Abstract
The objective of the study was to detect Babesia infections in pet dogs of a north-eastern state of India. The diagnostic efficacy of Babesia infection by polymerase chain reaction (PCR) technique has been compared with microscopy examination. For this, a total of 111 blood samples of pet dogs presented at clinical complex of the College of Veterinary Science, Guwahati, Assam with clinical signs suspected for Babesia infection subjected to the study. A total of 44 (39.63 %) dogs were diagnosed as positive for Babesia infections after microscopic examination. Among these, Babesia canis infection was diagnosed in 5 dogs (4.50 %) and B. gibsoni infection in 39 (35.13 %) dogs microscopically in Giemsa stained blood smears. Molecular diagnosis using PCR detected 63 (56.75 %) dogs positive for Babesia infection. Single infection with B. canis was found in 9 (8.10 %) dogs while B. gibsoni alone was detected in 3 (2.70 %) dogs. Mixed infections by both these species were detected in 51 (45.94 %) dogs. Overall, PCR detected 54 (48.64 %) dogs as B. gibsoni and 60 (54.05 %) dogs as B. canis positive.
Keywords: Babesiacanis, Babesiagibsoni, Detection, Dog, Microscopy, PCR, India
Introduction
Canine babesiosis is an emerging tick-borne life threatening disease caused by the intra-erythrocytic protozoan parasites under the genus Babesia in many parts of the world including India. There are two species of Babesia viz; Babesia canis and B. gibsoni which are morphologically differentiated on the basis of their size. B. canis rossi, B. canis canis and B. canis vogeli have been recognized so far as subspecies of B. canis on the basis of geographical distribution of vector tick, differences in pathological and clinical syndrome, antigenic property and molecular analysis (Boozer and Macintire 2003). Similarly recent molecular analyses have revealed three morphologically similar but genotypically distinct small Babesia of which B. gibsoni Asia type is endemic to Asia, North America, North and East Africa (Zahler et al. 2000). The identification of the causal agents involved in cases of canine babesiosis in a particular locality is important to define treatment planning for a successful outcome (Boozer and Macintire 2003). In India, the situation of canine babesiosis is not clear (Megat Abd Rani et al. 2010) except few reports (Sundar et al. 2004; Chaudhuri 2006; Chaudhuri and Varshney 2007; Senthil Kumar et al. 2009; Balachandran et al. 2010; Karunakaran et al. 2011) that are also based on conventional method of diagnosis and moreover very little study has been done in the North-Eastern region of India. The present communication reports the results of detection of Babesia infection in dogs of Assam, a north-eastern state of India, through conventional and molecular methods.
Materials and methods
Animals and sample collection
A total of 111 randomly selected pet dogs of either sex, different ages and breeds presented at the teaching veterinary clinical complex of the College of Veterinary Science, Guwahati, Assam during the year 2010–2011 and suspected for haemoparasite infection on the basis of clinical findings (depression, inappetance, lethargy, fever, abnormal coloration of urine and stool, anaemia) and history of tick exposure were included in the study. Blood samples were collected from all these dogs in vials containing EDTA (Ethylene diamine tetraacetic acid) as anticoagulant. A drop from each of well mixed fresh blood sample was used to prepare blood smear and rest was stored at −20 °C for extraction of DNA.
Diagnosis
Conventional method
Giemsa stained blood smears were examined under microscope for the presence of intra-erythrocytic Babesia organisms. In positive cases, species differentiation into B. canis and B. gibsoni was done on the basis of their size (large or small) and appearance inside the red blood cells (Soulsby 1982). Failure to detect parasite in a smear after evaluating at least 500 oil immersion fields in 20–30 min was declared as microscopically negative blood sample.
Molecular method
For diagnosis of infection by molecular technique, polymerase chain reaction (PCR) method was used. DNA was extracted from all blood samples using DNA extraction kit (Genei™ Blood Genomic DNA purification kit, Genei Bangalore, India) as per manufacturer’s instruction. PCR of all blood samples was done using B.canis and B.gibsoni specific primers as presented in Table 1.
Table 1.
Primer sets used for molecular diagnosis of B. canis and B. gibsoni infections using PCR
For molecular diagnosis of B. canis, PCR was set up in 50 μl reaction mixture consisting 25 μl of 2× PCR Master Mix (Promega) containing 50U/ml Taq Polymerase, 400 μM each dATP, dGTP, dCTP, dTTP and 3 mM Mgcl2, 25 pmol each (0.25 μl) of forward primer and reverse primer (GCC), 2 μl of Template DNA and 22.5 μl of autoclaved triple-distilled water. Amplification was performed in a thermal cycler (Gene Amp PCR System 9700, Applied Biosystem) with cyclic conditions comprising initial denaturation at 94 °C for 10 min, 40 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 30 s followed by final extension at 72 °C for 5 min (Foldvari et al. 2005).
