Abstract
Epidemiology of paramphistomosis in sheep and goats was studied using field and abattoir samples at Jammu, India. Abattoir examination revealed that 36.2 % of sheep and 30.9 % of goats were positive for paramphistomosis. The mean worm counts (±SEM) were 23.5 ± 5.7 in sheep and 19.9 ± 3.5 in goats. On coprological examination, 16.3 % of sheep and 13.6 % of goats were found positive for paramphistomosis. The mean egg counts (±SEM) were 4.4 ± 2.6 in sheep and 3.6 ± 2.1 in goats. Season was found to have a significant (p < 0.05) influence on the prevalence of paramphistomosis. A higher percentage of animals were found positive in rainy and post-rainy seasons as compared with summer and winter seasons. The distributions of eggs and adult flukes were significantly (p < 0.01) different among seasons. The prevalences observed according to age and sex of sheep and goats were not significant. The study conclude that the late summer months are major risk period for paramphistomosis in the pasture grazing sheep and goats and the administration of an efficient anthelmintic in May–June and September–October should be strongly recommended to reduce the infection and minimise the perpetuating financial losses to animal owners in the region.
Keywords: Egg counts, Epidemiology, Paramphistomosis, Sheep and goats, Worm burden
Introduction
Paramphistomosis is considered to be one of the most important parasitic diseases affecting livestock worldwide and the scenario is worst in tropical and subtropical regions (Prasad and Varma 1999; Rangel-Ruiz et al. 2003; Hassan et al. 2005; Phiri et al. 2007). The immature flukes are plug feeders in the upper part of small intestine and cause haemorrhage, resulting into anaemia, loss of weight gain and decreased production (Soulsby 1982; Singh et al. 1984). In the Indian subcontinent, immature paramphistomosis of domestic ruminants ranks next to fasciolosis and the mortality can reached up to 30 % in cattle or 75 to 88 % in sheep and goats (Dutt 1980; Choudhury 1994; Agrawal 2003).
For better and appropriate control strategies, it is important to identify the epidemiological aspects of the disease and the associated risk factors that are unique to a particular area and farming system (Tariq et al. 2008). The epidemiology of the fluke diseases is closely associated with environmental factors, and ecology and the infection of snail intermediate host in a particular area (Fegbemi 1984). In a certain geographical region, the microclimate determines the type and severity of parasitic infections in pasture grazing animals (Arambulo and Moran 1981). The studies on the prevalence of paramphistomosis are limited in Jammu region, despite the fact that the paramphistomosis constitutes a major health hazard to ruminants in low-lying areas, under paddy cultivation which provide a suitable habitat for the snail intermediate host. Therefore, the aim of the present study was to determine the epidemiological aspects and associated risk factors for paramphistomosis in sheep and goats in Jammu, India.
Materials and methods
Study area
The study was carried out in the plain irrigated region of Jammu province of Jammu and Kashmir state, located in north-western region of India, during a 12 month period from March 2010 to February 2011. The area lies about 332 m above sea level and experiences a subtropical humid climate. The area has four seasons; summer (March to May), rainy (June to August), post-rainy (September to November) and winter (December to February).
Abattoir study
Rumen and reticulum of slaughtered sheep (n = 199, range of 13–19 animals per month) and goats (n = 207, range of 11–22 animals per month) were examined at R.S. Pura, Jammu by weekly visits. Sheep and goats slaughtered at this abattoir are those reared by natives for meat purposes. The paramphistomes of each rumen and reticulum were collected with fine forceps. They were placed in plastic beakers containing 0.7 % saline solution and were labeled with month of collection, animal identification, sex and dental age. The collected paramphistomes were counted and preserved in 70 % alcohol. Some of them were flattened, fixed in 10 % formalin and stained with borax carmine for the preparation of permanent mounts and taxonomic identification according to the morphological features described by Dutt (1980) and Soulsby (1982).
