Abstract
This study was conducted to investigate the role of 1-aminocyclopropane-1-carboxylate (ACC) deaminase in Pseudomonas fluorescens strain REN1 and its ability to reduce ethylene levels produced during stress, endophytically colonize and promote the elongation of the roots of rice seedlings under gnotobiotic conditions. We isolated 80 bacteria from inside roots of rice plants grown in the farmers’ fields in Guilan, Iran. All of the isolates were characterized for plant growth promoting (PGP) traits. In addition, the colonization assay of these isolates on rice seedlings was carried out to screen for competent endophytes. The best bacterial isolate, based on ACC deaminase production, was identified and used for further study. 16S rDNA sequence analysis revealed that the endophyte was closely related to Pseudomonas fluorescens. The results of this study showed ACC deaminase containing P. fluorescens REN1 increased in vitro root elongation and endophytically colonized the root of rice seedlings significantly, as compared to control under constant flooded conditions. The trait of low amount of indole-3-acetic acid (IAA) production (<15 μg mL−1) and the high production of ACC deaminase by bacteria may be main factors in colonizing rice seedling roots compared to other PGP traits (siderophore production and phosphate solubilization) in this study. Endophytic IAA and ACC deaminase-producing bacteria may be preferential selections by rice seedlings. Therefore, it may be suggested that the utilization of ACC as a nutrient gives the isolates advantages in more endophytic colonization and increase of root length of rice seedlings.
Keywords: ACC deaminase, Colonization, Ethylene, Rice, Plant–bacteria interaction, Endophyte
Introduction
Global food production may soon become insufficient to feed all of the world’s people, since environmental damage and human population pressure have increased. Therefore, agricultural productivity must meaningfully be increased within the next few decades. One way to sustainably increase agricultural productivity is by the increased use of plant growth-promoting bacteria (PGPR) (Glick 2013). PGPR can motivate the growth of plants by one or more of different direct and indirect mechanisms (Laslo et al. 2012). Importance of IAA production in promoting root growth directly by stimulating plant cell elongation or indirectly by affecting bacterial ACC deaminase activity by PGPR has been greatly recognized (Kennedy et al. 2004; Pedraza et al. 2004). Similarly, role of phosphate-solubilizing bacteria in plant growth and development and the production of siderophores by PGPR especially under iron-limited conditions has been well documented (Ahmad et al. 2008; Dimkpa et al. 2008; Richardson et al. 2009). One of the most PGP traits shown in many PGPR is to stimulate plant growth by the activity of ACC deaminase that promotes plant growth by decreasing plant ethylene levels. The enzyme hydrolyzes ACC, the immediate biosynthetic precursor of the hormone ethylene in plant tissues, to ammonia and α-ketobutyrate (Laslo et al. 2012), thereby reducing the suppressive effect of ethylene on root elongation, and thus promoting plant growth (Glick et al. 1998). These bacteria not only directly promote plant growth; they also protect plants against flooding, drought, salt, flower wilting, metals, organic contaminants, and both bacterial and fungal pathogens (Glick 2013). The ability of ACC-utilizing PGPR to ameliorate plant growth inhibition caused by ethylene through a decrease in ACC content and ethylene production has been repeatedly demonstrated, particularly when the plants were subjected to stressful growth conditions (Belimov et al. 2001; Penrose et al. 2001; Belimov et al. 2002; Mayak et al. 2004; Safronova et al. 2006). However, the role of bacterial biosynthesis of ACC deaminase in endophytically colonizing of plant roots has not been reported under flooded conditions. The beneficial effects of the PGPR have been reported in many crops (Khalid et al. 2004; Malhotra and Srivastava 2009). Plants treated with ACC deaminase-containing bacteria have longer roots (Belimov et al. 2007) and can better resist the inhibitory effects of stress ethylene on plant growth imposed by heavy metals (Burd et al. 2000), pathogens (Wang et al. 2000) and flooding (Grichko and Glick 2001). It was previously proposed that rhizobacteria attached to the surface of plant roots or seeds can take up some of the ACC exuded by the plant and degrade it through the action of ACC deaminase (Glick et al. 1998). Rice is cultivated under constant flooding conditions in Iran and the farmers face several problems such as drought, pathogens, flooding, salinity, etc. Keeping in view the above discussion and with regard to role of ACC deaminase in lowering those stresses, the present study was undertaken (i) to isolate and determine the frequency of root endophytic isolates in terms of plant growth promoting (PGP) traits (especially ACC deaminase feature) in rice plants grown under constant flooded conditions in Iran soils, (ii) to evaluate the survival of the isolates and their ability to colonize rice seedlings under constant flooded conditions, (iii) to study the effect of P. fluorescence strain REN1, the most promising ACC deaminase isolate, and application of Cobalt chloride (CoCl2) as an inhibitor of ethylene biosynthesis on rice root elongation and (iv) to study the ability of utilization of ACC as a nutrient substance by isolate REN1 as an advantage in colonization of rice seedlings roots.
