Abstract
Sarcopenia is thought to play a major role in the functional impairment that occurs with old age. In clinical practice, sarcopenia is often determined by measuring handgrip strength. Here, we compared the lower limb quadriceps strength to the handgrip strength in their association with health outcomes in older adults in primary care. Our study population consisted of older adults (n = 764, 68.2 % women, median age 83) that participated in the Integrated Systemic Care for Older People (ISCOPE) study. Participants were visited at baseline to measure quadriceps strength and handgrip strength. Data on health outcomes were obtained at baseline and after 12 months (including life satisfaction, disability in daily living, GP contact-time and hospitalization). Quadriceps strength and handgrip strength showed a weak association (β = 0.42 [95 % CI 0.33–0.50]; R2 = 0.17). Quadriceps strength and handgrip strength were independently associated with health outcomes at baseline, including quality of life, disability in daily living, GP contact-time, hospitalization, and gait speed. Combined weakness of the quadriceps and handgrip distinguished a most vulnerable subpopulation that presented with the poorest health outcomes. At follow-up, handgrip strength showed an association with quality of life (β = 0.05; P = 0.002) and disability in daily living (β = −0.5; P = 0.004). Quadriceps weakness did not further contribute to the prediction of the measured health outcomes. We conclude that quadriceps strength is only moderately associated with handgrip strength in an older population and that the combination of quadriceps strength and handgrip strength measurements may aid in the identification of older adults in primary care with the poorest health outcomes. In the prediction of poor health outcomes, quadriceps strength measurements do not show an added value to the handgrip strength.
Keywords: Older adults, Handgrip/grip strength, Quadriceps strength, Sarcopenia
Introduction
Sarcopenia, defined as the degenerative loss of skeletal muscle tissue associated with aging, is thought to play a major role in the functional impairment that occurs with old age (Morley et al. 2001; Rolland et al. 2008). In clinical practice, handgrip strength is often used as a surrogate measure representative of the sarcopenic condition, as measuring handgrip strength is a simple, accessible, and inexpensive method to use (Cruz-Jentoft et al. 2010). Handgrip strength has been shown to associate with several adverse health outcomes in the elderly, including functional impairment (Femia et al. 1997; Giampaoli et al. 1999; Hairi et al. 2010; Rantanen et al. 1994), morbidity (Rantanen et al. 1998), hospitalization (Cawthon et al. 2009) and mortality (Laukkanen et al. 1995; Newman et al. 2006; Rantanen et al. 2003).
Although upper limb muscle measurements correlate with several health outcomes, lower limb muscle measurements may be a better parameter for mobility outcomes. In clinical practice, lower limb muscle strength measurements often consist of quadriceps strength measurements during knee extension and flexion (Cruz-Jentoft et al. 2010). A previous study investigating the relationship between handgrip strength and lower limb muscle strength reported a strong association with correlation coefficients varying from 0.70 to 0.72 (Cruz-Jentoft et al. 2010; Lauretani et al. 2003). Similar to handgrip strength, quadriceps strength correlates with various negative health outcomes in older adults. These include functional impairment (Hairi et al. 2010; Rantanen et al. 1994, 2001; Visser et al. 2005), hospitalization (Cawthon et al. 2009) and mortality (Laukkanen et al. 1995; Newman et al. 2006).
Several studies have investigated the correlation of handgrip strength and quadriceps strength on disability items related to mobility (Hairi et al. 2010; Rantanen et al. 1994). However, these studies all analyzed the correlation separately for the handgrip strength and quadriceps strength, and did not investigate the correlation of these parameters in relation to each other to determine whether the two muscle strength parameters have complementary value. In this study, we investigated both lower limb quadriceps strength and handgrip strength in their association with health outcomes in older adults in primary care.
Methods
Study population
The study population consisted of older adults aged 75 and over who participated in the Integrated Systematic Care for Older People (ISCOPE) study. The ISCOPE study is a pragmatic cluster-randomized controlled trial that compared a proactive approach by the general practitioner (GP) with the usual care provided by the GP in monitoring the health status of older adults with complex problems. The presence of complex problems was defined as having problems in at least three of the four predefined domains: functional, somatic, psychological and social, and was assessed by the ISCOPE screening questionnaire (Poot et al. 2014).
The ISCOPE study population was recruited from 59 participating primary care practices and all registered adults aged 75 and above were targeted (n = 12,066). Persons who were deceased, too ill, non-Dutch speaking, admitted to a nursing home, or judged unsuitable according to their GP were excluded (n = 590). Of the remaining 11,476 individuals who were sent a written screening questionnaire, 7285 were completed and returned.
A random sample of the 7285 participants was visited at home to obtain data on socio-demographic characteristics and to partake in additional questionnaires. Based on the outcomes of the screening questionnaire, individuals with problems in three or more domains were all visited and interviewed. Of the participants with problems in two domains, 60 % was interviewed. Of the individuals with no problems or problems in one domain, 15 % was interviewed. In total, 2713 participants were visited at home to obtain data on socio-demographic characteristics, residency, functional status, and the presence of disease. After 1 year, participants were revisited to partake in the same questionnaires to obtain follow-up information.
In the ISCOPE study, older adults with complex problems were provided the usual care by the GP or provided a proactive approach by the GP, which consisted of a care plan that was drafted together with the participant and informal caregiver. The priorities and goals of the older adult and informal caregiver were taken as a starting point. Actions to be taken and evaluation plans for follow-up were formulated by the GP together with the participant.
For the purpose of the present analyses, a subgroup of 823 participants was randomly selected from 22 of the 59 participating general practices (both control and intervention practices). Participants with complex problems were oversampled. The 823 participants received an additional questionnaire during the home visit at baseline, and additional measurements on handgrip strength, quadriceps strength, and gait speed were obtained by the research nurses. All participants gave written informed consent; for participants with severe cognitive impairment, informed consent was obtained from a proxy. The study was approved by the Medical Ethics Committee of Leiden University Medical Center. For the present analysis, 764 participants were included with valid quadriceps and handgrip strength measurements. Follow-up data was available for 570 participants.
