Abstract
OBJECTIVES
Body size is postulated to modulate type 1 diabetes as either a trigger of islet autoimmunity or an accelerator to clinical onset after seroconversion. As overweight and obesity continue to rise among children, the aim of this study was to determine whether human leukocyte antigen DQ (HLA-DQ) genotypes may be related to body size among children genetically at risk for type 1 diabetes.
METHODS
Repeated measures of weight and height were collected from 5 969 children 2–4 years of age enrolled in The Environmental Determinants of Diabetes in the Young prospective study. Overweight and obesity was determined by the International Obesity Task Force cutoff values that correspond to body mass index of 25 and 30 kg/m2 at age 18.
RESULTS
The average BMI was comparable across specific HLA genotypes at every age point. The proportion of overweight was not different by HLA, but percent obesity varied by age with a decreasing trend among DQ2/8 carriers (p for trend = 0.0315). A multivariable regression model suggested DQ2/2 was associated with higher obesity risk at age four (OR, 2.41; 95% CI, 1.21–4.80) after adjusting for the development of islet autoantibody and/or type 1 diabetes.
CONCLUSIONS
The HLA-DQ2/2 genotype may predispose to obesity among 2–4 year old children with genetic risk for type 1 diabetes.
Keywords: Body Mass Index, HLA Genotype, Type 1 Diabetes, Pediatric, Autoantibodies
Introduction
Type 1 diabetes is a multifactorial chronic disease that mostly but not exclusively manifests before 25 years.1 The human leukocyte antigen (HLA) class II immune recognition molecules, as indicators of genetic risk, are of particular importance as they are found to explain more than 50% of familial clustering in type 1 diabetes risk2 with certain HLA haplotypes, such as DQ A1*0301-B1*0302 (DQ8) and A1*0501-B1*0201 (DQ2) positively associated with type 1 diabetes.3 In recent decades, a global increase in the incidence of type 1 diabetes has been reported4–9 and a younger age at onset was also noted in multiple studies.10–12 It is unlikely that these phenomena are attributable to changes in genetic susceptibility because population prevalence of high risk HLA genotypes for type 1 diabetes has not risen over time.13, 14 Environmental factors are postulated to modify disease pathogenesis; in particular body mass index (BMI) has been hypothesized to be an accelerator in the development of type 1 diabetes.15, 16 It has been reported in several studies that the increase in type 1 diabetes incidence rate may be correlated with an increase of childhood obesity in the population.17–20 In Finland, positive associations between weight and the risk of type 1 diabetes were seen in children below the age of 15 years.17 In Sweden, susceptibility for childhood type 1 diabetes was associated with the A1*05:01-B1*02:01/A1*05:01-B1*02:01 (DQ2/2) genotype and an increased BMI.18 A report from a Mediterranean pediatric cohort further suggested a positive association between the age at diagnosis of type 1 diabetes and BMI score adjusted for age and gender.20 In the USA, the prevalence of overweight (defined as ≥85th percentile for BMI for age and gender) among children newly diagnosed with type 1 diabetes increased from 12.6% during 1979–1989 to 36.8% during 1990–1998.19 Among the Pediatric Diabetes Consortium participants who were diagnosed with type 1 diabetes between 2009 and 2011, 21% were overweight or obese at the time of diagnosis.21
Since both HLA class II genotypes and body size were found to be related to type 1 diabetes risk, it is of interest to examine the association between these two factors and investigate the role of HLA genotypes in physical growth. To our knowledge there is a paucity of investigations of the very young age group with respect to body size, weight, length, and the HLA associated risk for islet autoimmunity and the subsequent onset of type 1 diabetes. The Better Diabetes Diagnosis (BDD) study group reported a negative association between HLA risk level and BMI, however their data were from newly-diagnosed type 1 diabetes children aged 0–18 years.18 Larsson et al. examined HLA genotypes and physical growth prior to type 1 diabetes diagnosis among the Diabetes Prediction in Skåne (DiPiS) study participants and reported increased linear growth, but not BMI, was associated with this disease independent of HLA genotypes between 0–18 months of age.22 It was, therefore, important to examine BMI in the entire group of TEDDY children as they have a documented higher risk of developing islet autoimmunity. The objective of this analysis was to assess the prevalence of overweight and obesity by specific HLA genotypes associated with increased risk for type 1 diabetes.
Subjects and Methods
The Environmental Determinants of Diabetes in the Young (TEDDY) is a prospective cohort study funded by the National Institutes of Health with the primary goal to identify environmental causes of type 1 diabetes. It includes six clinical research centers - three in the USA: Colorado, Georgia/Florida, Washington and three in Europe: Finland, Germany, and Sweden. Newborn children were screened for type 1 diabetes risk HLA genotypes either in the cord blood or on heel sticks within three months after birth.23 If a child was determined to have increased genetic risk, the family was contacted by a TEDDY nurse and invited to participate in a 15-year-follow-up study before the child reached 4.5 months of age. Detailed study design and methods have been previously published.23, 24 Written informed consents were obtained for all study participants from a parent or primary caretaker, separately, for genetic screening and participation in prospective follow-up. The study was approved by local Institutional Review Boards and is monitored by an External Advisory Board formed by the National Institutes of Health.
