
| Ketamine | Placebo | |||||
|---|---|---|---|---|---|---|
| Baseline | +40 min | +240 min | Baseline | +40 min | +240 min | |
| Behavioral Changes | ||||||
| BPRS+ | 4.0 ± 0 | 4.3 ± 0.7 | 4.1 ± 0.5 | 4.0 ± 0 | 4.0 ± 0 | 4.0 ± 0 |
| CADSS | 0.8 ± 1.6 | 2.2 ± 3.7 | 0.6 ± 1.9 | 0.8 ± 2.3 | 1.2 ± 3.3 | 0.3 ± 1.2 |
| Hemodynamic Changes | ||||||
| Systolic BP | 122.8 ± 11.1 | 130.4 ± 12.8 | 117 ± 14.1 | 117.2 ± 12.2 | 117.2 ± 12.7 | 116.5 ± 14.6 |
| Diastolic BP | 68.5 ± 9.4 | 76.8 ± 10.1 | 67.5 ± 10.4 | 72.1 ± 10.6 | 71.2 ± 12.2 | 68.7 ± 9.1 |
| Heart Rate | 72.4 ± 10.4 | 73.9 ± 8.9 | 72.7 ± 11.2 | 69.8 ± 6.7 | 69.6 ± 6.3 | 72.4 ± 7.6 |
Table describes changes in acute behavioral and hemodynamic measures at +40 min and +240 min following a single intranasal administration of ketamine or placebo (saline) in the context of a randomized controlled trial.
Abbreviations: BP, blood pressure (measured in mmHg); BPRS+, Brief Psychiatric Rating Scale-Positive Subscale (scale range 4–28, higher values indicate increased psychotic-like symptoms); CADSS, Clinician-Administered Dissociative States Scale (scale range 0–92, higher values indicate increased dissociative symptoms); HR, heart rate (measured in beats per minute).
Hemodynamic Effects
Intranasal ketamine was associated with small increases in systolic blood pressure (mean increase of 7.6 mmHg at +40 min compared to baseline) (Table 2). Four participants experienced treatment-emergent increases in systolic BP above 130 mmHg following ketamine and 3 participants experienced systolic BP above 130 mmHg following placebo. No patients had diastolic BP above 100 mmHg. There were no clinically significant elevations in blood pressure or heart rate that required intervention and all hemodynamic changes resolved by 4 hours post infusion. No association was found between hemodynamic changes and antidepressant response to ketamine (all p<0.05).
General Adverse Events
The most common adverse events in the ketamine group for up to 4 hours after infusion were feeling strange or unreal, poor memory, and weakness or fatigue (Table 3). The majority of these symptoms resolved within 4 hours of administration. No serious adverse events occurred during the study.
Table 3.
General Adverse Events
| Ketamine | Placebo | |||
|---|---|---|---|---|
|
| ||||
| ≤240m | 240m–24h | ≤240m | 240m–24h | |
| Feeling strange or unreal (%) | 8 (42) | 0 (0) | 0 (0) | 0 (0) |
| Poor memory (%) | 7 (37) | 0 (0) | 1 (5) | 1 (5) |
| Weakness or fatigue (%) | 5 (26) | 1 (5) | 1 (5) | 0 (0) |
| Delayed or absent orgasm (%) | 4 (21) | 1 (5) | 0 (0) | 0 (0) |
| Loss of sexual interest (%) | 4 (21) | 0 (0) | 1 (5) | 0 (0) |
| Trouble concentrating (%) | 4 (21) | 0 (0) | 1 (5) | 1 (5) |
| Problems with sexual arousal (%) | 3 (16) | 1 (5) | 2 (11) | 1 (5) |
| Dizziness or fainting (%) | 3 (16) | 0 (0) | 0 (0) | 0 (0) |
| Dizziness when you stand up (%) | 3 (16) | 0 (0) | 1 (5) | 1 (5) |
| Diminished mental (%) | 3 (16) | 0 (0) | 2 (11) | 1 (5) |
| Feeling drowsy or sleepy (%) | 3 (16) | 0 (0) | 3 (16) | 1 (5) |
| Poor coordination or unsteadiness (%) | 2 (11) | 1 (5) | 0 (0) | 0 (0) |
| Difficulties finding words (%) | 2 (11) | 1 (5) | 2 (11) | 0 (0) |
| Abnormal sensations (%) | 2 (11) | 0 (0) | 0 (0) | 0 (0) |
| Numbness or tingling (%) | 2 (11) | 0 (0) | 0 (0) | 0 (0) |
