Abstract
The resistance status of Rhipicephalus (Boophilus) microplus collected from Noorpur Bet village of Ludhiana district, Punjab was evaluated against malathion by Adult Immersion Test. The adult female ticks showed an upward trend in the mortality percentage with increase in drug concentration. The regression graph of probit mortality of ticks plotted against log values of progressively increasing concentrations of malathion was utilized for the determination of slope of mortality (95 % confidence intervals) which was 2.469 ± 0.5744 (0.6413–4.297) whereas, the value of goodness of fit (R2) was 0.8603. The LC50 (95 % CI) and LC95 (95 % CI) were recorded as 1875.05 (1725.14–2,038) and 8,654 (7296.8–10263.8) ppm, respectively and the resistance factor was 3.46 (Level I). The slope of egg mass (95 % CI) was −0.1500 ± 0.04071 (−0.2795 to −0.02045) and was negative because with the increasing concentrations of acaricide the ticks died. The reproductive index when plotted against increasing log concentrations of malathion revealed a slope value of −0.414 ± 0.055. Further, a significant variation (p = 0.0049) was recorded in the inhibition of oviposition among the various groups treated with increasing concentrations of malathion.
Keywords: Malathion, Punjab, Resistance, Rhipicephalus (Boophilus) microplus
Introduction
The cattle tick Rhipicephalus (Boophilus) microplus is one of the most important ectoparasites infesting dairy animals of tropics and subtropics particularly Punjab state, India (Haque et al. 2011; Singh et al. 2012a). According to Minjauw and McLeod (2003) the cost of management of ticks and tick borne diseases in livestock of India is as high as US$ 498.7 million per annum. This parasite causes serious direct and indirect damages to cattle industry. Direct damage includes losses in milk production and body weight along with increased mortality, as well as damage to the leather industry due to hide punctures by this tick. Indirect damages include its role as vector transmitting the causative agents of various economically important haemoprotozoan diseases in dairy animals like babesiosis and anaplasmosis (Jongejan and Uilenberg 1994).
Chemical acaricides particularly synthetic pyrethroids (SP) and organophosphates (OP) are currently the most used method of tick control but the indiscriminate and incessant use with improper concentrations for an extended period of time has probably contributed to the development of resistance to these acaricides (FAO 2004). There are several reports available on acaricidal resistance in R. (B.) microplus from various parts of the country (Chaudhary and Naithani 1964; Basu and Haldar 1997; Vatsya and Yadav 2011; Singh et al. 2013) including Punjab state (Singh et al. 2010, 2012b). Recently, large scale resistance to OP compound diazinon (Kumar et al. 2011) and SP particularly cypermethrin and deltamethrin (Sharma et al. 2012) has been experimentally validated in Indian isolates of R. (B.) microplus. However, as reports of malathion resistance in R. (B.) microplus are scanty particularly from Punjab state (Rath et al. 2006), the current study was undertaken to detect malathion resistance in R. (B.) microplus collected from Ludhiana district, Punjab.
Materials and methods
Study area
Live engorged R. (B.) microplus adult female ticks were collected from sheds of dairy animals comprising cross bred cattle as well as buffaloes from Noorpur Bet village of Ludhiana district, Punjab in October, 2012. Data related to frequency, type and mode of acaricide treatment adopted by the owners and their experiences about the commonly used acaricides efficacy was recorded. The ticks were collected in vials, closed with muslin cloth to allow air and moisture exchange, brought to the Entomology Laboratory, Department of Veterinary Parasitology, GADVASU, Ludhiana, cleaned, labelled and kept at 28 ± 1 °C and 85 ± 5 % relative humidity.
Acaricide
Technical grade malathion (AccuStandard® Inc., USA) was used to prepare the stock solution in methanol. For the experimental bioassay, different concentrations of the malathion were prepared in distilled water from the stock solution and tested against R. (B.) microplus.