Questionnaire data
Subject information, including date of birth, gender, whether the subject had first degree relatives (FDR) with type 1 diabetes, birth weight and length, and number of fetuses in the pregnancy (singleton, twin, triplet, etc.), were collected by questionnaire at the time of HLA typing or at the first clinic visit when the infant was three months of age. The FDR status was considered positive if the child had a biological relative (mother, father or sibling) with type 1 diabetes. In the USA, subjects also identified their race/ethnicity using the USA census questions. Maternal factors, including maternal age, pre-pregnancy weight and height, presence of pre-gestational diabetes (type 1 or type 2) or gestational diabetes, gestational age, and gestational weight gain were collected by questionnaire at the same time. A subject is required to withdraw from the study after he or she is diagnosed with type 1 diabetes. The date of diagnosis was reported by the caretaker.
Weight and height
Body weight was measured in kilograms using an electronic scale calibrated monthly. From the age of two years height was measured to the nearest 0.1 centimeter using a wall-mounted stadiometer. For subjects who could not attend a clinical visit, anthropometric data were copied from their pediatricians’ records collected around the visit date. BMI was calculated using measured weight divided by measured height in meters squared. Overweight and obesity status was determined by the International Obesity Task Force (IOTF) cutoff values, which were derived from averaged centile curves that pass through BMI of 25 kg/m2 for overweight and 30 kg/m2 for obesity.25
HLA typing and antibody testing
Detailed description of HLA typing and antibody testing has been published previously.26 In brief, a total of 424 788 newborns were screened for HLA-DR, DQ genotypes associated with type 1 diabetes.24 Blood samples of newborn children were obtained in the maternity clinics either as cord blood or dry blood spots. HLA genotypes were determined using either a genotyping system with an asymmetric polymerase chain reaction and subsequent hybridization of allele-specific probes for HLA-DQA1, DQB1 and DRB1 as described27 using DELFIA reagents (Perkin-Elmer, Waltham, MA USA) or in a dot blot hybridization assay as detailed elsewhere.28
Newborns with no first degree relative with type 1 diabetes were eligible for the study if they had one of the following four HLA genotypes:
-
(1)
DR3/4 = DR4-DQA1*030X-DQB1*0302 (or *0304)/DR3-DQA1*0501-DQB1*0201 (abbreviated as DQ8/2);
-
(2)
DR4/4 = DR4-DQA1*030X-DQB1*0302(or *0304)/DR4-DQA1*030X-DQB1*0302(or *0304) (abbreviated as DQ8/8);
-
(3)
DR3/3 = DR3-DQA1*0501-DQB1*0201/DR3-DQA1*0501-DQB1*0201 (abbreviated as DQ2/2); and
-
(4)
DR4/8 = DR4-DQA1*030X-DQB1*0302(or *0304)/DR8-DQA1*0401-DQB1*0402 (abbreviated as DQ8/X where X is not DR3-DQA1*0501-DQB1*0201 or DR4-DQA1*030X-DQB1*0302(*0304)).
Infants who had a first degree relative with type 1 diabetes were eligible for enrollment if they had any of the above four HLA genotypes or one of the additional genotypes:
-
(5)
DR4-DQA1*030X-DQB1*0302(or *0304)/DR4-DQA1*030X-DQB1*020X (abbreviated as DQ8/X where X is not DR3-DQA1*0501-DQB1*0201 or DR4-DQA1*030X-DQB1*0302(*0304));
-
(6)
DR4-DQA1*030X-DQB1*0302(or *0304)/DR1(not DR10)-DQA1*0101-DQB1*0501 (abbreviated as DQ8/X where X is not DR3-DQA1*0501-DQB1*0201 or DR4-DQA1*030X-DQB1*0302(*0304));
-
(7)
DR4-DQA1*030X-DQB1*0302(or *0304)/DR13-DQA1*0102-DQB1*0604 (abbreviated as DQ8/X where X is not DR3-DQA1*0501-DQB1*0201 or DR4-DQA1*030X-DQB1*0302(*0304));
-
(8)
DR4-DQA1*030X-DQB1*0302/DR4-DQA1*030X-DQB1*0304 (abbreviated as DQ8/X where X is not DR3-DQA1*0501-DQB1*0201 or DR4-DQA1*030X-DQB1*0302(*0304));
-
(9)
DR4-DQA1*030X-DQB1*0302(or *0304)/DR9-DQA1*030X-DQB1*0303 (abbreviated as DQ8/X where X is not DR3-DQA1*0501-DQB1*0201 or DR4-DQA1*030X-DQB1*0302(*0304)); and
-
(10)
DR3-DQA1*0501-DQB1*0201/DR9-DQA1*030X-DQB1*0303 (abbreviated as DQ2/X where X is not DR3-DQA1*0501-DQB1*0201 or DR4-DQA1*030X-DQB1*0302(*0304)).
These additional HLA genotypes were present in only 276 (3%) of the TEDDY subjects who were included in the current analyses. Newborns carrying the DR4 subtype DRB1*0403 were excluded if they had no first degree relative with type 1 diabetes.