Materials and methods
Isolation of endophytic bacterial strains
Roots of the healthy cultivated rice plants (Oryza sativa L., Cv, Gohar), at flowering, were collected randomly from the Research Farm, Guilan Province, Iran, and conveyed to the laboratory in coolers. Roots were washed thoroughly with tap water, rinsed with deionized water to remove attached clay, and drained on absorbent towels. Roots were submerged in 70 % ethanol for 3 min, washed with fresh sodium hypochlorite solution (2.5 % available Cl−) for 5 min, rinsed with 70 % ethanol for 30 s, and finally washed ten times with sterile distilled water. Aseptic techniques were used in every step. Root slices (2–3 cm long) were placed in sterile 12.5 mM potassium phosphate buffer (pH 7.1) (Phosphate buffer) solution and macerated. The macerate was decanted into conical flasks, shaken on a rotatory shaker for 45 min (at 120 rpm). Tissue extracts were then serially diluted in phosphate buffer and plated in triplicate to recover any bacterial endophytes present in the plant tissue onto Nutrient Agar (NA). Serial dilutions (up to 10−8) were made and 0.1 mL aliquots were spread on to NA plates. All the plates were incubated at 28 ± 2 °C for 3–5 days and the number of colony-forming units (CFU) were counted. Numbers of endophytic bacterial cells recovered were expressed as CFU g−1 fresh tissue weight (FW). To confirm that the sterilization process was successful, the aliquots of the sterile distilled water used in the final rinse were set on NA medium plates. The plates were examined for bacterial growth after incubation at 28 °C for 3 days. Rice root samples that were not contaminated as detected by culture-dependent sterility test were used for this study. Bacterial isolates identified as individual CFU were selected and sub-cultured onto NA. Similar bacterial isolates were grouped based on phenotypic characteristics (shape, motility, color, growth rate, culture) and gram-staining reaction, and stored in a refrigerator at 4 °C for further studies. For long-term storage, bacterial cultures were maintained at −80 °C in Nutrient Broth (NB) that contained 20 % glycerol.
In vitro plant growth promoting (PGP) traits of isolates
Production of siderophore, IAA production (μg mL−1), ability of phosphate-solubilizing and hydrocyanic acid (HCN) production by the isolates were determined as described by Schwyn and Neilands (1987), Glickmann and Dessaux (1995), Pikovskaya (1948) and Lorck (1948) respectively. The ability of the isolates to produce ACC deaminase was also screened on minimal media containing ACC as their sole nitrogen source as described by Penrose and Glick (2003). Optical density (OD) was measured after 48 h at 540 nm by spectrophotometer (Specord 200, Analytic Jena, Germany) and considered as an index for evaluating ACC deaminase-producing isolates.