In the case of molecular diagnosis of B.gibsoni, PCR was set up in 25 μl reaction mixture which consisted 12.5 μl of 2× PCR Master Mix, 12.5 pmol each (0.125 μl) of forward and reverse primer, 5 μl of Template DNA and 7.25 μl of autoclaved triple-distilled water. Amplification was performed with cyclic conditions comprising initial denaturation at 95 °C for 5 min, 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 90 s followed by final extension at 72 °C for 5 min (Inokuma et al. 2004).
Electrophoresis of amplified DNA was done in 1.5 % agarose gel using 100 bp DNA marker for 2 h at 80 V using Tris Acetate EDTA (TAE) running buffer. The gels were stained with ethidium bromide (0. 5 μg/ml) and viewed in UV transilluminator for the expected size product.
For all amplifications, known positive and negative samples were used as controls.
Results
The results of blood examination through microscopy and PCR analysis are summarized in Table 2.
Table 2.
Results of examination of Giemsa stained blood smears and molecular diagnosis using PCR for detection of canine Babesiosis
| Total no. of samples tested | Nos. positive after microscopical examination | Nos. positive after molecular diagnosis using PCR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B. gibsoni | B. canis | Total | B. gibsoni (single) | B. canis (single) | Mixed | Total | Overall B. gibsoni |
Overall B. canis |
|
| 111 | 39 | 5 | 44 | 3 | 9 | 51 | 63 | 54 | 60 |
| (35.13 %) | (4.50 %) | (39.63 %) | (2.70 %) | (8.10 %) | (45.94 %) | (56.75 %) | (48.64 %) | (54.05 %) | |
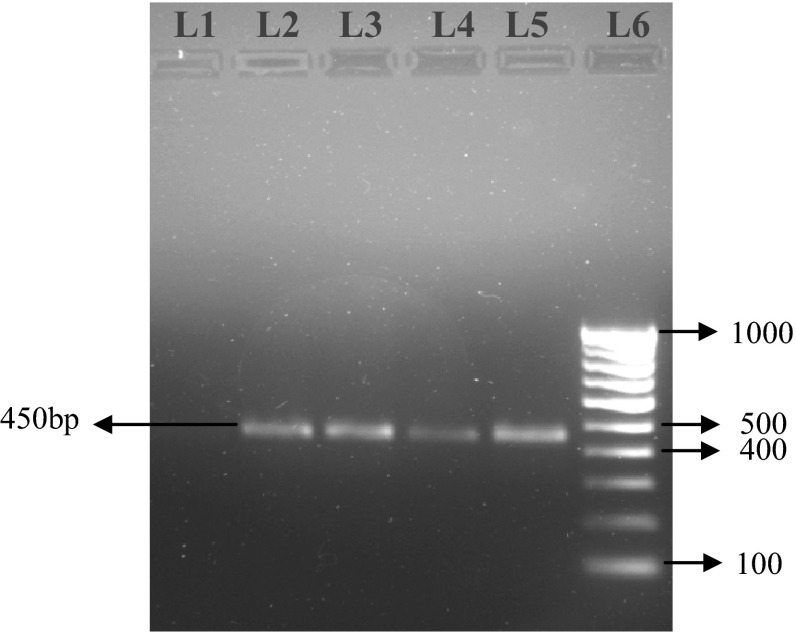
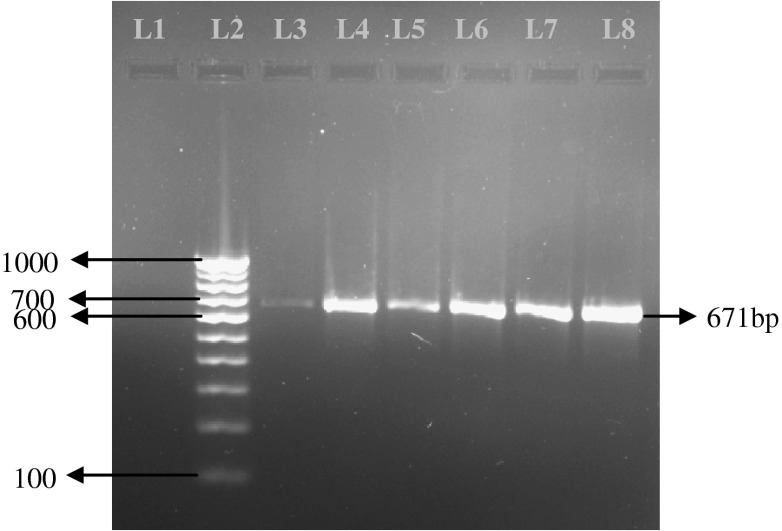
Microscopic examination of blood revealed 5 (4.50 %) and 39 (35.13 %) dogs positive to B. canis (Fig. 1) and B. gibsoni (Fig. 2) respectively, with a total 39.63 % Babesia positive. Rhipicephalus sanguineus ticks were identified from the body of the infected dogs. Molecular diagnosis using PCR detected 63 (56.75 %) dogs as positive to B. canis and B. gibsoni infections. Single infection with B. canis was found in 9 (8.10 %) dogs while single infection with B. gibsoni was detected in 3 (2. 70 %) dogs. Mixed infections by both these species were detected in 51 (45.94 %) dogs. In case of B. canis an expected PCR product of 450 bp band size (Fig. 3) and in case of B. gibsoni an expected PCR product of 671 bp band size (Fig. 4) were observed. Overall, PCR detected 54 (48.64 %) dogs as B. gibsoni and 60 (54.05 %) dogs as B. canis positive.