Islet autoantibodies to insulin (IAA), glutamic acid decarboxylase (GAD65) or insulinoma antigen-2 (IA-2) were measured by radiobinding assays in both central autoantibody laboratories, one located in the USA and one in Europe.24 All positive islet autoantibodies and 5% of negative samples were re-tested in the other laboratory and deemed confirmed if concordant. Persistent confirmed islet autoantibody was defined as confirmed positive IAA, GAD65, or IA-2 on at least two consecutive study visits.
Transglutaminase antibody testing was carried out annually in all children starting at 24 months of age to screen for celiac disease (CD). The testing was completed with the assessment of tissue transglutaminase autoantibodies (TGA) using radiobinding assays (RBA) analyzed at the Bristol Laboratory (University of Bristol, UK) for the European sites and the Barbara Davis Center (BDC) Laboratory (Aurora, Colorado) for the USA sites. At the BDC, the RBA uses anti-IgA agarose to capture IgA-TGA; whereas, in Bristol a mixture of both anti-IgA agarose and protein A sepharose (PAS) is used to assess both IgA-TGA and IgG-TGA. The discrepancy in methods does not impact detection of autoantibodies as the two IgA-TGA assays have proven highly concordant in previous TGA workshops.29
TEDDY enrolled 8 677 subjects between 2004 and 2010. After excluding those whose HLA genotypes do not meet TEDDY’s criteria (n=113), who were not followed for two or more years (n=2 378), who did not have repeated weight and height measurements collected at study clinics (n=195), and too few subjects with HLA genotype DQ2/X to draw inference (n=22), 5 969 subjects were included in this analysis as of March 2012.
Statistical analysis
Characteristics of subjects were summarized and compared as follows: Pearson’s χ2 tests or Fisher’s exact test was used to analyze the categorical variables, namely subject’s gender, country of residence, FDR, mother’s diabetes status during pregnancy, and race/ethnicity (USA subjects only). Analysis of variance (ANOVA) was used to analyze continuous variables, including gestational age, gestational weight gain, mother’s BMI before pregnancy, and subject’s birth weight. The temporal trend of varying proportions of overweight and obesity within every HLA genotype was tested using the Cochran-Armitage Trend Test. Multivariable logistic regression models adjusted for the above factors were used to estimate the risk of being overweight or obese by comparing the proportion of subjects with HLA genotype DQ2/2, DQ8/8, and DQ8/X subjects against the proportion of subjects with the DQ2/8 genotype. A birth weight z-score was developed to adjust for country, gender, mother’s height, gestational age, and birth type. We repeated all univariate analyses by including and excluding subjects who developed islet autoantibody, type 1 diabetes, and/or TGA and found no change in results to account for the possibility that individuals with type 1 diabetes might have different height/weight than the general population at the time of diagnosis18, 21 and that the presence of islet antibody and/or TGA may or may not affect growth trajectory.30 The logistic regression models were then adjusted for autoimmunity and disease diagnosis. All tests for significance were two-tailed with a significance level of 0.05. Analysis was performed using the Statistical Analysis System Software (Version 9.3, SAS Institute, Cary, NC).
Results
Among the 5 969 eligible subjects shown in Table 1, overall distribution of high risk genotypes was 39.2% for DQ2/8, 19.8% for DQ8/8, 20.8% for DQ2/2, and 20.1% for DQ8/X. DQ2/8 was the most common high risk genotype in every TEDDY country except in Finland, where DQ8/X was most common with DR4-DQA1*030X-DQB1*0302(or *0304)/DR8-DQA1*0401-DQB1*0402 being the predominant genotype. A larger proportion of subjects (n=5 294) without FDR carried DQ2/8 (40.2%), whereas FDR subjects (n=675) carried DQ8/X more frequently (37.3%) (p<0.0001). The distribution of high risk genotypes did not differ by the gender of the subject.
Table 1.
Characteristics of 5 969 2–4 year old children at genetic risk for type 1 diabetes.