Study parameters
Determinants
Muscle strength
Muscle strength measurements for the handgrip and the lower limb were obtained during the home visits at baseline.
Handgrip strength was measured using a Jamar hand dynamometer (Sammons Preston Inc., Bolingbrook, IL). Participants were asked whether they were left or right handed to determine their dominant side. To measure handgrip strength, the participant was instructed to stand up and hold the dynamometer in the dominant hand with the arm parallel to the body without squeezing the arm against the body. The width of the handle was adjusted to the size of the participant’s hand to ensure that the middle phalanx rested on the inner handle. The participant was allowed to perform one test trial. After this, three measurements followed and the best score was used for analysis (Hanten et al. 1999, Ling et al. 2010, Taekema et al. 2010). Handgrip strength was expressed in kilograms; cut-off points to determine weak (versus normal) handgrip strength were derived from Fried et al. (2001) (for male participants <30 kg and for female participants <18 kg). Subjects unable to complete the handgrip strength test due to physical limitations were considered to have weak handgrip strength.
Lower limb muscle strength was measured for the quadriceps using the Microfet2®, a hand-held dynamometer (Hoggan Health Industries Inc., Salt Lake City, UT). A hand-held dynamometer is a widely used, reliable, and valid instrument to measure isometric peak force, also in the elderly patient (Andrews et al. 1996, Stark et al. 2011). The Microfet2® has a high (inter-rater) reliability and validity in measuring quadriceps strength (Douma et al. 2014, Kwoh et al. 1997, Schaubert and Bohannon 2005). To measure knee extension, the participant was seated in a straight-back chair without armrests. The participants stabilized their position by holding on to the seat of the chair and knees were positioned at a resting angle of 90°. The dynamometer was placed just above the ankles against the shins. Participants were asked to push against the dynamometer to measure isometric strength. The participant was allowed to perform one test trial. After this, three measurements followed and the best score was used for analysis. The peak force was measured in kilograms. The median (adjusted for sex) was used as a cut-off point to determine weak (versus normal) quadriceps strength. Among the 764 subjects included in the analysis, 66 subjects were hindered by physical limitations during the quadriceps strength assessment (8.6 %). Subjects unable to complete the knee extension test due to physical limitations were considered to have weak quadriceps strength. Quadriceps strength was measured in both legs; measurements of the dominant and nondominant side were highly correlated (R2 = 0.75, β = 0.87; Table 2) indicating consistent quadriceps measurements. Participants were asked whether they were left or right handed to determine their dominant side. For further analyses, the quadriceps strength of the dominant leg was used.
Table 2.
Association between the quadriceps strength and the handgrip strength
| Per unita change in | Outcome | Crude | Adjusted for age and sex | ||||
|---|---|---|---|---|---|---|---|
| R 2 | β (95 % CI) | P value | R 2 | β (95 % CI) | P value | ||
| Handgrip strength | Quadriceps strength | 0.16 | 0.44 | <0.001 | 0.17 | 0.42 | <0.001 |
| (Dominant side) | (0.37; 0.52) | (0.33; 0.50) | |||||
| Quadriceps strength | Quadriceps strength | 0.75 | 0.87 | <0.001 | 0.75 | 0.87 | <0.001 |
| (Dominant side) | (Nondominant side) | (0.83; 0.91) | (0.83; 0.90) | ||||
aHandgrip and quadriceps strength measurements were normalized by square root transformation and standardized into Z scores
Health outcomes
Quality of life
Quality of life was assessed at baseline and follow-up using the EQ5D+c questionnaire, a generic instrument developed by the EuroQol Group to value health (extended with a cognitive dimension) (EuroQol Group 1990; Krabbe et al. 1999). Quality of life was additionally scored using the Visual Analogue Scale for perceived health status, ranging from 0 (worst imaginable health status) to 100 (best imaginable health status) and the Cantril ladder of life, a visual analogue scale on the perceived quality of life, ranging from 1 (very unsatisfied) to 10 (very satisfied) (Cantril 1966).
Functional status
Disability in basic (BADL) and instrumental activities of daily living (IADL) was measured at baseline and follow-up with the Groningen Activities Restriction Scale (GARS) (Kempen et al. 1996). The GARS is a questionnaire that assesses disability in the competence of nine BADL items and nine IADL items (Bootsma-van der Wiel et al. 2001). A sum score was calculated for BADL and IADL separately; each sum score ranged from 9 (competent in all activities) to 36 (unable to perform any activity without help).
GP contact-time
The number of GP contacts, including home visits and consultations, were extracted from the electronic patient records (EPR). GP contact-time at baseline was calculated from the GP contacts during the year before ISCOPE and GP contact-time at follow-up was calculated from the GP contacts in the year during follow-up. Consultations were estimated at 10 min of GP contact and home visits at 30 min. EPR data were available for 359 participants.
Hospitalization
Participants were asked whether they had been admitted in the hospital in the past 12 months.
Gait speed
Gait speed was assessed at baseline with a 12 m walking test (Bloem et al. 1992; van Houwelingen et al. 2013). A 3-m course was denoted by tape measurement, along which the participants were instructed to walk up and down. Participants were requested to walk as quickly as possible from a standing start position and total time was measured using a stopwatch. Use of a walking aid was allowed. Gait speed was calculated using distance in meters and time in seconds (meters per second [m/s]).
Other parameters
Socio-demographic characteristics
Participants were interviewed to obtain information on their sex, age, living situation (independent and alone, independent with others, residential care home or residential nursing home), income (state pension only or state pension with additional income), and educational level (primary school, high school, vocational school, or university).