| Strange taste in mouth (%) | 2 (11) | 0 (0) | 1 (5) | 0 (0) |
| Nightmares or other sleep disturbance (%) | 2 (11) | 0 (0) | 2 (11) | 3 (16) |
| Blurred vision (%) | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| Appetite decreased (%) | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| Dry mouth (%) | 1 (5) | 0 (0) | 0 (0) | 1 (0) |
| Heartbeat rapid or pounding (%) | 1 (5) | 0 (0) | 0 (0) | 1 (5) |
| Clenching of teeth at night (%) | 1 (5) | 0 (0) | 1 (5) | 0 (0) |
| Stuffy nose (%) | 1 (5) | 0 (0) | 1 (5) | 1 (5) |
| Overall (%) | 1 (5) | 0 (0) | 1 (5) | 3 (16) |
| Irritable (%) | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
| Headache (%) | 0 (0) | 1 (5) | 0 (0) | 1 (5) |
| Tremor or shakiness (%) | 0 (0) | 1 (5) | 0 (0) | 1 (5) |
| Constipation (%) | 0 (0) | 1 (5) | 0 (0) | 1 (5) |
| Trouble sitting still (%) | 0 (0) | 1 (5) | 1 (5) | 0 (0) |
| Feeling nervous or hyper (%) | 0 (0) | 1 (5) | 1 (5) | 1 (5) |
| Frequent need to urinate (%) | 0 (0) | 1 (5) | 1 (5) | 1 (5) |
| Apathy/Emotional indifference (%) | 0 (0) | 1 (5) | 1 (5) | 1 (5) |
| Trouble Sleeping (%) | 0 (0) | 1 (5) | 2 (11) | 7 (37) |
| Muscle twitching or movements (%) | 0 (0) | 0 (0) | 1 (5) | 0 (0) |
| Stomach or abdominal discomfort (%) | 0 (0) | 0 (0) | 1 (5) | 0 (0) |
| Appetite increased (%) | 0 (0) | 0 (0) | 1 (5) | 0 (0) |
| Ringing in ears or trouble hearing (%) | 0 (0) | 0 (0) | 1 (5) | 1 (5) |
| Unable to sit still (%) | 0 (0) | 0 (0) | 1 (5) | 1 (5) |
Adverse events reported on SAFTEE with intensity of moderate or severe and increased from baseline acutely following treatment and at 24 hours.
Blood Levels
Following ketamine administration, plasma levels of ketamine were detectable (≥ 40 ng/mL) in 14 individuals at 20 min, and in 17 individuals at 40 min. Norketamine was not detected at 20 min, and was detected in 8 individuals at 40 min. Mean ketamine plasma level was 72 ng/mL at 20 min and 84 ng/mL at 40 min. Mean norketamine plasma level was 46 ng/mL at 40 min (Supplemental Figure S2).
DISCUSSION
In the current study we found that a single dose of 50 mg of ketamine administered via intranasal route was associated with a rapid antidepressant response in patients with major depression who had failed at least one prior antidepressant trial. A significant antidepressant effect of ketamine was detected as early as 40 min following administration and there was a large difference in depression severity between the treatment conditions at the 24-hour primary outcome (mean difference in MADRS score of 7.6 ± 3.7). In aggregate, there was significant antidepressant benefit following ketamine compared to placebo over the full 7-day assessment period, although when comparing individual time points the treatment conditions no longer separated at 72 hours or 7 days. Ketamine was associated with significant improvement in anxiety symptoms and self-reports of depressive symptoms at 24 hours. Intranasal ketamine was well tolerated with only very minimal increases in dissociation, psychosis-like symptoms or hemodynamic parameters. This study provides the first randomized, controlled evidence that intranasal ketamine is safe, well tolerated, and effective for rapid reduction of depressive symptoms in patients with MDD and at least mild treatment resistance.