| DQ2/8 (n=2341) | DQ8/8 (n=1180) | DQ2/2 (n=1241) | DQ8/X (n=1207) | P | |
|---|---|---|---|---|---|
| Participant | |||||
| Sex (male/female) | 1195/1146 | 593/587 | 676/565 | 617/590 | 0.1477 |
| Age (months) | 55.8(17.2) | 55.7(17.2) | 55.1(16.9) | 55.6(17.0) | 0.7167 |
| Country | |||||
| USA (n=2314) | 923 (40.0%) | 473 (20.4%) | 556 (24.0%) | 362 (15.6%) | <0.0001 |
| Finland (n=1374) | 465 (33.8%) | 222 (16.2%) | 205 (14.9%) | 482 (35.1%) | |
| Germany (n=357) | 141 (39.5%) | 64 (17.9%) | 71 (19.9%) | 81 (22.77%) | |
| Sweden (n=1924) | 812 (42.2%) | 421 (21.9%) | 409 (21.3%) | 282 (14.6%) | |
| Having FDR with Type 1 Diabetes | |||||
| Yes (n=675) | 212 (31.4%) | 112 (16.6%) | 99 (14.7%) | 252 (37.3%) | <0.0001 |
| No (n=5294) | 2129 (40.2%) | 1068 (20.2%) | 1142 (21.6%) | 955 (18.0%) | |
| Birth weight (g) | |||||
| 3516.2 (554.3) | 3510.4 (530.5) | 3500.6 (546.8) | 3506.5 (550.4) | 0.8742 | |
| Maternal | |||||
| Diabetes during pregnancy | |||||
| Type 1(n=235) | 71 (30.2%) | 41 (17.4%) | 26 (11.1%) | 97 (41.3%) | <.0001 |
| Type 2 (n=16) | 3 (18.7%) | 4 (25.0%) | 5 (31.3%) | 4 (25.0%) | |
| GDM (n=330) | 137 (41.5%) | 63 (19.1%) | 59(17.9%) | 71(21.5%) | |
| None (n=5203) | 2064 (39.6%) | 1039 (20.0%) | 1111(21.4%) | 989 (19.0%) | |
| Pre-pregnancy BMI | |||||
| 24.7(5.1) | 24.7(5.1) | 25.2(5.7) | 24.8(5.3) | 0.0759 | |
| Gestational age (weeks) | |||||
| 39.6(1.6) | 39.5(1.5) | 39.4(1.7) | 39.5(1.7) | 0.0539 | |
| Gestational weight gain (kg) | |||||
| 14.9(6.0) | 14.5(6.5) | 14.9(6.4) | 14.1(6.2) | 0.0051 | |
Data are n(%) or means (SD). All percentages are by row. FDR = First degree relatives. GDM = gestational diabetes mellitus.
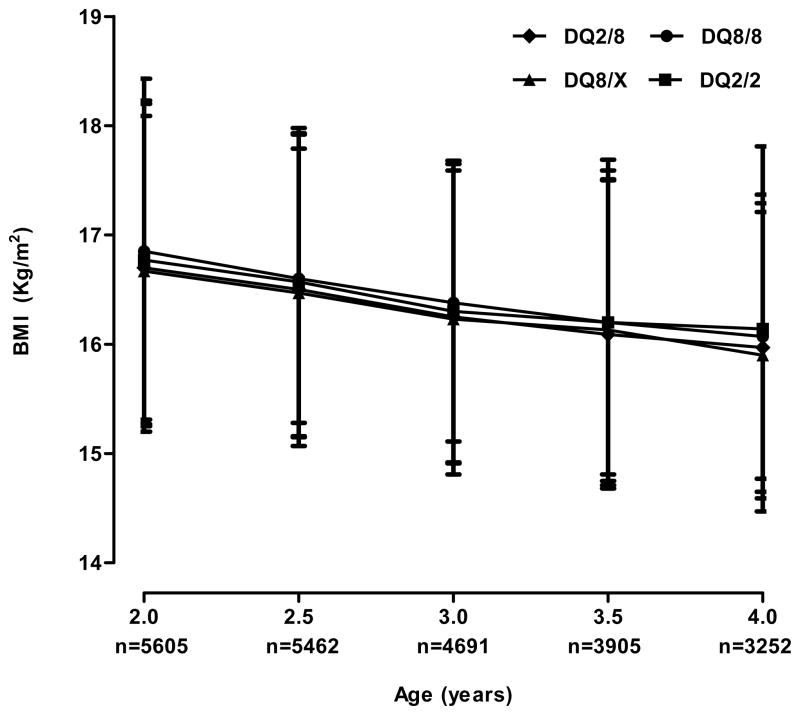
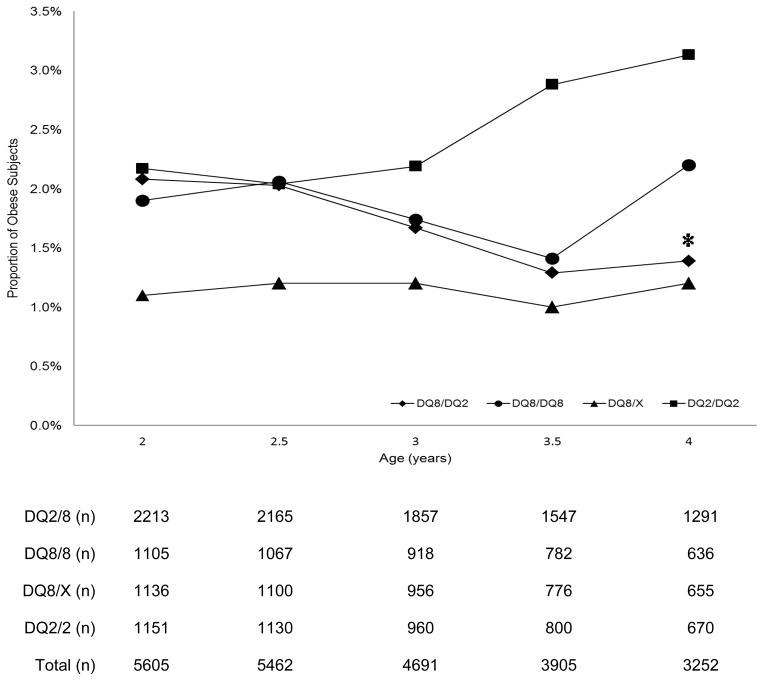
Gestational weight gain among the TEDDY mothers differed significantly when examined by their children’s HLA genotypes (p<0.01); however, our previous report indicated TEDDY subjects had comparable birth weight (data not shown).31 The average BMI at each cross-sectional assessment point between ages two and four was not statistically different and followed a normal pattern of decline with increasing age (Figure 1). According to the IOTF cutoff values, 12.1%, 12.2%, 11.1%, 11.7%, and 12.0% of subjects at 2, 2.5, 3, 3.5, and 4 years were overweight but not obese. The lowest prevalence, 10.4%, was seen in 3-year old subjects with DQ8/X genotype and the highest prevalence, 13.3%, was seen in 2.5-year old children with the DQ2/2 genotype. The overall proportion of obese subjects was 1.9%, 1.9%, 1.7%, 1.6%, and 1.9% at 2, 2.5, 3, 3.5, and 4 years, respectively. Univariate analysis indicated that the proportion of overweight was not significantly different by age, HLA genotypes, or country of residence, but a decreasing trend of obesity was also observed among those that carried the DQ2/8 genotype (p for trend = 0.0315, Figure 2).