Coprological examination
Faecal samples of sheep (n = 398, range of 25−45 animals per month) and goats (n = 360, range of 21–49 animals per month) of the study area were collected directly from the rectum by weekly visits. Samples were collected in air tight bottles containing 5 % formalin and were labeled with month of collection, animal identification, sex and dental age. Samples were brought to the laboratory and were examined using qualitative and quantitative examination as given by Soulsby (1982).
Meteorological data
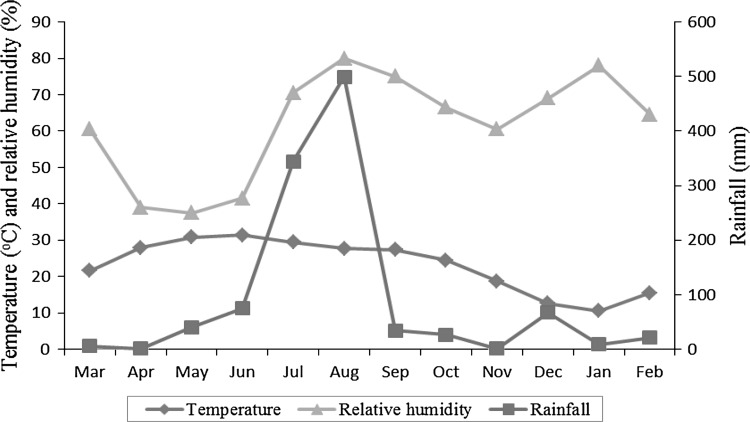
The data regarding mean monthly temperature, rainfall and relative humidity were obtained from the Division of Meteorology, Faculty of Agriculture Sciences, Jammu and are presented in Fig. 1. The annual rainfall for the year March 2010 to February 2011 was 1124 mm. The mean relative humidity was ranged from 37.5 % in May to 80 % in August. The mean annual minimum and maximum temperature was 10.5°C (January) and 31.4°C (June), respectively.
Fig. 1.
Meteorological data of Jammu during 2010/2011
Statistical analysis
The data obtained on the coprological and abattoir prevalences of paramphistomosis were compared by Chi square test. The mean egg counts (the total number of fluke egg counts divided by the total number of animals examined) and mean worm counts (the total number of flukes divided by the total number of animals examined) in sheep and goats were compared by ANOVA using SPSS 16.0 for windows. A p value of <0.05 was considered significant.
Results
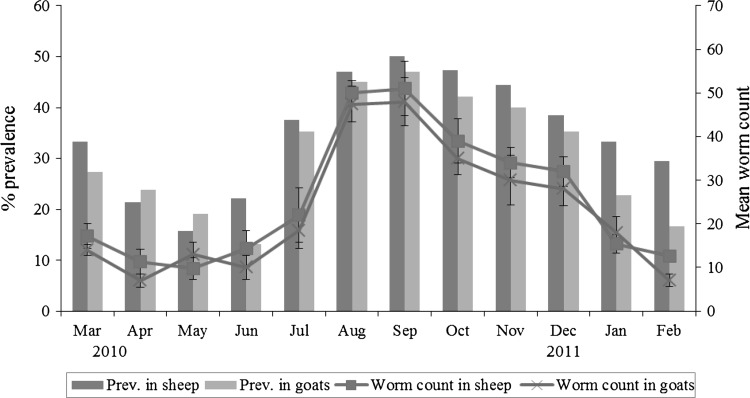
The abattoir based prevalence and mean worm counts (±SEM) of paramphistomes in sheep and goats are shown in Table 1 and Fig. 2. Sheep had a higher prevalence (36.2 %) as compared to goats (30.9 %). A significantly (p < 0.05) higher prevalence was found in the post-rainy season (47.3 % in sheep and 43.1 % in goats) as compared to other seasons.
Table 1.