Chronic diseases
Data on common chronic diseases were self-reported and obtained during the interview at baseline. Chronic diseases included self-reported diabetes, heart failure, cancer, chronic obstructive pulmonary disease (COPD), incontinence, arthritis, osteoporosis, dizziness, lower urinary tract symptoms (LUTS), depression, anxiety, dementia, vision, deafness, fracture, stroke/transient ischemic attack (TIA), and myocardial infarction. The number of chronic diseases was used as a measure of multimorbidity.
Body weight
The body weight of the participants was measured in kilograms (kg) during the home visits.
Statistical analyses
Medians and interquartile ranges (IQR) were used to describe continuous variables and proportions were used for categorical variables. Handgrip and quadriceps strengths were normalized using square root transformation followed by standardization into Z scores.
First, we analyzed the correlation between quadriceps strength and handgrip strength using linear regression. The cross-sectional association with health outcomes was analyzed both separately and combined for the handgrip and quadriceps strength using linear or logistic regression models adjusting for age and sex. In addition, we performed a longitudinal analysis investigating the predictive value of the handgrip and quadriceps strength on health outcomes after 1 year of follow-up, separately and combined. This analysis was additionally adjusted for the baseline values of the health outcomes measured.
Second, we compared characteristics and health outcomes for different handgrip and quadriceps strength categories, both cross-sectionally and prospectively. Groups were defined by having either normal handgrip and normal quadriceps strength, weak handgrip and normal quadriceps strength, weak quadriceps and normal handgrip strength, or weak handgrip and weak quadriceps strength. The four groups were compared using general linear model (GLM) univariate analyses. Health outcomes at baseline were corrected for age and sex, and health outcomes at follow-up were corrected for age, sex and baseline values of the health outcome. A P value of <0.05 was considered statistically significant. Data were analyzed using SPSS 20.0 for Windows.
Results
Study population
The baseline characteristics of our population are described in Table 1. The median age of the participants was 83 (interquartile range [IQR] 79–87). Among the participants, 68.2 % were female, 57.2 % were living alone, and 9.7 % were living in a care home. The median number of chronic diseases was 4 (IQR 2–6). Perceived life satisfaction as measured by Cantril’s ladder was 7 (IQR 7–8) and quality of life measured by the EQ5D+c index was 0.81 (IQR 0.65–0.84). The median scores of disability in the basic (BADL) and instrumental activities of daily living (IADL) were 9 (IQR 9–11) and 21 (IQR 15–27), respectively. Of the participants, 30.9 % was considered frail according to the definition by Fried et al. (2001).
Table 1.
Baseline characteristics of the study population
| Total | Men | Women | |
|---|---|---|---|
| (n = 764) | (n = 243; 31.8 %) | (n = 521; 68.2 %) | |
| Age (year) | 83 [79; 87] | 82 [78; 87] | 83 [79; 87] |
| Living situation | |||
| Living alone | 434 (57.2) | 86 (36.1) | 348 (66.8) |
| Residence in a long term facility | 74 (9.7) | 13 (5.5) | 61 (11.7) |
| Low incomea | 101 (13.3) | 23 (9.6) | 78 (15.0) |
| Low educationb | 266 (35.0) | 61 (25.6) | 205 (39.3) |
| Number of chronic diseasesc | 4 [2; 6] | 3 [2; 5] | 4 [3; 6] |
| Weight (kg) | 72 [63; 82] | 80 [71; 87] | 69 [60; 77] |
| Quality of life | |||
| Cantril’s ladder | 7 [7; 8] | 7 [7; 8] | 7 [7; 8] |
| EQ5D+c | 0.81 [0.65; 0.84] | 0.81 [0.68; 0.89] | 0.78 [0.65; 0.84] |
| Visual analogue scale | 70 [60; 75] | 70 [60; 75] | 70 [60; 75] |
| Functioning | |||
| GARS | 30 [24; 38] | 29 [24; 39] | 30 [24; 38] |
| BADL | 9 [9; 11] | 9 [9; 11] | 9 [9; 12] |
| IADL | 21 [15; 27] | 20 [15; 27] | 21 [15; 27] |
| GP contact-time (min)d | 110 [50; 220] | 80 [40; 160] | 120 [60; 260] |
| Gait speed (m/s) | 0.52 [0.31; 0.71] | 0.59 [0.40; 0.77] | 0.48 [0.30; 0.66] |
| Handgrip strength (kg)e | 20 [16; 28] | 30 [25; 36] | 18 [14; 22] |
| Quadriceps strength (kg)e | 25 [17; 36] | 32 [20; 41] | 23 [16; 33] |
Data are numbers (%) or median [IQR]
aState pension only
bPrimary school only
cIncluding self-reported diabetes, heart failure, cancer, chronic obstructive pulmonary disease, incontinence, arthritis, osteoporosis, dizziness, lower urinary tract symptoms, depression, anxiety, dementia, impaired vision, deafness, fracture, stroke/transient ischemic attack, myocardial infarction
dGP contact information was available for 359 participants
eMedian handgrip and quadriceps strength differed between men and women (Mann-Whitney U, P < 0.001)
The median handgrip strength was 30 kg (IQR 25–36) for male participants and 18 kg (IQR 14–22) for female participants (P < 0.001). The median quadriceps strength was 32 kg (IQR 20–41) for men and 23 kg (IQR 16–33) for women (P <0.001).