Figure 1.
Cross-sectional comparison of body mass index by age and type 1 diabetes high risk HLA genotypes among 5 969 2–4 year old children genetically at risk for type 1 diabetes.
Figure 2.
Prevalence of obesity among 5 969 2–4 year old children genetically at risk for type 1 diabetes by age and by type 1 diabetes high risk HLA genotypes. The asterisk denotes a significant declining trend in obesity within the DQ 2/8 genotype (p = 0.0315).
Race/ethnicity comparison was conducted only among USA participants due to data availability. The prevalence of overweight and obesity did not differ by HLA genotypes in USA Non-Hispanic White (NHW) and Hispanic subjects in univariate analysis except that the obesity rate declined from 3.2% (age two) to 1.7% (age four) in the NHW DQ2/8 group (p=0.032) and peaked at 6.3% (age three) compared to 3.3% at age two and 3.3% at age four (p<0.0001) in the Hispanic DQ2/8 group.
Children carrying the DQ2/2 genotypes were independently associated with significantly higher risk of obesity at age four (n=3 252, odds ratio (OR) = 2.41, 95% CI 1.21–4.80) after adjusting for mother’s pre-pregnancy BMI, birth weight z-score, and the development of type 1 diabetes or persistent confirmed islet autoantibody (Figure 3). In the meanwhile, mother’s pre- pregnancy BMI was associated with risk of obesity between age two and four with an adjusted OR of 1.05 (95% CI 1.01–1.08) at age two (n=5 605), OR 1.10 (95% CI 1.06–1.13) at age three (n=4 691), and OR 1.09 (95% CI 1.06–1.13) at age four (n=3 252).
Figure 3.
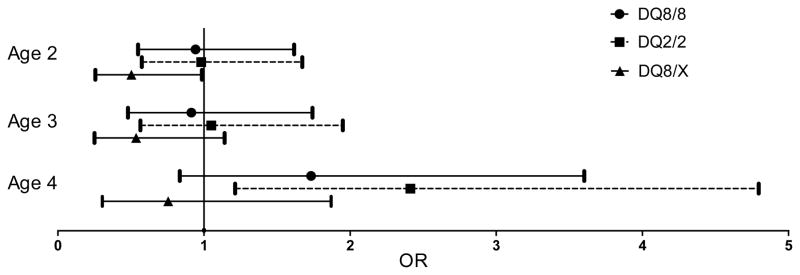
Association between obesity and HLA-DQ genotypes among 5 969 2–4 year old children genetically at risk for type 1 diabetes. Reference group = DQ2/8. Birth weight Z score was created to adjust for country, sex, mother’s height, gestational age and the number of fetuses in the pregnancy (singleton, twin, triplet, etc.) The development of persistent confirmed islet autoantibody and type 1 diabetes diagnosis was also adjusted for in the logistic regression model.
Discussion
In this international study of two to four years old children who are at increased genetic risk for type 1 diabetes, we report two major findings. First, a trend of continuously declining obesity was seen among DQ2/8 children (p=0.0315) compared to other genotype groups. Second, the DQ2/2 genotype was independently associated with higher risk of obesity at age four (OR = 2.21, 95% CI 1.12 – 4.33) after adjusting for maternal BMI before pregnancy, a widely acknowledged factor that influences body size.
The first finding that HLA-DQ8/2 genotype being associated with decreasing prevalence of obesity as the subjects aged was consistent with the result reported by Carlsson et al18 which indicated the frequency of high risk HLA genotypes declined with increasing BMI. Taking into account that children with HLA DQ8/2 genotype tend to develop type 1 diabetes at a younger age,32–34 it is reasonable to hypothesize that carriers of DQ8/2 may have a more progressive disease progress and/or a more rapid loss of insulin production which would lead to a classic diabetes symptom - weight loss.