Adult immersion test (AIT)
AIT was conducted according to the method of Sharma et al. (2012) with minor modifications. Briefly, the pre weighed engorged females of R. (B.) microplus were immersed in different concentrations of malathion (1,250, 2,500, 5,000, 1,0000 and 20,000 ppm) for 2 min and then dried on filter paper before transferring into the petri dishes. After 24 h, ticks were transferred to the glass tubes covered with muslin cloth and were kept in desiccators kept in BOD incubator maintained at 28 ± 1 °C and 85 ± 5 % RH. The ticks which did not oviposit even after 14 days post treatment were considered as dead. The control group was treated in similar manner in distilled water and the following parameters were compared:
Mortality: recorded up to 14 days post treatment
The egg masses laid by the live ticks.
Reproductive index (RI) = egg mass wt. (EW)/engorged female wt. (EFW).
Percentage inhibition of oviposition (IO %) = ((RI control−RI treated)/RI control × 100).
Dose response data were analyzed by probit method (Finney 1962) using Graph Pad Prism 4 software. The LC50 and LC95 value of malathion was determined by applying regression equation analysis to the probit transformed data of mortality.
Resistance diagnosis in field isolates
Resistance factors (RF) were worked out by the quotient between LC95 of field isolates and LC95 of susceptible isolate (half the recommended dose of malathion as discriminating dose is twice the LC95). On the basis of RF, the resistance status was classified as susceptible (RF < 1.4), level I resistant (RF = 1.5–5.0), level II resistant (RF = 5.1–25.0), level III resistant (RF = 25.1–40) and level IV resistance (RF > 40.1) as per Sharma et al. (2012).
Results and discussion
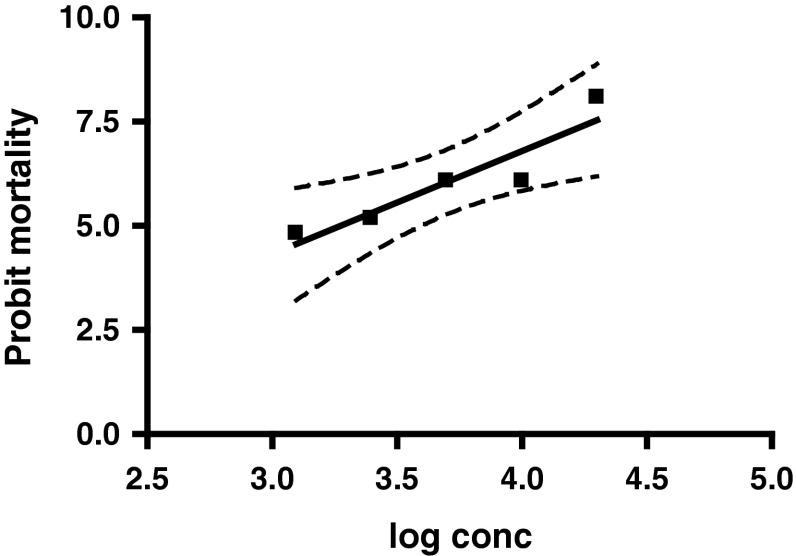
An increase in the mortality of ticks was recorded along with the increase in concentrations of malathion and 100 % mortality was recorded at 20,000 ppm. It was observed that exposure of ticks to the concentration at which commercial malathion is used (5,000 ppm) could not achieve 100 % mortality and even much higher concentration of 10,000 ppm failed to produce cent percent mortality, thus indicating development of resistance against malathion in these ticks. The regression graph of probit mortality of ticks was plotted against log values of progressively increasing concentrations of malathion (Fig. 1). The dotted lines in the regression curve represented the 95 % confidence limits. The slope of mortality (95 % confidence intervals) was 2.469 ± 0.5744 (0.6413–4.297) whereas, the value of goodness of fit (R2) was recorded as 0.8603. From the regression equation the various lethal concentration values of malathion were calculated. The LC50 (95 % CI) and LC95 (95 % CI) were recorded as 1875.05 (1725.14–2,038) and 8,654 (7296.8–10263.8) ppm, respectively and the resistance factor was 3.46 which indicated level I resistance status.