Fig. 1.
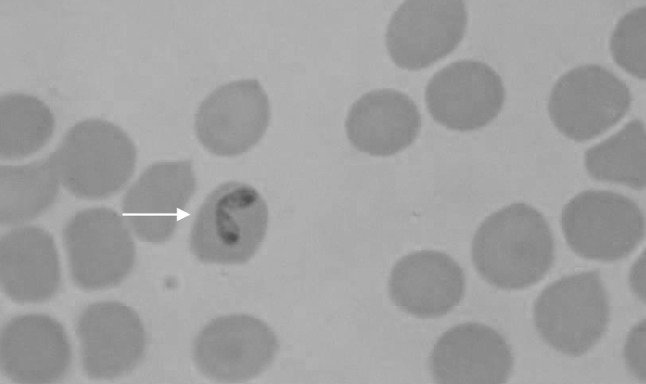
B. canis within RBC in Giemsa stained blood smear of a dog (×1,000)
Fig. 2.

B. gibsoni within RBC in Giemsa stained blood smear of a dog (×1,000)
Fig. 3.
Electrophoresis gel (1.5 % agarose, stained with ethidium bromide), showing lanes from left to right: L1, Negative Control; L2 to L5, PCR product showing positive for B. canis (450 bp); L6, 100 bp DNA ladder
Fig. 4.
Electrophoresis gel (1.5 % agarose, stained with ethidium bromide), showing lanes from left to right : L1, Negative Control; L2, 100 bp DNA ladder; L3 to L8, PCR product showing positive for B. gibsoni (671 bp)
Discussion
In the present study a high percentage (39.63 %) of clinically ill dogs was found positive for Babesia infection after microscopical examination. Species-wise, the percentage of infection with B. gibsoni was more (35. 13 %) than that of B. canis (4.50 %). The prevalence of canine babesiosis in Assam has been reported as 21.7 % by Chaudhuri and Varshney (2007) without mentioning the species. The incidence reported from Northern part of India varied between 0.66 and 8.9 % (Varshney and Dey 1998; Chaudhuri 2006) while from Southern India Senthil Kumar et al. (2009) recorded 11.6 % prevalence of haemoprotozoa and among haemoprotozoa B. canis and B. gibsoni were recorded 3.9 % and 84.9 %, respectively. Wide variation in climatic conditions prevailing in different parts of India might be responsible for varying percentage of these tick borne infections.
On the basis of present findings microscopy could detect 39.63 % cases as Babesia positive against 56.75 % detection by PCR. So 17.12 % cases remained false negative to Babesia during microscopy. Higher detection of Babesia in PCR compared to microscopy as observed in the present study was also reported by several authors (Birkenheuer et al. 2003; Gotsch et al. 2009; O’Dwyer et al. 2009). The prevalence pattern of this two species recorded in the present study was found similar to those microscopic and serologic findings reported by other workers (Bansal et al. 1985; Varshney et al. 2003; Senthil Kumar et al. 2009). PCR analysis of the present study was found more sensitive than microscopy. This is in agreement with Matsuu et al. (2005) who reported detection limit of light microscopy as approximately 0.001 % parasitaemia compared to PCR capable of detecting 9 parasites per μl of blood similar to 50 organisms/ml of blood reported by Birkenheuer et al. (2003).