Correlation handgrip strength and quadriceps strength
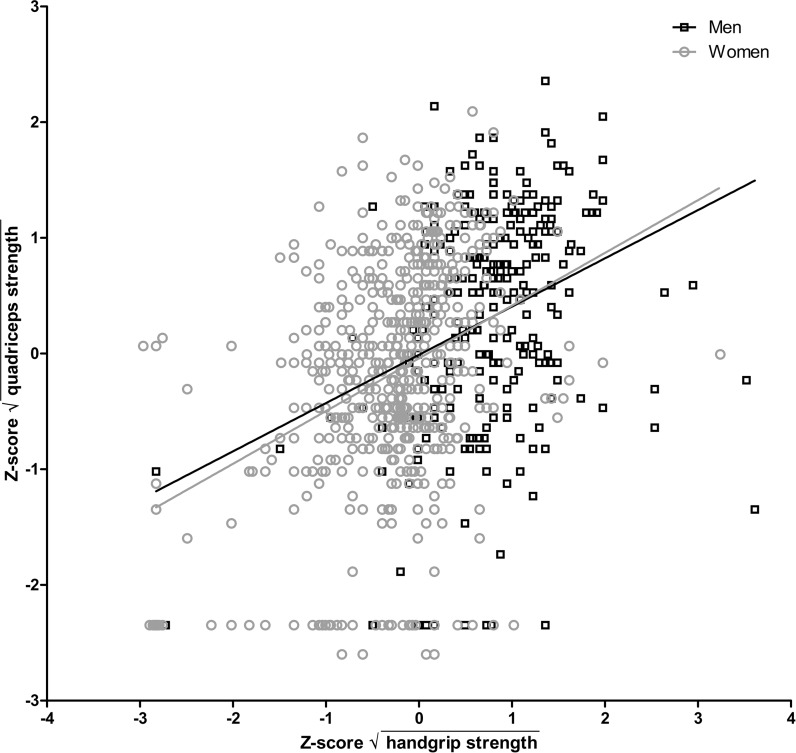
In this study population, the correlation between handgrip strength and quadriceps strength was weak; the effect estimate (β) of the handgrip strength on the quadriceps strength was 0.42 (95 % confidence interval [CI] 0.33–0.50) and the coefficient of determination (R2) was 0.17 (adjusted for age and sex; Table 2). When outliers were excluded from the analysis, the correlation between handgrip and quadriceps strength was even poorer (β = 0.32 [95 % CI 0.22–0.42], R2 = 0.13). Figure 1 depicts the correlation between handgrip and quadriceps strength, separately for men and women. The effect estimate and coefficient of determination for men (adjusted for age) were 0.34 (95 % CI 0.18–0.51) and 0.12, and for women 0.44 (95 % CI 0.34–0.55) and 0.14.
Fig. 1.
Scatter plot showing the relationship between the handgrip strength and the quadriceps strength. Regression lines are marked separately for men and women. Handgrip and quadriceps strength measurements were normalized using square root transformation followed by standardization into Z scores. Correlation estimates for men (adjusted for age): R 2 = 0.12, β = 0.34 (95 % CI 0.18–0.51); women (adjusted for age): R 2 = 0.14, β = 0.44 (95 % CI 0.34–0.55)
Association between the handgrip or quadriceps strength and health outcomes at baseline
Table 3 shows the cross-sectional analysis of the association between the handgrip strength and several health outcomes, unadjusted (Model I) and adjusted (Model II) for quadriceps strength. In the univariate analyses, handgrip strength was significantly associated with quality of life measured by EQ5D+c (β = 0.08 [95 % CI 0.06; 0.10]), disability in daily living as scored using GARS (β = −5.2 [95 % CI −6.2; −4.3]), GP contact-time (β = −34 min [95 % CI −63; −5]), hospitalization (odds ratio (OR) = 0.6 [95 % CI 0.5; 0.8]) and gait speed (β = 0.12 m/s [95 % CI 0.10; 0.15]). When quadriceps strength was included in the multivariate model, the predictive value improved as demonstrated by elevated R2 scores and significant F ratios and model χ2. Handgrip strength remained significantly associated with most health outcomes except for GP contact-time (P = 0.082). Quadriceps strength was also significantly associated with poorer scores on the measured health outcomes, independently of the handgrip strength: EQ5D+c (β = 0.09 [95 % CI 0.07; 0.11]), GARS (β = −3.4 [95 % CI −4.1; −2.7]), GP contact-time (β = −28 min [95 % CI −52; −4]), hospitalization (OR = 0.8 [95 % CI 0.7; 1.0]) and gait speed (β = 0.10 m/s [95 % CI 0.08; 0.12]). The effect estimates of the quadriceps strength were comparable to those for the handgrip strength except for EQ5D+c: β for quadriceps strength was 0.09 (95 % CI 0.07; 0.11) and 0.04 (95 % CI 0.02; 0.07) for handgrip strength.
Table 3.