Fig. 1.
Dose mortality curve of R. (B.) microplus against malathion
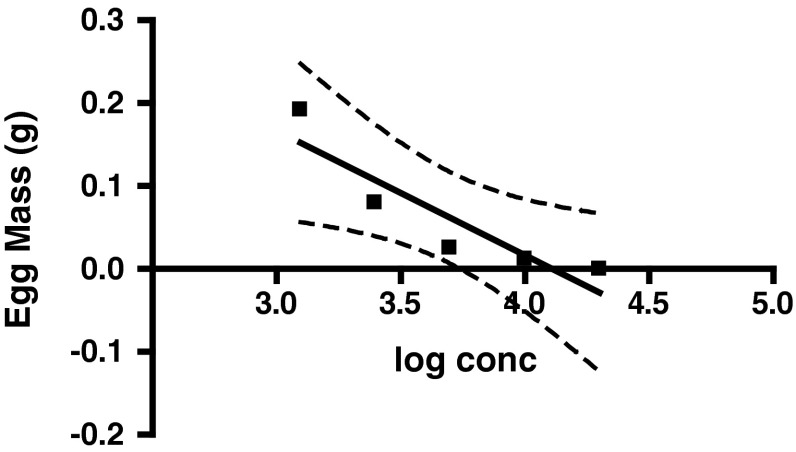
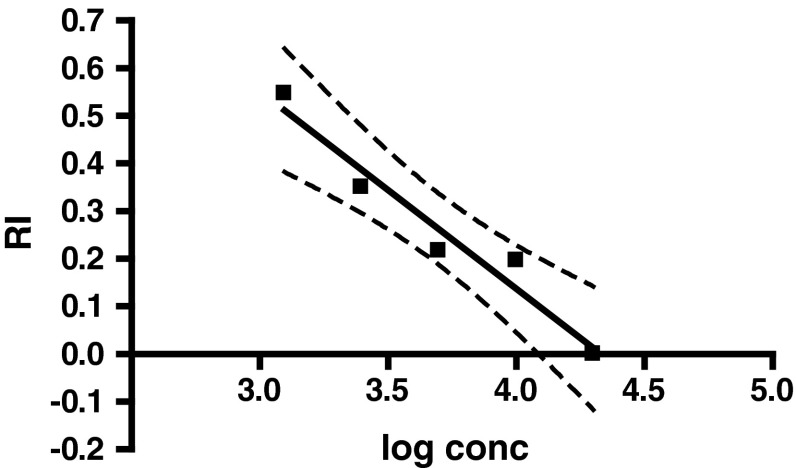
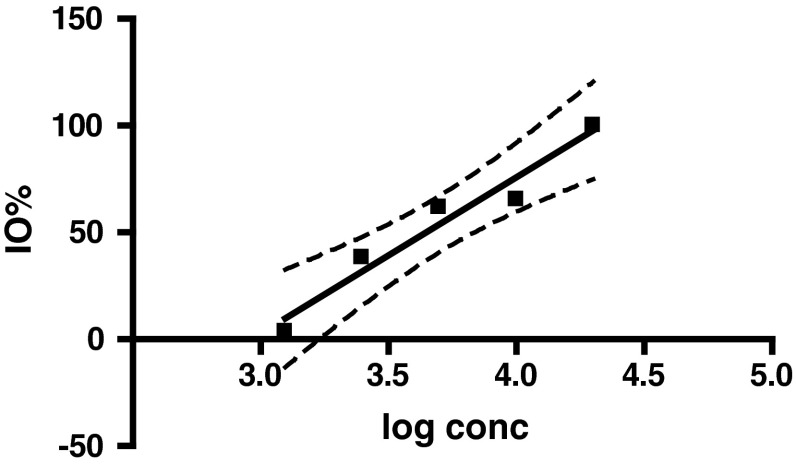
The dose response curves for egg masses, reproductive index and IO % of R. (B.) microplus were also validated by AIT. The egg mass of ticks treated with different concentrations of malathion decreased with increasing concentrations of drug and the variation was statistically significant (p = 0.0347) as shown in Fig. 2. The slope of egg mass (95 % CI) was −0.1500 ± 0.04071 (−0.2795 to −0.02045) and was negative because with the increasing concentrations of acaricide the ticks died. The reproductive index of ticks treated with different concentrations of malathion decreased with increasing concentrations of drug and the slope (95 % CI) was −0.4142 ± 0.05515 (−0.5896 to −0.2387). This indicated that although the increase in concentration of the acaricide may have not caused mortality in ticks but the survived ticks showed significant decrease (p = 0.0049) in their efficiency to convert the live weight into egg mass (Fig. 3). Further, there was a significant increase (p = 0.0049) in the IO % in ticks with increase in drug concentration and thus a positive slope (95 % CI) of 73.05 ± 9.725 (42.10–104.0) was recorded (Fig. 4).