The seasonal prevalence and mean worm counts (±SEM) of paramphistomes in sheep and goats based on abattoir examinations
| Season | Sheep | Goats | ||||
|---|---|---|---|---|---|---|
| Number examined | Infected (%) | Mean worm count (±SEM) | Number examined | Infected (%) | Mean worm count (±SEM) | |
| Summer | 48 | 22.9a | 12.8 ± 3.8p | 53 | 22.6g | 11.1 ± 3.6s |
| Rainy | 51 | 35.3ab | 28.8 ± 4.4q | 52 | 32.7gh | 25.3 ± 3.9t |
| Post-rainy | 55 | 47.3b | 41.1 ± 7.1r | 51 | 43.1h | 37.6 ± 6.9v |
| Winter | 45 | 33.3ab | 20.0 ± 5.3pq | 51 | 25.5gh | 19.5 ± 4.9st |
Different superscripts indicate significant difference (p < 0.05) in prevalence in sheep (a, b) and goats (g, h) during different seasons
Different superscripts indicate significant difference (p < 0.05) in worm counts in sheep (p, q, r) and goats (s, t, v) during different seasons
Fig. 2.
The prevalence (%) and mean worm counts (±SEM) of paramphistomes in sheep and goats based on abattoir examinations
The mean worm count (±SEM) in sheep was 23.5 ± 5.7 (range 0–201) while that of goats was 19.9 ± 3.5 (range 0–175). The highest worm counts (±SEM) in sheep (51 ± 6.2) and goats (48 ± 5.6) were found in the month of September. Seasonally, both sheep and goats had a significant (p < 0.05) higher worm counts (±SEM) in post-rainy season (41.1 ± 7.1 and 37.6 ± 6.9, respectively) as compared to other seasons.
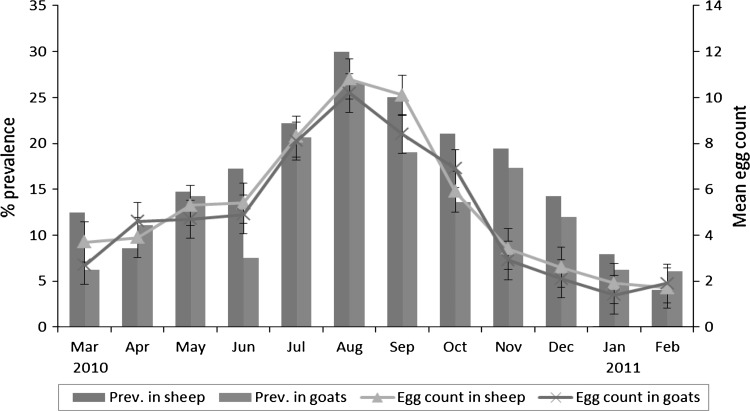
Coprological prevalence and the mean egg counts (±SEM) in sheep and goats are shown in Table 2 and Fig. 3. Sheep had a higher prevalence rate (16.3 %) as compared to goats (13.6 %). The highest prevalence was found in the rainy season (23.1 % in sheep and 18.6 % in goats) and the lowest was in winter (8.8 % in sheep and 7.8 % in goats). The observations were statistically significant (p < 0.05).
Table 2.
The seasonal prevalence and mean egg counts (±SEM) of paramphistomes in sheep and goats based on coprological examinations
| Season | Sheep | Goats | ||||
|---|---|---|---|---|---|---|
| Number examined | Infected (%) | Mean egg count (±SEM) | Number examined | Infected (%) | Mean egg count (±SEM) | |
| Summer | 101 | 11.9ac | 4.3 ± 1.7p | 80 | 10.0gh | 4.0 ± 1.2s |
| Rainy | 104 | 23.1b | 8.6 ± 2.4q | 118 | 18.6h | 7.9 ± 2.6t |
| Post-rainy | 102 | 21.6ab | 6.5 ± 4.3pq | 72 | 16.7gh | 6.1 ± 3.7st |
| Winter | 91 | 8.8c | 2.1 ± 0.9p | 90 | 7.8g | 1.8 ± 1.1s |
Different superscripts indicate significant difference (p < 0.05) in prevalence in sheep (a, b, c) and goats (g, h) during different seasons
Different superscripts indicate significant difference (p < 0.05) in egg counts in sheep (p, q) and goats (s, t) during different seasons
Fig. 3.