Analysis of the handgrip and quadriceps strength on health outcomes at baseline
| Model Ia | Model IIa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (handgrip strength) | (combination of handgrip and quadriceps strength) | |||||||||||
| Model statistics | Per unit handgrip strength | Model statistics | Per unit handgrip strength | Per unit quadriceps strength | ||||||||
| R 2,b | F ratio/χ 2 | P value | β/OR (95 % CI) | P value | R 2,b | F ratio/χ 2 | P value | β/OR (95 % CI) | P value | β/OR (95 % CI) | P value | |
| EQ5D+c | 0.07 | 18.01 | <0.001 | 0.08 (0.06; 0.10) | <0.001 | 0.17 | 91.03 | <0.001 | 0.04 (0.02; 0.07) | 0.001 | 0.09 (0.07; 0.11) | <0.001 |
| GARS | 0.28 | 98.41 | <0.001 | −5.2 (–6.2; −4.3) | <0.001 | 0.36 | 85.39 | <0.001 | −3.8 (–4.8; −2.9) | <0.001 | −3.4 (–4.1; −2.7) | <0.001 |
| BADL | 0.14 | 39.60 | <0.001 | −1.6 (–2.0; −1.2) | <0.001 | 0.20 | 61.93 | <0.001 | −1.1 (−1.5;–0.7) | <0.001 | −1.2 (–1.6; −0.9) | <0.001 |
| IADL | 0.31 | 111.86 | <0.001 | −3.6 (–4.3; −3.0) | <0.001 | 0.37 | 70.90 | <0.001 | −2.8 (–3.4; −2.1) | <0.001 | −2.1 (–2.6; −1.6) | <0.001 |
| GP contact-time (min)c | 0.14 | 18.70 | <0.001 | −34 (–63; −5) | 0.024 | 0.15 | 5.30 | 0.022 | −27 (–56; 3) | 0.082 | −28 (–52; −4) | 0.022 |
| Hospitalization | 0.05 | 26.30 | <0.001 | 0.6 (0.5; 0.8) | <0.001 | 0.06 | 4.77 | 0.029 | 0.7 (0.5;0.9) | 0.002 | 0.8 (0.7; 1.0) | 0.028 |
| Gait speed (m/s) | 0.26 | 88.92 | <0.001 | 0.12 (0.10; 0.15) | <0.001 | 0.37 | 121.55 | <0.001 | 0.08 (0.06; 0.11) | <0.001 | 0.10 (0.08; 0.12) | <0.001 |
aBoth models are adjusted for sex and age
bFor the outcome “Hospitalization”, the Nagelkerke R 2 was used to estimate R 2
cGP contact information was available for 359 participants
Predictive value of baseline handgrip and quadriceps strength on health outcomes at follow-up
The longitudinal analysis of the association between handgrip or quadriceps strength at baseline and health outcomes at year one is shown in Table 4. Handgrip strength was significantly associated with EQ5D+c (β = 0.05 [95 % CI 0.02; 0.08]) and the BADL items of the GARS (β = −0.5 [95 % CI −0.8; −0.2]) after 1 year of follow-up (adjusted for baseline, Model I). When quadriceps strength was included in the analysis, handgrip strength remained significantly associated with these parameters (Model II). Quadriceps strength was not significantly associated with EQ5D+c, GARS, GP contact-time or hospitalization at follow-up.
Table 4.
Prospective analysis of baseline handgrip and quadriceps strength on health outcomes after 1 year of follow-up
| Model Ia | Model IIa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (handgrip strength) | (combination of handgrip and quadriceps strength) | |||||||||||
| Model statistics | Per unit handgrip strength | Model statistics | Per unit handgrip strength | Per unit quadriceps strength | ||||||||
| R 2,b | F ratio/χ 2 | P value | β/OR (95 % CI) | P value | R 2,b | F ratio/χ 2 | P value | β/OR (95 % CI) | P value | β (95 % CI) | P value | |
| EQ5D+c | 0.29 | 58.54 | <0.001 | 0.05 (0.02; 0.08) | <0.001 | 0.30 | 2.09 | 0.149 | 0.05 (0.02; 0.08) | 0.002 | 0.02 (-0.01; 0.04) | 0.149 |
| GARS | 0.77 | 460.27 | <0.001 | −0.4 (–1.1; 0.3) | 0.295 | 0.77 | 1.03 | 0.311 | −0.4 (–1.2; 0.3) | 0.246 | 0.3 (–0.3; 0.9) | 0.311 |
| BADL | 0.70 | 317.31 | <0.001 | −0.5 (–0.8; −0.1) | 0.008 | 0.70 | 1.86 | 0.173 | −0.5 (–0.8; −0.2) | 0.004 | 0.2 (–0.1; 0.4) | 0.174 |
| IADL | 0.73 | 370.19 | <0.001 | −0.1 (–0.7; 0.5) | 0.727 | 0.73 | 0.00 | 0.986 | −0.1 (–0.7; 0.5) | 0.731 | 0 (–0.4; 0.4) | 0.986 |
| GP contact-time (min)c | 0.56 | 112.64 | <0.001 | 1 (–18; 20) | 0.940 | 0.56 | 0.04 | 0.834 | 0 (–19; 20) | 0.974 | 2 (–14; 17) | 0.834 |
| Hospitalization | 0.08 | 30.54 | <0.001 | 0.9 (0.7; 1.2) | 0.521 | 0.08 | 0.02 | 0.886 | 0.9 (0.7; 1.2) | 0.901 | 1.0 (0.8; 1.3) | 0.886 |
aBoth models are adjusted for sex, age and baseline
bFor the outcome “Hospitalization”, the Nagelkerke R 2 was used to estimate R 2
cGP contact information was available for 359 participants
Characteristics and health outcomes among the different handgrip and quadriceps strength categories
Participants were grouped according to their handgrip and quadriceps strength status (weak or normal). Table 5 shows the characteristics and health outcomes of the four handgrip and quadriceps strength categories. Participants with weak handgrip and normal quadriceps strength (“weak handgrip strength only”) were older (P < 0.001) and had a lower body weight (P < 0.001) in comparison to participants with normal handgrip strength and normal quadriceps strength (“normal handgrip and quadriceps strength”). The “weak handgrip strength only” group scored more poorly on GARS (including IADL) and gait speed at baseline (all P < 0.001). At follow-up, the “weak handgrip strength only” group scored lower on EQ5D+c and BADL (all P ≤ 0.044).
Table 5.