Microscopic detection of B. gibsoni, though smaller in size than B. canis, was easier due to its frequent appearance in circulating blood as evidenced by 35.13 % positivity against 48.64 % molecular detection, thus raising its false negative as 13.51 % by microscopy, although they showed clinical manifestations. This might be due to parasitaemia beyond the level of microscopic detection during very early or carrier stage (Birkenheuer et al. 2003; Bourdoiseau 2006; Irwin 2009). However in the present study, it was a difficult task to find B. canis during microscopy which showed only 4.50 % cases against 54.05 % positivity in PCR; thus raising its false negativity to 49.55 % by microscopy. Very less detection of B.canis similar to the present finding was also reported by Senthil Kumar et al. (2009) who recorded only 3.9 % B.canis against 84.9 % occurrence of B. gibsoni in haemoparasite positive dogs. Higher percentage of false negativity in the case of B. canis might be due to its transient appearance in circulating blood during febrile stage of acute infection only as reported by Bourdoiseau (2006) who failed to observe the parasite during chronic infection. Schalm et al. (1975) also reported demonstration of B. canis under microscope as a fortunate occurrence. Baneth et al. (1998) failed to diagnose B. canis in blood smears although 90 % dogs remained sero-reactive in subclinical state of infection. False negativity in the present microscopic study was found more with B. canis than in B. gibsoni. Scrutiny of the results of microscopy and PCR analysis of individual blood sample in the present study revealed that out of 39, 5 microscopic positive B. gibsoni cases remained negative in PCR and the false positivity was 12.82 %.
Confirming the vector specific geographic distribution (Uilenberg et al. 1989; Zahler et al. 1998) of the subspecies of B. canis, several workers reported significant association of Dermacentor reticulatus with B. canis canis prevalent in Europe (Caccio et al. 2002; Porchet et al. 2007; Zygner et al. 2008; Welc-Faleciak et al. 2009) and Rhipicephalus sanguineus with B. canis vogeli in tropical and subtropical countries (O’Dwyer et al. 2009; Gotsch et al. 2009). Using PIRO-A1 and PIRO-B primers Foldvari et al. (2005) reported Babesia DNA having 99.8 or 100 % similarity with B. canis canis in sequencing. On the contrary O’Dwyer et al. (2009) using the same sets of primers reported PCR products 100 % identical to B. canis vogeli. Although no attempt was made in the present study to characterize B. canis at the subspecies level, in view of detection of R. sanguineus ticks in the body of suspected dogs during the present investigation, it is suggested that B. canis might belong to B. canis vogeli. Megat Abd Rani et al. (2010) opined that B. vogeli and B. gibsoni were likely co-endemic in India. Our study also revealed that a large number of Babesia positive cases had co-infections with B. gibsoni and B. canis.
Although parasite detection by microscopy is a gold standard in terms of specificity (Ravindran et al. 2007), this method in the present study failed to detect B. canis in blood smear thus giving an incorrect diagnosis in maximum cases. Molecular analysis of dog’s blood in the present study not only confirmed the existence of B. canis and B. gibsoni but also, conforming to the other reports (Birkenheuer et al. 2003; Garcia de Sa et al. 2006), proved to be superior to the blood smear evaluation, which did not provide definite diagnostic information in regards to B. canis infection. The results are in agreement with Martin et al. (2006) who opined it as a promising tool with 100 % specificity and sensitivity. On the basis of present PCR results, it could be mentioned that highest number (45.94 %) of positive cases having mixed infection of B. canis and B. gibsoni. This is important for the purpose of obtaining maximum response to therapy in clinical cases as observed by Shaw et al. (2001) and Walter et al. (2002) who reported drug regimen against the two species were different and treatment against one species might be unsuccessful due to usual presence of mixed infection. Molecular analysis in the present study provided powerful information over blood smears examination, which did not provide definite diagnostic information on B. canis compared to that of B. gibsoni. It could be presumed that large numbers of dogs of India might be suffering from canine babesiosis which remained undiagnosed or under reported and molecular diagnosis using PCR could unveil its actual prevalence.
Acknowledgments
The authors are thankful to the Director, ICAR Research Complex for NEH Region, Umiam, Meghalaya and Dean and the Director of Clinics, Faculty of Veterinary Science, Guwahati-781022, for proving facilities to carry out this research work.