Fig. 2.
Pattern of egg masses laid by R. (B.) microplus against malathion
Fig. 3.
Pattern of reproductive index of R. (B.) microplus against malathion
Fig. 4.
Pattern of inhibition of oviposition (% IO) of R. (B.) microplus against malathion
For conducting the bioassay, technical grade malathion was selected over commercial formulation as commercial products are prepared with many proprietary ingredients and it is difficult to assess the responses due to active ingredients (Shaw 1966). The stock solutions were prepared by dissolving in 100 % methanol and the working concentrations were prepared with distilled water. Use of organic solvent (methanol) facilitates adsorption of compound over the surface area of target biological materials and also enhances penetration of active ingredients of the acaricide across the exoskeleton (Sharma et al. 2012).
The AIT uses engorged females which are immersed in acaricides and are based on rate of oviposition between females of two groups, treated and control. The eggs are analyzed by weight and viability. Additionally, direct mortality can be taken into consideration by comparing females that oviposit or not (Benavides et al. 1999). In AIT, the results are obtained in 1–2 weeks in contrast with Larval Packet Test which requires 5–6 weeks for completion and hence requires less time (Castro-Janer et al. 2009). AIT has been utilized by various workers for estimation of efficacy of various acaricides against R. (B.) microplus (Jonsson et al. 2007; Kumar et al. 2011; Sharma et al. 2012; Singh et al. 2012b). Among the various acaricides used in India for the control of ticks in livestock, resistance was first reported against γBHC in R. (B.) microplus (Chaudhary and Naithani 1964; Khan and Srivastava 1977) followed by dieldrin (Sangwan et al. 1993), sevin (Basu and Haldar 1997), lindane (Kumar 1999), diazinon (Kumar et al. 2011), SP (Singh et al. 2010, 2012b Sharma et al. 2012) and amitraz (Singh et al. 2013). Reports of resistance against OP compounds are available against R. (B.) microplus ticks from various parts of world (Miller et al. 2005; Mendes et al. 2007; Baffi et al. 2008) but are scanty from India particularly malathion resistance (Rath et al. 2006).
The current study reports the detection of resistance status of R. (B.) microplus against malathion and the mechanism involved in the development of this resistance is further being investigated. Several mechanisms have been evolved through which ticks acquire resistance to OP acaricides particularly malathion. OP compounds inhibit the enzyme acetyl cholinesterase (AChE) which plays a key role in nerve impulse transmission by hydrolyzing the neurotransmitter acetylcholine. Further, elevated oxidative detoxification mechanism of cytochrome P450 monooxygenase (Li et al. 2004) and increased GST activity which may also be involved in a metabolic detoxification mechanism of OP compounds (Li et al. 2003) and lead to resistance. The alteration of AChE to an insensitive form in the cattle tick, R (B.) microplus has been demonstrated as an important mechanism for resistance (Baxter and Barker 1998).
The results revealed development of resistance in R. (B.) microplus to malathion and addresses an emerging problem of resistance hence, recommendations aiming for effective tick control should be accordingly formulated and implemented. This will lead to judicious use of the drug and would further cause decreased environmental pollution and reduction in the residual effect of acaricides in the animal products like milk and meat.