Health outcomes among the different handgrip and quadriceps strength categories
| Normal handgrip and normal quadriceps strength (n = 267) | Weak handgrip and normal quadriceps strength (n = 134) | Normal handgrip and weak quadriceps strength (n = 165) | Weak handgrip and weak quadriceps strength (n = 198) | ||||
|---|---|---|---|---|---|---|---|
| Median [IQR] | Median [IQR] | P valuea | Median [IQR] | P valuea | Median [IQR] | P valueb | |
| Baseline characteristics | |||||||
| Femalec | 184 (68.9) | 90 (67.2) | 0.723 | 117 (70.9) | 0.662 | 130 (65.7) | 0.776 |
| Age (year) | 81 [78; 85] | 84 [80; 88] | <0.001 | 82 [79; 86] | 0.007 | 85 [80; 89] | 0.165 |
| N chronic diseasesd | 3 [2; 5] | 4 [3; 5] | 0.346 | 4 [2; 6] | 0.193 | 5 [3; 6] | <0.001 |
| Weight (kg) | 76 [67; 84] | 71 [61; 80] | <0.001 | 71 [62; 81] | 0.002 | 69 [60; 80] | 0.826 |
| Health outcomes at baselinee | |||||||
| EQ5D+c | 0.81 [0.72; 0.89] | 0.81 [0.69; 0.86] | 0.948 | 0.81 [0.68; 0.84] | <0.001 | 0.68 [0.30; 0.81] | <0.001 |
| GARS | 25 [21; 31] | 31 [25; 38] | <0.001 | 29 [24; 36] | <0.001 | 40 [32; 49] | <0.001 |
| BADL | 9 [9; 10] | 9 [9; 11] | 0.141 | 9 [9; 11] | 0.001 | 11 [9; 17] | <0.001 |
| IADL | 16 [12; 21] | 21 [16; 27] | <0.001 | 20 [15; 25] | <0.001 | 28 [22; 33] | <0.001 |
| GP contact-time (min)f | 100 [40; 170] | 100 [43; 198] | 0.753 | 90 [40; 170] | 0.693 | 185 [103;368] | 0.005 |
| Hospitalizationc | 40 (15.0) | 28 (21.1) | 0.152 | 27 (16.4) | 0.667 | 62 (31.6) | 0.034 |
| Gait speed (m/s) | 0.67 [0.50; 0.84] | 0.52 [0.36; 0.66] | <0.001 | 0.50 [0.31; 0.65] | <0.001 | 0.30 [0.05; 0.46] | <0.001 |
| Health outcomes at follow-upg | |||||||
| Mortalityc | 8 (3.0) | 7 (5.2) | 0.812 | 6 (3.6) | 0.708 | 10 (5.1) | 0.825 |
| EQ5D+c | 0.81 [0.69; 0.89] | 0.73 [0.56; 0.84] | 0.044 | 0.81 [0.65; 0.84] | 0.954 | 0.65 [0.26; 0.81] | 0.479 |
| GARS | 28 [22; 35] | 35 [29; 43] | 0.156 | 33 [25; 41] | 0.995 | 43 [34; 51] | 0.379 |
| BADL | 9 [9; 11] | 11 [9; 14] | 0.017 | 10 [9; 12] | 0.785 | 13 [10; 19] | 0.819 |
| IADL | 18 [13; 25] | 24 [19; 29] | 0.294 | 23 [16; 29] | 0.870 | 30 [24; 35] | 0.419 |
| Hospitalizationc | 41 (19.6) | 23 (23.5) | 0.495 | 26 (20.3) | 0.778 | 30 (21.7) | 0.567 |
| GP contact-time (min)f | 100 [50; 170] | 105 [53; 245] | 0.706 | 120 [60; 240] | 0.384 | 210 [73; 398] | 0.697 |
aNumbers were compared to the normal handgrip and normal quadriceps strength group using general linear model (GLM) univariate analysis
bNumbers were compared to the weak handgrip and normal quadriceps strength group using GLM univariate analysis
cNumber (%)
dIncluding self-reported diabetes, heart failure, cancer, chronic obstructive pulmonary disease, incontinence, arthritis, osteoporosis, dizziness, lower urinary tract symptoms, depression, anxiety, dementia, impaired vision, deafness, fracture, stroke/transient ischemic attack, myocardial infarction
eGLM univariate analyses were corrected for age and sex
fGP contact information was available for 359 participants; the numbers of participants analyzed in the respective groups are 134, 67, 79, and 79
gGLM univariate analyses were corrected for age, sex and health outcome at baseline. Follow-up information was available for 570 participants: the numbers of participants analyzed in the respective groups are 208, 98, 127, and 137
Participants with weak quadriceps and normal handgrip strength (“weak quadriceps strength only”) were older (P = 0.007) and had a lower body weight (P = 0.002) in comparison to the “normal handgrip and quadriceps strength” group. With regard to the health outcomes, the “weak quadriceps strength only” group scored more poorly on EQ5D+c, GARS (including both BADL and IADL) and gait speed at baseline (all P ≤ 0.001) when compared to the “normal handgrip and quadriceps strength” group. Health outcomes measured at follow-up, however, did not differ significantly.
Participants scoring weak on both the handgrip and quadriceps strength (“weak handgrip and quadriceps strength”) scored more poorly on all measured health parameters at baseline when compared to the “normal handgrip and quadriceps strength”, “weak handgrip strength only” or the “weak quadriceps strength only” groups (all P ≤ 0.034). The baseline characteristics of the “weak handgrip and quadriceps strength” group differed most with the “normal handgrip and quadriceps strength” group; participants were older, had more chronic diseases and a lower body weight (all P < 0.001). When compared to the “weak handgrip strength only” group, the “weak handgrip and quadriceps strength” participants only differed in the number of chronic diseases (5 versus 4, P < 0.001). In comparison to the “weak quadriceps strength only” group, the “weak handgrip and quadriceps strength” participants had more chronic diseases and were older (all P < 0.001).
At follow-up, the “weak handgrip and quadriceps strength” group only scored lower on EQ5D+c when compared to the “normal handgrip and quadriceps strength” and the “weak quadriceps strength only” groups (all P ≤ 0.006). The health outcomes at follow-up did not differ between the “weak handgrip and quadriceps strength” and the “weak handgrip strength only” groups.
Following the frailty criteria by Fried et al. (2001), 74.2 % of the “weak handgrip and quadriceps strength” group were considered frail compared to 38.8 % in the “weak handgrip strength only” group. In the “normal handgrip and quadriceps strength” and “weak quadriceps strength only” groups respectively 8.2 % and 9.1 % were considered frail.