References
- Balachandran C, Sridhar R, Pazhanivel N, Anooraj R. A note on the incidence of Babesia canis in a 10 day old pup on post mortem examination in Chennai, Tamil Nadu. Indian J Animal Res. 2010;44:73–75. [Google Scholar]
- Baneth G, Breitschwerdt EB, Hegarty BC, Pappalardo B, Ryan J. A survey of tick-borne bacteria and protozoa in naturally exposed dogs from Israel. Vet Parasitol. 1998;74:133–142. doi: 10.1016/S0304-4017(97)00149-0. [DOI] [PubMed] [Google Scholar]
- Bansal SR, Gautam OP, Banerjee DP. Prevalence of Babesia canis and Hepatozoon canis infection in dogs of Hissar (Haryana) and Delhi and attempts to isolate Babesia from human beings. Indian Vet J. 1985;62:748–751. [Google Scholar]
- Birkenheuer AJ, Levy MG, Breitschwerdt EB. Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian genotype) J Clin Microbiol. 2003;41:4172–4177. doi: 10.1128/JCM.41.9.4172-4177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boozer AL, Macintire DK. Canine babesiosis. Vet Clin N Am Small Animal Pract. 2003;33:885–904. doi: 10.1016/S0195-5616(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Bourdoiseau G. Canine babesiosis in France. Vet Parasitol. 2006;138:118–125. doi: 10.1016/j.vetpar.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Caccio SM, Antunovic B, Moretti A, Mangili V, Marinculic A, Baric RR, Slemenda SB, Pieniazek NJ. Molecular characterization of Babesia canis canis and Babesia canis vogeli from naturally infected European dogs. Vet Parasitol. 2002;106:285–292. doi: 10.1016/S0304-4017(02)00112-7. [DOI] [PubMed] [Google Scholar]
- Chaudhuri S (2006) Studies on clinico-therapeutic aspects of babesiosis in dogs. In MVSC Thesis. Indian Veterinary Research Institute
- Chaudhuri S, Varshney JP. Clinical management of babesiosis in dogs with homeopathic Crotalus horridus 200C. Homeopathy. 2007;96:90–94. doi: 10.1016/j.homp.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Foldvari G, Hell E, Farkas R. Babesia canis canis in dogs from Hungary: detection by PCR and sequencing. Vet Parasitol. 2005;127:221–226. doi: 10.1016/j.vetpar.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Garcia de Sa A, Cerqueira AMF, O’Dwyer LH, Macieira DB, Abreu FS, Ferreira RF, Pereira AM, Velho PB, Almosny NRP. Detection and molecular characterization of Babesia canis vogeli from naturally infected Brazilian dogs. Intern J Appl Res Vet Med. 2006;2:163–168. [Google Scholar]
- Gotsch S, Leschnik M, Duscher G, Burgstaller JP, Willie-Piazzai W, Joachim A. Ticks and haemoparasites of dog from Praia, Cape Verde. Vet Parasitol. 2009;166:171–174. doi: 10.1016/j.vetpar.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Inokuma H, Yoshizaki Y, Matsumoto K, Okuda M, Onishi T, Nakagome K, Kosugi R, Hirakawa M. Molecular survey of Babesia infection in dogs in Okinawa, Japan. Vet Parasitol. 2004;121:341–346. doi: 10.1016/j.vetpar.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Irwin PJ. Canine babesiosis: from molecular taxonomy to control. Parasite Vectors. 2009;2(Suppl 1):S4. doi: 10.1186/1756-3305-2-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran S, Pillai UN, Sasidharan HP. Babesia gibsoni infection in a German Shepherd dog. Vet World. 2011;4:269–270. doi: 10.5455/vetworld.4.269. [DOI] [Google Scholar]
- Martin AR, Dunstan RH, Roberts TK, Brown GK. Babesia canis vogeli: a novel PCR for its detection in dogs in Australia. Exp Parasitol. 2006;112:63–65. doi: 10.1016/j.exppara.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Matsuu A, Ono S, Ikadi H, Uchide T, Imamura S, Onuma M, Okano S, Higuchi S. Development of a SYBR green real-time Polymerase Chain Reaction assay for quantitative detection of Babesia gibsoni (Asian genotype) DNA. J Vet Diagn Investig. 2005;17:569–573. doi: 10.1177/104063870501700608. [DOI] [PubMed] [Google Scholar]