Acknowledgments
The authors are grateful to Department of Science and Technology, New Delhi for funding through Women Scientist Scheme (WOS-A) Project no. SR/WOS-A/LS-493/2011 to senior author. Sincere thanks are also due to the Director of Research, GADVASU, Ludhiana for providing facilities to carry out the research study.
References
- Baffi MA, de Souza GRL, de Souza CS, Ceron CR, Bonetti AM (2008) Esterase enzymes involved in pyrethroid and organophosphate resistance in a Brazilian population of Rhipicephalus (Boophilus) microplus (Acari, Ixodidae). Mol Biochem Parasitol 160:70–73 [DOI] [PubMed]
- Basu A, Haldar DP. A note on the effect of continuous use of Sevin 50 WP on some cattle ticks. J Vet Parasitol. 1997;11:183–184. [Google Scholar]
- Baxter GD, Barker SC. Acetylcholinesterase cDNA of the cattle tick, Boophilus microplus: characterization and role in organophosphate resistance. Insect Biochem Mol Biol. 1998;28:581–589. doi: 10.1016/S0965-1748(98)00034-4. [DOI] [PubMed] [Google Scholar]
- Benavides OE, Romero NA, Rodriguez JL, Silva ZJ (1999) Evidencia preliminar de la aparicion de resistenciaa lactonas macrociclicas en cepas de garrapata Boophilus microplus en Colombia. In: Memorias IV Seminarios Internacional de Parasitologia, Puerto Vallarta, Jalisco, Mexico, pp 264–266
- Castro-Janer E, Rifran L, Piaggio J, Gil A, Miller RJ, Schumaker TTS. In vitro tests to establish LC50 and discriminating concentrations for fipronil against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) and their characterization. Vet Parasitol. 2009;162:120–128. doi: 10.1016/j.vetpar.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Chaudhary RP, Naithani RC. Resistance to BHC in the cattle tick Boophilus microplus in India. Bull Ent Res. 1964;55:405–410. doi: 10.1017/S0007485300049555. [DOI] [Google Scholar]
- FAO . Resistance management and integrated parasite control in ruminants guidelines. Rome: Animal Production and Health Division, FAO; 2004. pp. 25–77. [Google Scholar]
- Finney DJ. Probit analysis−a statistical treatment of the response curve. Cambridge: Cambridge University Press; 1962. pp. 1–318. [Google Scholar]
- Haque M, Jyoti, Singh NK, Rath SS, Ghosh S (2011) Epidemiology and seasonal dynamics of ixodid ticks of dairy animals of Punjab state, India. Indian J Anim Sci 81:661–664
- Jongejan F, Uilenberg G. Ticks and control methods. Rev Sci Tech Off Int Epiz. 1994;13:1201–1226. doi: 10.20506/rst.13.4.818. [DOI] [PubMed] [Google Scholar]
- Jonsson NN, Miller RJ, Robertson JL. Critical evaluation of the modified-adult immersion test with discriminating dose bioassay for Boophilus microplus using American and Australian isolates. Vet Parasitol. 2007;146:307–315. doi: 10.1016/j.vetpar.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Khan MH, Srivastava SC. In vitro tests with some ixodicides against cattle tick B. microplus. Indian J Anim Health. 1977;16:137–140. [Google Scholar]
- Kumar S (1999) Studies on acaricides resistance in common ixodid ticks of Punjab. M.V.Sc thesis, PAU, Ludhiana
- Kumar S, Paul S, Sharma AK, Kumar R, Tewari SS, Chaudhuri P, Ray DD, Rawat AKS, Ghosh S. Diazinon resistant status in Rhipicephalus (Boophilus) microplus collected from different agroclimatic zones of India. Vet Parasitol. 2011;181:274–281. doi: 10.1016/j.vetpar.2011.04.030. [DOI] [PubMed] [Google Scholar]