Discussion
We compared the lower limb quadriceps strength with the handgrip strength in their association with health outcomes in older adults in primary care. We showed that in an older population the association between quadriceps strength and handgrip strength is weak, and that quadriceps strength and handgrip strength are both independently associated with several health outcomes at baseline. Further, combined weakness of the quadriceps and handgrip identified a subpopulation of older adults that presents with the poorest health scores at baseline, as demonstrated by increased morbidity, lower quality of life, increased disability in daily living, increased GP contact, increased hospitalization and slower gait speed. This group of vulnerable older adults scored significantly poorer on all measured health parameters compared to those characterized by handgrip weakness only.
Prospectively, quadriceps strength showed no association with the measured health outcomes, whereas handgrip strength was associated with quality of life and BADL only. Quadriceps weakness did not contribute to the prediction of lower health scores, and handgrip weakness was sufficient to distinguish older adults with lower health, who presented with a lower quality of life and decreased BADL.
In clinical practice, handgrip strength measurements are often preferred over lower limb muscle measurements in determining sarcopenia (Cruz-Jentoft et al. 2010). This has several reasons: first, measuring the handgrip strength is an easy, accessible, and inexpensive method to use. In addition, handgrip strength has been reported to correlate well with a number of health parameters, as well as leg strength, which suggests an interchangeability of upper and lower limb muscle measurements (Cruz-Jentoft et al. 2010; Lauretani et al. 2003). However, our data in a population of older adults did not show a strong correlation between handgrip and quadriceps strength. This discrepancy may be explained by the study population used, as our population consisted of older adults aged 75 and above, whereas the previous study demonstrating a strong correlation between upper and lower limb muscle strength consisted of a study population with an age range from 20 to 102 (Lauretani et al. 2003). Older people progressively show more physical impairments, which may distort the correlation between upper and lower limb muscle strength.
In addition, we observed that both quadriceps strength and handgrip strength associated independently with several health outcomes at baseline. These health outcomes included disability in daily living and gait speed (mobility-related items) and quality of life. Surprisingly, the effect estimates of the quadriceps and handgrip strength were very comparable for disability in daily living, GP contact-time, hospitalization and gait speed, whereas quality of life showed a stronger association with quadriceps strength. Our prospective analysis showed an overall limited association with health outcomes. Handgrip strength was only associated with quality of life and BADL, and quadriceps strength was associated with none of the measured health outcomes.
When grouping the participants according to handgrip and quadriceps weakness, we observed that weakness in the handgrip strength contributed to poorer health scores at baseline and follow-up, whereas quadriceps weakness contributed only to poorer health scores at baseline. The lack of a strong correlation between handgrip and quadriceps strength, together with their independent associations with distinct health outcomes suggest that the two muscle parameters reflect different properties at old age. By measuring either handgrip strength or quadriceps strength vulnerable older adults can be identified, however, it is the combined use of both muscle parameters that distinguishes the most vulnerable subpopulation.
Strengths and limitations
Our study population consists of older adults who participated in the ISCOPE study. As this is a large population-based study with few exclusion criteria, it augments the external validity of our results, which is a strength of this study. In addition, we analyzed the association of the quadriceps and handgrip strength with respect to one another to distinguish their individual effects on health outcomes. Although previous studies have reported the association of quadriceps and handgrip strength with several negative health outcomes, none of these studies analyzed the association independent of the other muscle parameter (Cawthon et al. 2009; Hairi et al. 2010; Laukkanen et al. 1995; Newman et al. 2006; Rantanen et al. 1994). A limitation of our study is the short follow-up period and the relatively small sample size, which may explain the lack of association between baseline quadriceps strength and health outcomes at follow-up.
Persons with complex problems were oversampled in our study, which could be considered a limitation as it may have resulted in the overestimation of negative health outcomes. However, handgrip and/or quadriceps weakness are likely to be similarly overestimated due to the oversampling. Oversampling may have affected the association between handgrip and quadriceps strength, as health problems do not always equally affect handgrip strength and quadriceps strength. We addressed this matter by analyzing the effect of the individual muscle parameters independently of each other.
Implications
Our results confirm that handgrip weakness is a good parameter for the association and prediction of poor health status in the elderly as reported previously (Femia et al. 1997; Giampaoli et al. 1999; Hairi et al. 2010; Rantanen et al. 1994). In addition, our study shows that handgrip weakness is not automatically linked to quadriceps weakness suggesting that measuring quadriceps strength next to handgrip strength may have added value in assessing sarcopenia more accurately. Measuring quadriceps strength aids in the assessment of poorer health status in older adults in primary care, as it helps to identify those older adults at increased risk of negative health outcomes. Although it has been suggested that lower limb muscle strength associates more strongly with health outcomes related to mobility (Cruz-Jentoft et al. 2010), we did not observe a difference in the association of mobility-related health outcomes (e.g., gait speed and GARS) with handgrip strength or quadriceps strength. This suggests that other determinants such as balance may play a more significant role in mobility than previously hypothesized (Cruz-Jentoft et al. 2010).
Conclusions
Our study shows that in an older population quadriceps strength and handgrip strength are only moderately associated, and that quadriceps strength and handgrip strength associate independently with several health outcomes. Although quadriceps strength measurements do not contribute to the prediction of adverse health outcomes, the combination of quadriceps strength measurements with handgrip strength measurements, may aid in the identification of older adults in primary care with the poorest health. These older people present with the poorest scores on quality of life, disability in daily living, GP contact-time, hospitalization, and gait speed.
Acknowledgments
Author contributions
Guarantor: WPJ den Elzen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design
J Gussekloo, JW Blom, WPJ den Elzen.
Acquisition of data
OYA Chan, AH van Houwelingen, J Gussekloo, JW Blom, WPJ den Elzen.
Analysis and interpretation of data
OYA Chan, AH van Houwelingen, J Gussekloo, JW Blom, WPJ den Elzen.
Drafting of the manuscript
OYA Chan, WPJ den Elzen.