The second observation that genotype DQ2/2 was associated with higher risk of obesity suggested individuals with this genotype may be more susceptible to become obese during physical development and their BMI may mediate their likelihood of developing type 1 diabetes imposed by genotype. Such mediation may be realized by some cytokines secreted by adipocytes (e.g. IL-6) affecting the risk of islet autoimmunity through a direct effect on the function of autoimmunity suppression T regulatory cells,35 or by other cytokines influencing beta cell function.36 Additionally, increase in adiposity was found to contribute to prepubertal insulin resistance.37 All of the above factors may account for the reported correlation between higher prevalence of childhood obesity in the population and elevated type 1 diabetes incidence rate.17–19 Another possible contributor to the relationship between DQ2/2 and higher risk of obesity observed in the TEDDY sample might be the role HLA genotype plays in the pathogenesis of celiac disease (CD). Class II HLA-DQA1 and HLA-DQ81 loci are strong genetic components of CD with approximately 90% of celiac subjects having HLA-DQ2 heterodimers.38
The major findings in this paper were observed in more than 5000 children from four different countries in which the proportion of overweight children ranged from 10.4% to 13.3% and the proportion of obese children varied from 1.0% to 3.1% across four high risk HLA genotypes and five age points. Within each TEDDY country, the prevalence of overweight and obesity was lower than the country-specific data from similar study cohorts.39–41 Compared to national data from the age-comparable general population in the USA, the prevalence of overweight and obesity among the TEDDY USA cohort was lower than the estimates from the 2007–2008 National Health and Nutrition Examination Survey which noted 21.2% of 2–5 years old children were overweight and 10.4% of the same group were obese.39 The Pediatric Diabetes Consortium study also observed that newly-diagnosed type 1 diabetes patients had lower BMI than the general population after adjusting for age and gender.21 TEDDY Swedish participants had an overweight rate of 16.8% and an obesity rate of 1.9%, which were lower than the rates in another pediatric cohort from the same region in Sweden.40 The TEDDY subjects in Finland also had lower proportion of obesity (0.92 at age two and 0.75% at age four) compared to the observations from a Finnish population without diabetes.41 The lower prevalence in TEDDY may be attributed to the different genetic background between the TEDDY cohort and general public. Parental awareness of their children’s disease risk might also have caused the TEDDY parents to follow health recommendations more closely, which in turn, might have resulted in lower BMI. An examination among multiple birth cohorts from a same area indicated longer breast feeding duration and reduced energy and dietary fat intake might have contributed to a decreasing trend for overweight at two years of age.42
In conclusion, HLA-DQ genotype was found to be associated with risk of obesity early in life when controlling for country, gender, birth weight, mother’s height, maternal prepregnancy weight, gestational age, and number of fetuses in the pregnancy. Effect of HLA-DQ genotype on developing type 1 diabetes may be mediated by BMI as individuals carrying different genotypes may have different susceptibility to obesity. Longitudinal examinations of BMI as the TEDDY cohort ages will be valuable to verify findings in this study and to evaluate whether age and adiposity rebound affect the relationship between HLA genotypes and BMI. Data collected from similar populations such as the Diabetes Prediction in Skåne (DiPis) study and the Diabetes Autoimmunity Study in the Young (DAISY) study could also be used to test the findings. Investigation of the relationship between growth velocity and HLA genotypes will further shed light on alternative mediators in the development of type 1 diabetes.
Supplementary Material
Acknowledgments
Funding/Financial Support
This study was funded by U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, and UC4 DK95300 and Contract No. HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Juvenile Diabetes Research Foundation (JDRF), and Centers for Disease Control and Prevention (CDC).
Footnotes
J.Y. and K.V. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. A.L. contributed to discussion, reviewed and edited the manuscript. U.M.U., K.F.L., R.V., C.W., H.E.L., M.R., J.X.S., A.G.Z., O.G.S., W.A.H., B.A., and J.P.K. reviewed the manuscript. J.Y. is the guarantor of this work and, as such, had full access to all the data in the study and takes final responsibility for the decision to submit for publication.
Conflict of Interest
The authors declare no conflict of interest.
Supplementary information is available at the journal’s website.