- Li AY, Davey RB, Miller RJ, George JE. Resistance to coumaphos and diazinon in Boophilus microplus (Acari: Ixodidae) and evidence for the involvement of an oxidative detoxification mechanism. J Med Entomol. 2003;40:482–490. doi: 10.1603/0022-2585-40.4.482. [DOI] [PubMed] [Google Scholar]
- Li AY, Davey RB, Miller RJ, George JE. Detection and characterization of amitraz resistance in the southern cattle tick, Boophilus microplus (Acari: Ixodidae) J Med Entomol. 2004;41:193–200. doi: 10.1603/0022-2585-41.2.193. [DOI] [PubMed] [Google Scholar]
- Mendes MC, Pereira JR, Prado AP. Sensitivity of Boophilus microplus (Acari: Ixodidae) to pyrethroids and organophosphate in farms in the vale do paraiba region, Sao Paulo, Brazil. Arq Inst Biol. 2007;74:81–85. [Google Scholar]
- Miller RJ, Davey RB, George JE. First report of organophosphate-resistant Boophilus microplus (Acari: Ixodidae) within the United States. J Med Entomol. 2005;42:912–917. doi: 10.1603/0022-2585(2005)042[0912:FROOBM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Minjauw B, McLeod A (2003) Tick-borne diseases and poverty. The impact of ticks and tick borne diseases on the livelihood of small scale and marginal livestock owners in India and eastern and southern Africa. Research report, DFID Animal Health Programme, Centre for Tropical Veterinary Medicine, University of Edinburgh, U K, pp 59–60
- Rath SS, Kumar S, Joia BS. Resistance to diazinon and malathion in Boophilus microplus (Acari: Ixodidae) populations from Punjab, India. J Insect Sci. 2006;19:74–81. [Google Scholar]
- Sangwan AK, Chhabra MB, Singh S. Acaricide resistance status of common livestock ticks in Haryana. Indian Vet J. 1993;70:20–24. [Google Scholar]
- Sharma AK, Kumar R, Kumar S, Nagar G, Singh NK, Rawat SS, Dhakad ML, Rawat AKS, Ray DD, Ghosh S. Deltamethrin and cypermethrin resistance status of Rhipicephalus (Boophilus) microplus collected from six agro-climatic regions of India. Vet Parasitol. 2012;188:337–345. doi: 10.1016/j.vetpar.2012.03.050. [DOI] [PubMed] [Google Scholar]
- Shaw RD. Culture of an organophosphorus resistant strain of Boophilus microplus (Canestrini) and assessment of its resistance spectrum. Bull Entomol Res. 1966;56:398–405. doi: 10.1017/S0007485300056480. [DOI] [PubMed] [Google Scholar]
- Singh NK, Jyoti Haque M, Rath SS. Studies on acaricide resistance in Rhipicephalus (Boophilus) microplus against synthetic pyrethroids by adult immersion test with a discriminating dose. J Vet Parasitol. 2010;24:207–208. [Google Scholar]
- Singh NK, Jyoti, Haque M, Singh H, Rath SS (2012a) Prevalence of ixodid ticks in dairy animals of different farm types in Punjab. In: Sandhu, SK et al (eds) Proc. International Conference on Sustainable Agriculture for Food and Livelihood Security, Crop Improvement, Ludhiana, pp 1491–1492
- Singh NK, Haque M, Rath SS, Jyoti Deltamethrin resistance in Rhipicephalus microplus in Ludhiana. Indian Vet J. 2012;89:23–25. [Google Scholar]
- Singh NK, Gelot IS, Singh V, Jyoti, Rath SS. Detection of amitraz resistance in Rhipicephalus (Boophilus) microplus from North Gujarat, India. J Parasites Dis. 2013 doi: 10.1007/s12639-013-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsya S, Yadav CL. Evaluation of acaricide resistance mechanisms in field populations of Rhipicephalus (Boophilus) microplus collected from India. Int J Acarol. 2011;37:405–410. doi: 10.1080/01647954.2010.521518. [DOI] [Google Scholar]