Critical revision of the manuscript for important intellectual content
OYA Chan, AH van Houwelingen, J Gussekloo, JW Blom, WPJ den Elzen.
Obtained funding
JW Blom and J Gussekloo.
Competing interests
None declared.
Funding
This study was funded by a grant from the Netherlands Organization for Health Research and Development (ZonMw; grant number 60-61900-98-126).
Role of the Sponsor
The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Ethical approval
The Medical Ethical Committee of Leiden University Medical Center approved the study in 2009.
Trial registration
Netherlands Trial Register: NTR1946.
References
- Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther. 1996;76:248–259. doi: 10.1093/ptj/76.3.248. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Haan J, Lagaay AM, et al. Investigation of gait in elderly subjects over 88 years of age. J Geriatr Psychiatry Neurol. 1992;5:78–84. doi: 10.1177/002383099200500204. [DOI] [PubMed] [Google Scholar]
- Bootsma-van der Wiel A, Gussekloo J, de Craen AJ, et al. Disability in the oldest old: "can do" or "do do"? J Am Geriatr Soc. 2001;49:909–914. doi: 10.1046/j.1532-5415.2001.49181.x. [DOI] [PubMed] [Google Scholar]
- Cantril H. (1966) The Pattern of Human Concerns. Rutgers University Press.
- Cawthon PM, Fox KM, Gandra SR, et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411–1419. doi: 10.1111/j.1532-5415.2009.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma RK, Soer R, Krijnen WP, et al. Reference values for isometric muscle force among workers for the Netherlands: a comparison of reference values. BMC Sports Sci Med Rehabil. 2014;6:10. doi: 10.1186/2052-1847-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EuroQol Group EuroQol–a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- Femia EE, Zarit SH, Johansson B. Predicting change in activities of daily living: a longitudinal study of the oldest old in Sweden. J Gerontol B Psychol Sci Soc Sci. 1997;52:294–302. doi: 10.1093/geronb/52B.6.P294. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Giampaoli S, Ferrucci L, Cecchi F, et al. Hand-grip strength predicts incident disability in non-disabled older men. Age Ageing. 1999;28:283–288. doi: 10.1093/ageing/28.3.283. [DOI] [PubMed] [Google Scholar]
- Hairi NN, Cumming RG, Naganathan V, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58:2055–2062. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- Hanten WP, Chen WY, Austin AA, et al. Maximum grip strength in normal subjects from 20 to 64 years of age. J Hand Ther. 1999;12:193–200. doi: 10.1016/S0894-1130(99)80046-5. [DOI] [PubMed] [Google Scholar]
- Kempen GI, Miedema I, Ormel J, et al. The assessment of disability with the Groningen Activity Restriction Scale. Conceptual framework and psychometric properties. Soc Sci Med. 1996;43:1601–1610. doi: 10.1016/S0277-9536(96)00057-3. [DOI] [PubMed] [Google Scholar]
- Krabbe PF, Stouthard ME, Essink-Bot ML, et al. The effect of adding a cognitive dimension to the EuroQol multiattribute health-status classification system. J Clin Epidemiol. 1999;52:293–301. doi: 10.1016/S0895-4356(98)00163-2. [DOI] [PubMed] [Google Scholar]
- Kwoh CK, Petrick MA, Munin MC. Inter-rater reliability for function and strength measurements in the acute care hospital after elective hip and knee arthroplasty. Arthritis Care Res. 1997;10:128–134. doi: 10.1002/art.1790100208. [DOI] [PubMed] [Google Scholar]
- Laukkanen P, Heikkinen E, Kauppinen M. Muscle strength and mobility as predictors of survival in 75–84-year-old people. Age Ageing. 1995;24:468–473. doi: 10.1093/ageing/24.6.468. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- Ling CH, Taekema D, de Craen AJ, et al. Handgrip strength and mortality in the oldest old population: the Leiden 85-plus study. CMAJ. 2010;182:429–435. doi: 10.1503/cmaj.091278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Baumgartner RN, Roubenoff R, et al. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- Poot AJ, den Elzen WP, Blom JW, et al. Level of satisfaction of older persons with their general practitioner and practice: role of complexity of health problems. PLoS One. 2014;9:e94326. doi: 10.1371/journal.pone.0094326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen T, Era P, Heikkinen E. Maximal isometric strength and mobility among 75-year-old men and women. Age Ageing. 1994;23:132–137. doi: 10.1093/ageing/23.2.132. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Masaki K, Foley D, et al. Grip strength changes over 27 yr in Japanese-American men. J Appl Physiol. 1998;85:2047–2053. doi: 10.1152/jappl.1998.85.6.2047. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Ferrucci L, et al. Coimpairments as predictors of severe walking disability in older women. J Am Geriatr Soc. 2001;49:21–27. doi: 10.1046/j.1532-5415.2001.49005.x. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Volpato S, Ferrucci L, et al. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- Rolland Y, Czerwinski S, Van Abellan KG, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaubert KL, Bohannon RW. Reliability and validity of three strength measures obtained from community-dwelling elderly persons. J Strength Cond Res. 2005;19:717–720. doi: 10.1519/R-15954.1. [DOI] [PubMed] [Google Scholar]
- Stark T, Walker B, Phillips JK, et al. Hand-held dynamometry correlation with the gold standard isokinetic dynamometry: a systematic review. PM R. 2011;3:472–479. doi: 10.1016/j.pmrj.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Taekema DG, Gussekloo J, Maier AB, et al. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing. 2010;39:331–337. doi: 10.1093/ageing/afq022. [DOI] [PubMed] [Google Scholar]
- van Houwelingen AH, den Elzen WP, Mooijaart SP, et al. Predictive value of a profile of routine blood measurements on mortality in older persons in the general population: the Leiden 85-plus Study. PLoS One. 2013;8:e58050. doi: 10.1371/journal.pone.0058050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]