The prevalence (%) and mean egg counts (±SEM) of paramphistomes in sheep and goats based on coprological examinations
The mean egg count (±SEM) in sheep was 4.4 ± 2.6 (range 0–75) while in goats was 3.6 ± 2.1 (range 0–91). The mean egg counts of above 10 eggs per gram were recorded during the months of August and September in sheep and during the month of August in goats. The rainy season both in sheep and goats (median 112 and 108, respectively) had significantly (p < 0.05) higher egg counts as compared with summer (median 94 and 88, respectively) and winter (median 82 and 81, respectively).
The overall percentage infection as revealed by abattoir and coprological examinations was found to be 22.6 % in sheep (n = 597) and 19.9 % in goats (n = 567) and was statistically not significant. The infection rate, with few exceptions, was found to be moderate to severe. No significant differences in worm counts and faecal egg counts were observed between sheep and goats. No attempt was made to correlate the egg counts with the worm counts because the faecal samples were collected randomly from the sheep and goat flocks and were not linked to necropsy worm counts.
The prevalence of paramphistomosis in sheep and goats according to age and sex is given in Table 3. The animals below 1 year of age had high prevalences in both methods (coprological and necropsy examinations) as compared to animals between 1–3 years and above 3 years of age. The females had higher infection rate than the males.
Table 3.
Influence of age and sex on the prevalence of paramphistomes in sheep and goats using coprological and abattoir examinations
| Coprological examination | Abattoir examination | |||||||
|---|---|---|---|---|---|---|---|---|
| Sheep | Goats | Sheep | Goats | |||||
| Number examined | Infected (%) | Number examined | Infected (%) | Number examined | Infected (%) | Number examined | Infected (%) | |
| Age | ||||||||
| <1 year | 162 | 18.5 | 157 | 15.3 | 82 | 39.0 | 76 | 35.5 |
| 1–3 years | 101 | 14.9 | 91 | 8.8 | 53 | 37.7 | 61 | 27.9 |
| >3 years | 135 | 15.6 | 112 | 15.2 | 64 | 28.1 | 70 | 28.6 |
| Sex | ||||||||
| Female | 162 | 17.3 | 168 | 13.7 | 103 | 36.9 | 87 | 34.5 |
| Male | 236 | 16.1 | 192 | 13.5 | 96 | 33.3 | 120 | 28.3 |
Discussion
In the present study, the coprological examination showed the highest prevalence in rainy season while the highest prevalence using necropsy examination was found in post-rainy season. However, the infection was encountered throughout the year. The weather pattern of the region with ambient temperature and sufficient moisture, even during the dry spell of the year due to the perennial irrigation of the fields were important contributing factors for the translation and transmission of paramphistome species all round year. These results are in accordance to earlier reports (Phiri et al. 2006; Yadav et al. 2006; Tariq et al. 2008).
Based on data on coprological and necropsy examinations, it is evident that the highest egg counts were found in rainy season while the highest worm counts were recorded in post-rainy season. Perhaps, with the rise in ambient temperature during early summer months, there is an enhanced recruitment of infection by the miracidial stage in the snail hosts, which is followed by the emergence of a large number of cercariae and their encystment on pastures during late summer months (Lima et al. 2001). In terms of disease transmission dynamics, this is a major risk period for paramphistomosis in the pasture grazing nomadic sheep and goat flocks. With the onset of rainy season in June, a new crop of snails is produced which further contributes to the availability of infective stages of the flukes on the pastures, resulting in a gradual build-up of fluke population during rainy and post-rainy months as observed herein. On the other hand, the highest egg count was found in rainy season. The egg count was started increasing from May (late summer), attained the peak in August (end of rains) and thereafter declined sharply during post-rainy and winter months. However, an increase in egg counts was started earlier (May onwards) as compared to increase in worm counts (July onwards). It could be attributed to the appearance of gonads and other reproductive structures of flukes which developed rapidly from an incipient stage in late summer and regressed rapidly during post-rainy months, resulting in lower egg counts during post-rainy and winter months (Hanna et al. 1988).