In comparison with prior studies of ketamine administered IV (at a dose of 0.5 mg/kg) in depression, our observed magnitude of antidepressant effect with intranasal administration may be somewhat reduced. Murrough et al. reported a mean ketamine-placebo difference of 7.95 points (95% CI: 3.20–12.71) on the MADRS 24 hours following a single IV infusion and a response rate of 64% (15). Response rates as high as 70% following IV administration have been reported in some studies (11, 15), though other studies have reported response rates from 50% to as low as 30% following IV ketamine (28, 29). Our mean drug-placebo difference is in line with what has been previously reported (7.6 ± 3.7 points on the MADRS), although the proportion of responders in our study may be somewhat lower at 44%. This lower proportion of treatment responders may be consistent with the lower blood ketamine levels achieved in our study compared to levels previously reported following IV administration. In our sample, the mean ketamine blood level was 72 ng/mL at 20 min and 84 ng/mL at 40 min. In contrast, mean ketamine levels reported following IV infusion (0.5mg/kg) are approximately 150 ng/mL at 30 min and 200 ng/mL at 40 min. (27, 30, 31). It is currently not known if efficacy equivalent to IV administration can be obtained by intranasal administration in the case that comparable blood levels can be achieved.
We report a significant improvement in anxiety symptoms at 24 hours, assessed with the HAM-A. Two studies of IV ketamine for bipolar depression reported a significant improvement in anxiety symptoms measured with the HAM-A and a visual analog scale(27, 32). However, previous studies of patients with unipolar TRD have not described effects of IV ketamine on anxiety, with the exception of an early RCT (11) and an open label study (33) reporting significant improvement in psychic anxiety measured as an individual symptom on the Hamilton Depression Rating Scale, and another open-label study reporting significant decrease in anxiety symptoms on the HAM-A at +230 minutes (34).
Previous studies of IV ketamine in depression have reported elevations in measures of psychotomimetic, dissociative and hemodynamic parameters (11, 13, 35). In our study, the ketamine group experienced a very limited increase in dissociation at +40 min as measured by the CADSS (mean 1.4 points; scale range 0–92). In comparison, Murrough et al. reported a larger dissociative effect 40 min following ketamine administered IV [mean CADSS score of 14.7 points (95% CI: 10.6–18.8)] (15). A similar pattern was observed for psychotic-like effects measured using the BPRS+ (11, 15). We also observed comparatively small changes in hemodynamic parameters. No patient met protocol criteria for interventions. Studies of IV ketamine in depression have reported relatively greater changes in hemodynamic parameters (mean systolic BP increase of 19.0 versus our 7.6 mmHg at +40mins relative to baseline) (15). The reduced magnitude of acute behavioral and hemodynamic changes observed in the current study may be consistent with the lower blood levels achieved compared to prior studies with ketamine administered IV, as discussed above.
The bioavailability of ketamine administered via an intranasal route has been reported to be between 25–50% (36). A study in healthy volunteers comparing administration methods found intranasal ketamine bioavailability of 45%, higher than subligual, oral, or rectal administration and found no significant differences in pharmacokinetics between preparations, including injection (37). Additionally, this study found conversion to norketamine was more similar between intranasal and injection than the other administration methods, suggesting that first-pass metabolism is relatively absent with intranasal administration. The area under the ketamine and norketamine plasma concentration-time curves in that study was lowest for intranasal administration but was found to increase almost linearly with doses from 25 to 50mg (37). In previous studies of IV ketamine in depression, peak norketamine blood levels of approximately 20–50 ng/mL have been reported (30, 31). In line with these findings, the mean norketamine level in our study was 46 ng/mL at 40 min.
We selected our dose of 50 mg largely based on a previous study using a similar design and the same dose in patients with a chronic pain disorder (23). Based on an expected bioavailability of intranasal ketamine between 25–50% (36), our dose may be approximately equivalent to 0.15 – 0.34 mg/kg administered IV. Although this is lower than the standard 0.5 mg/kg IV frequently used in ketamine depression studies, we reasoned that this dose was appropriate from a safety perspective given that the administration period in the current study is relatively short (20 min versus 40 min or longer in IV studies). Clearly, much more research is required in order to determine the optimal dose, duration, frequency and route of administration of ketamine for depression.
Regarding our study sample, some participants had lower levels of treatment resistance compared to prior studies since we required only a single antidepressant treatment failure in the current episode. Treatment-resistant depression has been defined by some authors as failure to respond to a single treatment (38), though published IV ketamine studies have frequently required a minimum of 2–3 treatment failures (11, 15). Despite a wider range, our sample as a whole was highly treatment resistant with an average of 4.1 ± 3.9 antidepressant failures. This compares to 5.7 ± 3.4 and 5.1 ± 2.0 in previous studies (11, 15). Notably, other pertinent clinical characteristics, including duration of illness, length of current depressive episode, and history of ECT are not appreciably different compared to prior studies (11, 15).