References
- 1.Tuomilehto J. The Emerging Global Epidemic of Type 1 Diabetes. Current diabetes reports. 2013:1–10. doi: 10.1007/s11892-013-0433-5. [DOI] [PubMed] [Google Scholar]
- 2.Sheehy MJ, Scharf SJ, Rowe JR, Neme de Gimenez MH, Meske LM, Erlich HA, et al. A diabetes-susceptible HLA haplotype is best defined by a combination of HLA-DR and -DQ alleles. The Journal of clinical investigation. 1989;83(3):830–835. doi: 10.1172/JCI113965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Notkins AL, Lernmark Å. Autoimmune type 1 diabetes: resolved and unresolved issues. The Journal of clinical investigation. 2001;108(9):1247–1252. doi: 10.1172/JCI14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes--the analysis of the data on published incidence trends. Diabetologia. 1999;42(12):1395–1403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- 5.Keenan H, el Deirawi K, Walsh M, Grover VV, Alva E, Onyemere K, et al. Are trends in diabetes incidence changing for minority children? Annals of epidemiology. 2000;10(7):459. doi: 10.1016/s1047-2797(00)00091-0. [DOI] [PubMed] [Google Scholar]
- 6.Libman IM, LaPorte RE, Becker D, Dorman JS, Drash AL, Kuller L. Was there an epidemic of diabetes in nonwhite adolescents in Allegheny County, Pennsylvania? Diabetes Care. 1998;21(8):1278–1281. doi: 10.2337/diacare.21.8.1278. [DOI] [PubMed] [Google Scholar]
- 7.Waldhor T, Schober E, Karimian-Teherani D, Rami B. Regional differences and temporal incidence trend of Type I diabetes mellitus in Austria from 1989 to 1999: a nationwide study. Diabetologia. 2000;43(11):1449–1450. doi: 10.1007/s001250051553. [DOI] [PubMed] [Google Scholar]
- 8.Stanescu DE, Lord K, Lipman TH. The epidemiology of type 1 diabetes in children. Endocrinology and metabolism clinics of North America. 2012;41(4):679–694. doi: 10.1016/j.ecl.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Borchers AT, Uibo R, Gershwin ME. The geoepidemiology of type 1 diabetes. Autoimmunity Reviews. 2010;9(5):A355–A365. doi: 10.1016/j.autrev.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Knerr I, Wolf J, Reinehr T, Stachow R, Grabert M, Schober E, et al. The ‘accelerator hypothesis’: relationship between weight, height, body mass index and age at diagnosis in a large cohort of 9,248 German and Austrian children with type 1 diabetes mellitus. Diabetologia. 2005;48(12):2501–2504. doi: 10.1007/s00125-005-0033-2. [DOI] [PubMed] [Google Scholar]
- 11.Charkaluk ML, Czernichow P, Levy-Marchal C. Incidence data of childhood-onset type I diabetes in France during 1988–1997: the case for a shift toward younger age at onset. Pediatric research. 2002;52(6):859–862. doi: 10.1203/00006450-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Vehik K, Hamman RF, Lezotte D, Norris JM, Klingensmith GJ, Dabelea D. Childhood growth and age at diagnosis with Type 1 diabetes in Colorado young people. Diabetic medicine : a journal of the British Diabetic Association. 2009;26(10):961–967. doi: 10.1111/j.1464-5491.2009.02819.x. [DOI] [PubMed] [Google Scholar]
- 13.Vehik K, Hamman RF, Lezotte D, Norris JM, Klingensmith GJ, Rewers M, et al. Trends in High-Risk HLA Susceptibility Genes Among Colorado Youth With Type 1 Diabetes. Diabetes Care. 2008;31(7):1392–1396. doi: 10.2337/dc07-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fourlanos S, Varney MD, Tait BD, Morahan G, Honeyman MC, Colman PG, et al. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care. 2008;31(8):1546–1549. doi: 10.2337/dc08-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between Type I and Type II diabetes. Diabetologia. 2001;44(7):914–922. doi: 10.1007/s001250100548. [DOI] [PubMed] [Google Scholar]
- 16.Wilkin TJ. Diabetes: 1 and 2, or one and the same? Progress with the accelerator hypothesis Pediatric diabetes. 2008;9(3 Pt 2):23–32. doi: 10.1111/j.1399-5448.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 17.Hyppönen E, Virtanen SM, Kenward MG, Knip M, Akerblom HK Group CDiFS. Obesity, increased linear growth, and risk of type 1 diabetes in children. Diabetes Care. 2000;23(12):1755–1760. doi: 10.2337/diacare.23.12.1755. [DOI] [PubMed] [Google Scholar]
- 18.Carlsson A, Kockum I, Lindblad B, Engleson L, Nilsson A, Forsander G, et al. Low risk HLA-DQ and increased body mass index in newly diagnosed type 1 diabetes children in the Better Diabetes Diagnosis study in Sweden. Int J Obes (Lond) 2012;36(5):718–724. doi: 10.1038/ijo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care. 2003;26(10):2871–2875. doi: 10.2337/diacare.26.10.2871. [DOI] [PubMed] [Google Scholar]
- 20.Gimenez M, Aguilera E, Castell C, de Lara N, Nicolau J, Conget I. Relationship between BMI and age at diagnosis of type 1 diabetes in a Mediterranean area in the period of 1990–2004. Diabetes Care. 2007;30(6):1593–1595. doi: 10.2337/dc06-2578. [DOI] [PubMed] [Google Scholar]