The present findings revealed that age and sex had no significant influence on the prevalence of paramphistomosis. However, animals below 1 year of age had a high prevalence rate than the animals between 1–3 years and above 3 years of age which agrees with those of Patel et al. (2001) and Tariq et al. (2008). The propensity of sheep and goats in young age group to higher infection rate may be attributed to high susceptibility and low resistance. The adult animals afford some protection against reinfection duo to development of immunity, which is initially low but increases with the intensity and duration of exposure of infection and hence acute disease is usually seen in younger animals while the older animals, capable of withstanding massive exposure (Horak 1971; Soulsby 1982). The higher infection rate in females than the males could be attributed to genetic predisposition and differential susceptibility owing to hormonal effects (Tariq et al. 2008). Further the females generally get more attention in the hands of farmer on account of economic reasons and those experience problems with their health and production are usually culled while it is a standard production procedure for males to be eliminated (Phiri et al. 2006).
The abattoir prevalences of paramphistomosis in sheep and goats were significantly higher (p < 0.001) than that of coprological prevalences in sheep and goats. A low prevalence in coprological examination could have been arisen because of harbouring immature flukes and/or light infections. Further variation in egg counts found to be influenced by feed composition, faecal consistency and the time of day of faecal collection (Coyle 1958) with neither uniformity nor consistency in low, medium and high fluke burdens (Duwell and Reisenleiter 1984). Furthermore the egg production may be suppressed after acquisition of immunity resulting in low egg counts even in the presence of high numbers of adult worms in the host (Winks et al. 1983).
In conclusion, paramphistomosis was highly prevalent in the study region and the late summer months are the major risk period in the pasture grazing nomadic sheep and goat flocks. The administration of an efficient anthelmintic in late summer (May) and early post-rainy (September) seasons should be strongly recommended to reduce the infection, and to sustain the optimal growth and productivity of sheep and goats and minimise the perpetuating financial losses to animal owners in the region.
References
- Agrawal MC. Epidemiology of fluke infections. In: Sood ML, editor. Helminthology in India. 1. Dehradun: International Book Distributors; 2003. pp. 511–542. [Google Scholar]
- Anish Yadav, Khajuria JK, Raina AK. Seasonal prevalence of gastrointestinal parasites in sheep and goats of Jammu. J Vet Parasitol. 2006;20:65–68. [Google Scholar]
- Arambulo PV, Moran M. The tropics and parasitic diseases of animals-their impact on animal and human health. Int J Zoonoses. 1981;8:5–19. [PubMed] [Google Scholar]
- Choudhury N. Helminths of domesticated animals in Indian subcontinent. In: Chowdhury N, Tada I, editors. Helminthology. 1. New Delhi: Narosa; 1994. pp. 73–120. [Google Scholar]
- Coyle TJ. Experiments in the diagnosis and treatment of fascioliasis in Uganda cattle. Bull of Epizoot Dis Afr. 1958;6:255–272. [Google Scholar]
- Dutt SC. Paramphistomes and paramphistomiasis of domestic ruminants in India. Ludhiana: Monograph, Punjab Agricultural University; 1980. pp. 1–162. [Google Scholar]