The current study has several limitations. We allowed ongoing treatment with psychotropic medication at stable doses, including antidepressant medications. Our augmentation approach makes it difficult to distinguish an intrinsic effect of ketamine from benefits resulting from the combination of ketamine with other antidepressant agents. On the other hand, this augmentation design more likely reflects the potential clinical use of ketamine in treatment refractory populations should it gain approval for this indication. This approach enabled us to gather preliminary data to address concerns about risks of ketamine administration in depressed patients who are currently taking other psychotropic medications (39). We utilized a crossover design, which can result in carry-over effects, including subjects whose response to the first treatment prevents them from completing the second. In our study, 2 of 20 patients were unable to complete all study procedures (one in each randomization group). The relatively small sample size is also a limitation. The use of saline as a placebo control in ketamine treatment studies is a limitation since the integrity of the blind may be compromised. The very low levels of treatment-emergent psychotomimetic and dissociative symptoms observed in the current study may have helped to mitigate this limitation. The use of a single dose and a single drug administration in the current study does not address important questions related to optimal dosing and the longer-term safety or efficacy of this intervention.
In conclusion, MDD patients treated with intranasal ketamine experienced rapid improvement of depressive symptoms and limited adverse effects. To our knowledge, this study represents the first controlled investigation of intranasal administration for an NMDA antagonist in depression. While these findings are suggestive of efficacy and of a favorable tolerability profile, much more research is required before the true efficacy and safety of this intervention can be assessed. Future studies designed to optimize dosing while identifying relapse prevention strategies and biomarkers of treatment response will provide additional needed data to maximize benefit for patients and minimize side effects.
Supplementary Material
Footnotes
Contributions: This study was supported by NIH grant UL1TR000067 (Mount Sinai Clinical and Translational Science Award). Dr. Murrough is supported by NIH grant K23MH094707. Dr. Lapidus receives support from a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation. A portion of the data from the current study was previously presented at the 52nd annual meeting of the American College of Neuropsychopharmacology.
Financial/Conflict of Interest Disclosures: The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other sponsoring entity. In the past two years, Dr. Murrough has received research support from the National Institutes of Health, the National Institute of Mental Health, the Department of Veterans Affairs, the Doris Duke Charitable Foundation, the American Foundation for Suicide Prevention, the Brain and Behavior Research Foundation, Janssen Research and Development and Avanir Pharmaceuticals; he has served on advisory boards for Janssen Research and Development and Genentech and has provided consultation services for ProPhase, LLC and Impel Neuropharma. Dr. Lapidus has received research support from the Brain and Behavior Research Foundation, APIRE/Janssen, and the Le Foundation; he has received consulting fees from LCN consulting and serves on the advisory board for Halo Neuro Inc. Dr. Iosifescu has received research funding through Icahn School of Medicine at Mount Sinai from AstraZeneca, Brainsway, Euthymics, Neosync, Roche, and Shire; he has received consulting fees for Avanir, CNS Response, Otsuka, Servier, and Sunovion. Dr. Charney has received consulting fees or research support from the US Dept. of Defense, NIH, NIMH, NARSAD, USAMRAA, and CNS Spectrums; he a member of the Advisory and Editorial Boards of the Institute of Medicine Committee on DHS Workforce Resilience. Dr. Dennis Charney (Dean of Icahn School of Medicine at Mount Sinai and co-investigator on this study), and Icahn School of Medicine at Mount Sinai have been named on a use patent on Ketamine for the treatment of depression. The Icahn School of Medicine has entered into a licensing agreement for the use of ketamine as therapy for treatment-resistant depression. Dr. Charney and Icahn School of Medicine at Mount Sinai could potentially benefit from the results of this study. In addition, Dr. Charney and Dr. Adriana Feder (a Co-Investigator on this study) are named co-inventors on a use patent application filed by Mount Sinai for the use of ketamine as a treatment for PTSD. If Ketamine were shown to be effective in the treatment of PTSD and received approval from the Food and Drug Administration (FDA) for this indication, Dr. Charney, Dr. Feder, and the Icahn School of Medicine at Mount Sinai could benefit financially. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, et al. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapidus KA, Soleimani L, Murrough JW. Novel glutamatergic drugs for the treatment of mood disorders. Neuropsychiatr Dis Treat. 2013;9:1101–1112. doi: 10.2147/NDT.S36689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murrough JW, Charney DS. Is there anything really novel on the antidepressant horizon? Curr Psychiatry Rep. 2012;14:643–649. doi: 10.1007/s11920-012-0321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg DR, Macmaster FP, Mirza Y, Smith JM, Easter PC, Banerjee SP, et al. Reduced anterior cingulate glutamate in pediatric major depression: a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:700–704. doi: 10.1016/j.biopsych.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61:105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 12.Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 14.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, Aan Het Rot M, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weksler N, Ovadia L, Muati G, Stav A. Nasal ketamine for paediatric premedication. Can J Anaesth. 1993;40:119–121. doi: 10.1007/BF03011307. [DOI] [PubMed] [Google Scholar]
- 17.Louon A, Reddy VG. Nasal midazolam and ketamine for paediatric sedation during computerised tomography. Acta Anaesthesiol Scand. 1994;38:259–261. doi: 10.1111/j.1399-6576.1994.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 18.Diaz JH. Intranasal ketamine preinduction of paediatric outpatients. Paediatr Anaesth. 1997;7:273–278. doi: 10.1046/j.1460-9592.1997.d01-93.x. [DOI] [PubMed] [Google Scholar]
- 19.Weber F, Wulf H, el Saeidi G. Premedication with nasal s-ketamine and midazolam provides good conditions for induction of anesthesia in preschool children. Can J Anaesth. 2003;50:470–475. doi: 10.1007/BF03021058. [DOI] [PubMed] [Google Scholar]
- 20.Roelofse JA, Shipton EA, de la Harpe CJ, Blignaut RJ. Intranasal sufentanil/midazolam versus ketamine/midazolam for analgesia/sedation in the pediatric population prior to undergoing multiple dental extractions under general anesthesia: a prospective, double-blind, randomized comparison. Anesth Prog. 2004;51:114–121. [PMC free article] [PubMed] [Google Scholar]
- 21.Kaube H, Herzog J, Kaufer T, Dichgans M, Diener HC. Aura in some patients with familial hemiplegic migraine can be stopped by intranasal ketamine. Neurology. 2000;55:139–141. doi: 10.1212/wnl.55.1.139. [DOI] [PubMed] [Google Scholar]
- 22.Huge V, Lauchart M, Magerl W, Schelling G, Beyer A, Thieme D, et al. Effects of low-dose intranasal (S)-ketamine in patients with neuropathic pain. Eur J Pain. 2010;14:387–394. doi: 10.1016/j.ejpain.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Carr DB, Goudas LC, Denman WT, Brookoff D, Staats PS, Brennen L, et al. Safety and efficacy of intranasal ketamine for the treatment of breakthrough pain in patients with chronic pain: a randomized, double-blind, placebo-controlled, crossover study. Pain. 2004;108:17–27. doi: 10.1016/j.pain.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association., American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 25.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- 26.Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- 27.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlson PJ, Diazgranados N, Nugent AC, Ibrahim L, Luckenbaugh DA, Brutsche N, et al. Neural Correlates of Rapid Antidepressant Response to Ketamine in Treatment-Resistant Unipolar Depression: A Preliminary Positron Emission Tomography Study. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 30.Horacek J, Brunovsky M, Novak T, Tislerova B, Palenicek T, Bubenikova-Valesova V, et al. Subanesthetic dose of ketamine decreases prefrontal theta cordance in healthy volunteers: implications for antidepressant effect. Psychol Med. 2010;40:1443–1451. doi: 10.1017/S0033291709991619. [DOI] [PubMed] [Google Scholar]
- 31.Zarate CA, Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, et al. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry. 2012;72:331–338. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CM, Perez AM, et al. Antidepressant Efficacy of Ketamine in Treatment-Resistant Major Depression: A Two-Site Randomized Controlled Trial. Am J Psychiatry. 2013 doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, Ostermeyer B, et al. Ketamine for treatment-resistant unipolar depression: current evidence. CNS Drugs. 2012;26:189–204. doi: 10.2165/11599770-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanagihara Y, Ohtani M, Kariya S, Uchino K, Hiraishi T, Ashizawa N, et al. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Biopharm Drug Dispos. 2003;24:37–43. doi: 10.1002/bdd.336. [DOI] [PubMed] [Google Scholar]
- 38.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 39.Rush AJ. Ketamine for treatment-resistant depression: ready or not for clinical use? Am J Psychiatry. 2013;170:1079–1081. doi: 10.1176/appi.ajp.2013.13081034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.