- 21.Kaminski BM, Klingensmith GJ, Beck RW, Tamborlane WV, Lee J, Hassan K, et al. Body mass index at the time of diagnosis of autoimmune type 1 diabetes in children. The Journal of pediatrics. 2013;162(4):736–740. e731. doi: 10.1016/j.jpeds.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Larsson HE, Hansson G, Carlsson A, Cederwall E, Jonsson B, Larsson K, et al. Children developing type 1 diabetes before 6 years of age have increased linear growth independent of HLA genotypes. Diabetologia. 2008;51(9):1623–1630. doi: 10.1007/s00125-008-1074-0. [DOI] [PubMed] [Google Scholar]
- 23.The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatric diabetes. 2007;8(5):286–298. doi: 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 24.Hagopian WA, Lernmark A, Rewers MJ, Simell OG, She JX, Ziegler AG, et al. TEDDY--The Environmental Determinants of Diabetes in the Young: an observational clinical trial. Annals of the New York Academy of Sciences. 2006;1079:320–326. doi: 10.1196/annals.1375.049. [DOI] [PubMed] [Google Scholar]
- 25.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vehik K, Fiske SW, Logan CA, Agardh D, Cilio CM, Hagopian W, et al. Methods, quality control and specimen management in an international multicentre investigation of type 1 diabetes: TEDDY. Diabetes/Metabolism Research and Reviews. 2013;29(7):557–567. doi: 10.1002/dmrr.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiviniemi M, Hermann R, Nurmi J, Ziegler AG, Knip M, Simell O, et al. A high-throughput population screening system for the estimation of genetic risk for type 1 diabetes: an application for the TEDDY (the Environmental Determinants of Diabetes in the Young) study. Diabetes technology & therapeutics. 2007;9(5):460–472. doi: 10.1089/dia.2007.0229. [DOI] [PubMed] [Google Scholar]
- 28.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57(4):1084–1092. doi: 10.2337/db07-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Yu L, Tiberti C, Bonamico M, Taki I, Miao D, et al. A report on the International Transglutaminase Autoantibody Workshop for Celiac Disease. The American journal of gastroenterology. 2009;104(1):154–163. doi: 10.1038/ajg.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winkler C, Marienfeld S, Zwilling M, Bonifacio E, Ziegler AG. Is islet autoimmunity related to insulin sensitivity or body weight in children of parents with type 1 diabetes? Diabetologia. 2009;52(10):2072–2078. doi: 10.1007/s00125-009-1461-1. [DOI] [PubMed] [Google Scholar]
- 31.Sterner Y, Torn C, Lee HS, Larsson H, Winkler C, McLeod W, et al. Country-specific birth weight and length in type 1 diabetes high-risk HLA genotypes in combination with prenatal characteristics. Journal of perinatology : official journal of the California Perinatal Association. 2011;31(12):764–769. doi: 10.1038/jp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanjeevi CB, Sedimbi SK, Landin-Olsson M, Kockum I, Lernmark Å, et al. on behalf of the Swedish Childhood D. The Risk Conferred by HLA-DR and DQ for Type 1 Diabetes in 0–35-Years Age Group are Different in Different Regions of Sweden. Annals of the New York Academy of Sciences. 2008;1150(1):106–111. doi: 10.1196/annals.1447.061. [DOI] [PubMed] [Google Scholar]
- 33.Graham J, Hagopian WA, Kockum I, Li LS, Sanjeevi CB, Lowe RM, et al. Genetic Effects on Age-Dependent Onset and Islet Cell Autoantibody Markers in Type 1 Diabetes. Diabetes. 2002;51(5):1346–1355. doi: 10.2337/diabetes.51.5.1346. [DOI] [PubMed] [Google Scholar]
- 34.Redondo MJ, Fain PR, Eisenbarth GS. Genetics of Type 1A Diabetes. Recent Prog Horm Res. 2001;56(1):69–90. doi: 10.1210/rp.56.1.69. [DOI] [PubMed] [Google Scholar]
- 35.Guzik TJMD, Korbut R. Adipocytokines - novel link between inflammation and vascular function? J Physiol PHarmacol. 2006;57(4):505–528. [PubMed] [Google Scholar]
- 36.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nature reviews Endocrinology. 2009;5(4):219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 37.Jeffery AN, Metcalf BS, Hosking J, Streeter AJ, Voss LD, Wilkin TJ. Age Before Stage: Insulin Resistance Rises Before the Onset of Puberty: A 9-year longitudinal study (EarlyBird 26) Diabetes Care. 2012;35(3):536–541. doi: 10.2337/dc11-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kagnoff MF. Celiac disease: pathogenesis of a model immunogenetic disease. The Journal of clinical investigation. 2007;117(1):41–49. doi: 10.1172/JCI30253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA : the journal of the American Medical Association. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 40.Norberg C, Hallstrom Stalin U, Matsson L, Thorngren-Jerneck K, Klingberg G. Body mass index (BMI) and dental caries in 5-year-old children from southern Sweden. Community dentistry and oral epidemiology. 2012;40(4):315–322. doi: 10.1111/j.1600-0528.2012.00686.x. [DOI] [PubMed] [Google Scholar]
- 41.Vuorela N, Saha M-T, Salo M. Prevalence of overweight and obesity in 5- and 12-year-old Finnish children in 1986 and 2006. Acta Pædiatrica. 2009;98(3):507–512. doi: 10.1111/j.1651-2227.2008.01110.x. [DOI] [PubMed] [Google Scholar]
- 42.Vuorela N, Saha M-T, Salo MK. Change in prevalence of overweight and obesity in Finnish children – comparison between 1974 and 2001. Acta Paediatrica. 2011;100(1):109–115. doi: 10.1111/j.1651-2227.2010.01980.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.