- Duwell W, Reisenleiter R. Fasciola hepatica: coproscopic diagnosis compared with the worm burden in the sheep. Helminthologia. 1984;21:151–159. [Google Scholar]
- Fegbemi BO. The effect of environmental factors on the development, behaviour and survival of Paramphistomum microbothrium miracidia. Vet Parasitol. 1984;16:71–81. doi: 10.1016/0304-4017(84)90009-8. [DOI] [PubMed] [Google Scholar]
- Hanna REB, Williamson DS, Mattison RG, Nizami WA. Seasonal reproduction in Paramphistomum epiclitum and Gastrothylax crumenifer, rumen paramphistomes of Indian water buffalo and comparison with biliary paramphistome Gigantocotyle explanatum. Int J Parasitol. 1988;18:513–521. doi: 10.1016/0020-7519(88)90016-1. [DOI] [PubMed] [Google Scholar]
- Hassan SS, Kaur K, Joshi K, Juyal PD. Epidemiology of paramphistomosis in domestic ruminants in different districts of Punjab and other adjoining areas. J Vet Parasitol. 2005;19:43–46. [Google Scholar]
- Horak IC. Paramphistomiasis of domestic ruminants. Adv Parasitol. 1971;9:3–72. doi: 10.1016/s0065-308x(08)60159-1. [DOI] [PubMed] [Google Scholar]
- Lima WS, Coelho LH, Nunes RF, Guimaraes MP (2001) The epidemiology of fasciolosis in Minas Gerais State, south-east of Brazil. In: proceedings of the 18th international conference of the World association for the advancement of veterinary parasitology, Stressa, p 58
- Patel MD, Nauriyal DS, Hasnani JJ, Gupta RS. Prevalence of gastrointestinal parasitism in goats maintained under semi-intensive and field management systems. Indian J Vet Med. 2001;21:99–101. [Google Scholar]
- Phiri AM, Phiri IK, Monrad J. Prevalence of amphistomiasis and its association with Fasciola gigantica infections in Zambian cattle from communal grazing areas. J Helminthol. 2006;80:65–68. doi: 10.1079/JOH2005313. [DOI] [PubMed] [Google Scholar]
- Phiri AM, Chota A, Phiri IK. Seasonal pattern of bovine amphistomosis in traditionally reared cattle in the Kafue and Zambezi catchment areas of Zambia. Trop Anim Health Prod. 2007;39:97–102. doi: 10.1007/s11250-007-4406-z. [DOI] [PubMed] [Google Scholar]
- Prasad A, Varma TK. On the prevalence and community dominance among paramphistomes infecting domestic ruminants. J Vet Parasitol. 1999;13:129–133. [Google Scholar]
- Rangel-Ruiz LJ, Albores-Brahms ST, Gamboa-Aguilar J. Seasonal trends of Paramphistomum cervi in Tabasco, Mexico. Vet Parasitol. 2003;116:217–222. doi: 10.1016/j.vetpar.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Singh RP, Sahai BN, Jha GJ. Histopathology of the duodenum and rumen of goats during experimental infection with Paramphistomum cervi. Vet Parasitol. 1984;15:39–46. doi: 10.1016/0304-4017(84)90108-0. [DOI] [PubMed] [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7. London: Bailliere and Tindal; 1982. pp. 771–773. [Google Scholar]
- Tariq KA, Chishti MZ, Ahmad F, Shawl AS. The epidemiology of paramphistomosis of sheep (Ovis aries L) in the north-west temperate Himalayan region of India. Vet Res Commun. 2008;32:383–391. doi: 10.1007/s11259-008-9046-x. [DOI] [PubMed] [Google Scholar]
- Winks R, Bremner KC, Barger IA. Epidemiology and control of parasitic gastroenteritis of cattle in the tropical/sub-tropical zone. In: Anderson N, Waller PJ, editors. The epidemiology and control of gastrointestinal parasites of cattle in Australia. Australia: Commonwealth Scientific and Industrial Research Organisation; 1983. pp. 65–72. [Google Scholar]