- Megat Abd Rani PA, Irwin PJ, Gatne M, Coleman GT, Traub RJ. Canine vector-borne diseases in India: a review of the literature and identification of existing knowledge gas. Parasites Vectors. 2010;3:28–34. doi: 10.1186/1756-3305-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dwyer LH, Lopes VVA, Rubini AS, Paduan KDS, Ribolla PEM. Babesia spp. infection in dogs from rural areas of Sao Paulo State, Brazil. Rev Bras Parasitol Vet Jaboticabal. 2009;18:23–26. doi: 10.4322/rbpv.01802005. [DOI] [PubMed] [Google Scholar]
- Porchet MJ, Sager H, Muggli L, Oppliger A, Muller N, Frey C, Gottstein B. A descriptive epidemiological study of canine babesiosis in the Lake Geneva region. Schweiz Arch Tierheilkd. 2007;149:457–465. doi: 10.1024/0036-7281.149.10.457. [DOI] [PubMed] [Google Scholar]
- Ravindran R, Lakshmanan B, Sreekumar C, John L, Gomathinayagam S, Mishra AK, Tewari AK, Rao JR. Acridine orange staining for quick detection of blood parasites. J Vet Parasitol. 2007;21:85–86. [Google Scholar]
- Schalm OW, Jain NC, Carrol EL. Veterinary haematology. 3. Philadelphia: Lea and Febiger; 1975. pp. 453–458. [Google Scholar]
- Senthil Kumar K, Vairamuthu S, Kathiresanl D. Prevalence of Haemoprotozoans in canines in Chennai City, Tamilnadu. J Vet Anim Sci. 2009;5:104–108. [Google Scholar]
- Shaw SE, Day MJ, Birtles RJ, Breitschwerdt EB. Tick-borne infectious diseases of dogs. Trends Parasitol. 2001;17:74–80. doi: 10.1016/S1471-4922(00)01856-0. [DOI] [PubMed] [Google Scholar]
- Soulsby EJL. Helminths, Arthropods and Protozoa of domesticated animals. 7. London: Bailliere Tindall; 1982. [Google Scholar]
- Sundar N, Balachandran C, Senthivelan A. Incidence of Babesia gibsoni infection in dogs in Tamil Nadu. J Vet Parasitol. 2004;18:79–80. [Google Scholar]
- Uilenberg G, Franssen FF, Perie NM, Spanjer AA. Three groups of Babesia canis distinguished and a proposal for nomenclature. Vet Quart. 1989;11:33–40. doi: 10.1080/01652176.1989.9694194. [DOI] [PubMed] [Google Scholar]
- Varshney JP, Dey S. A clinical study on haemoprotozoan infections in referral canines. J Remount Vet Corp. 1998;37:83–89. [Google Scholar]
- Varshney JP, Varshney VP, Hoque M. Clinico-haematological, biochemical, endocrinological and ultrasonographic findings in canine babesiosis. Indian J Animal Sci. 2003;73:1099–1101. [Google Scholar]
- Walter S, Mehlhorn H, Zweygarth E, Schein E. Electron microscopic investigations on stages of dog piroplasm cultured in vitro: Asian isolates of Babesia gibsoni and strains of B. canis from France and Hungary. Parasitol Res. 2002;88:32–37. doi: 10.1007/s004360100495. [DOI] [PubMed] [Google Scholar]
- Welc-Faleciak R, Rodo A, Sinski E, Bajer A. Babesia canis and other tick-borne infections in dogs in central Poland. Vet Parasitol. 2009;166:191–198. doi: 10.1016/j.vetpar.2009.09.038. [DOI] [PubMed] [Google Scholar]
- Zahler M, Schein E, Rinder H, Gothe R. Characteristic genotypes discriminate between Babesia canis isolates of differing vector specificity and pathogenicity in dogs. Parasitol Res. 1998;84:544–548. doi: 10.1007/s004360050445. [DOI] [PubMed] [Google Scholar]
- Zahler M, Rinder H, Zweygarth E, Fukata T, Maede Y, Schein E, Gothe R. Babesia gibsoni of dogs from North America and Asia belong to different species. Parasitol. 2000;120:365–369. doi: 10.1017/S0031182099005557. [DOI] [PubMed] [Google Scholar]
- Zygner W, Jaros S, Wedrychowiez H. Prevalence of Babesia canis, Borrelia afzelii and Anaplasma phagocytophilum infection in hard ticks removed from dogs in Warsaw (Poland) Vet Parasitol. 2008;145:146–151. doi: 10.1016/j.vetpar.2006.11.008. [DOI] [PubMed] [Google Scholar]