Colonization assay of isolated strains
We studied root endophytic colonization on all of the isolates obtained from rice roots to screen for competent endophytes. For the colonization assay, we used the method described by Yanni et al. (1997) with some modification. For preparing the bacterial cultures, each bacterial isolate was grown in 250-mL flasks containing 100 mL NB medium and incubated for 24 h at 28 °C. After incubation, the cell suspension was centrifuged at 5,000×g for 10 min and the pellet was resuspended in 0.85 % sterilized saline solution. The bacterial culture was standardized to 5 × 108 CFU mL−1. Surface-sterilized dehulled seeds were transported to 20 × 200 mm tubes containing 20 mL of Hoagland’s plant growth medium solidified with 1 % purified agar. Each tube was incubated for 3 days in the dark at 28 °C for seed germination. The bacterial cell pellet of 80 strains were washed and resuspended in 0.5 mL sterile 0.03 M MgSO4 and placed on ice separately. The absorbance of the samples was adjusted evenly at 600 nm. The 100 μL of bacterial inoculum grown separately on NB medium for 2 days at 30 °C was suspended in sterile Hoagland’s medium as different mixtures of strains (isolates without any antagonistic effect with each other). To find isolates without any antagonistic effect with each other, we performed an in vitro antagonistic assay on all isolates as described by Etesami et al. (2014). Each seedling root was inoculated with 5 × 108 cells mL−1 (eight replicates were used for each treatment) and incubated in a growth chamber. Rice seedlings in tube culture were gently uprooted 20 days after inoculation, and then excised at the stem base. Roots were rinsed free of agar, weighed, surface-sterilized with 70 % ethanol followed by 5 % sodium hypochlorite solution, rolled over plates of NA to check for surface sterility, and then macerated in a sterilized commercial blender in potassium phosphate solution for 5 min. Viable plate counts of the rice endophyte populations were made 3 days after incubation of diluted root macerates plated on NA as an indicator of bacterial invasion capacity. Then, colonies were picked, re-streaked on NA, and stocked in pure culture. The endophytic isolates recovered from rice seedlings roots were screened for PGP traits again.
Molecular identification of isolate REN1
Morphological and cultural properties of the most promising ACC deaminase- producing isolate, REN1, were studied according to methods for general and molecular bacteriology (Gerhardt et al. 1994). Genomic DNA was isolated according to Sandhu et al. (2009). The amplification of 16S rDNA gene was done by using universal bacterial primer 1492R (50-TAC GGY TACCTT GTT ACG ACT T-30) and 27F (50-AGA GTT TGATCM TGG CTC AG-30) in accordance with the conditions described by Sandhu et al. (2009). The sequence of 16S rDNA gene was determined after genomic DNA extraction and polymerase chain reaction amplification. The PCR product was purified and sequenced on a DNA sequencer (Iowa State University, USA). The homology of partial sequence was compared with the sequences from the DNA databases and similar sequences showing above 95 % were recovered by nucleotide Basic Local Alignment Search Tool (BLAST) program at the National Center for Biotechnology Information (NCBI) BLAST server (http://www.ncbi.nih.gov/BlAST/). The 16S rDNA gene sequences were aligned using the ClustalW2 and the phylogenetic tree was bootstrapped by the program MEGA5.
Marking of Pseudomonas fluorescence REN1 with gusA
We selected the most promising ACC deaminase-producing isolate, REN1, among the re-isolated strains from inside rice seedlings roots for further study. To monitor rice root and tissue colonization by this isolate and since rice seeds can be an important source of endophytic bacteria (Kaga et al. 2009), we marked P. fluorescence REN1 with gusA. Briefly for this purpose, Escherichia coli DH5α containing transposon-based b-glucuronidase (GUS) marker pNpt, which has the gusA gene under the control of a constitutive tetracycline and ampicillin resistance gene promoter (Chen et al. 2010), was maintained on Luria agar plates. For generation of the GUS-tagged strain, pNpt-GUS was transferred to P. fluorescence REN1 using the triparental mating method (Ditta et al. 1980). The transconjugants were selected on minimal medium MinA (Miller 1972) plates containing 20 μg mL−1 of tetracycline. Color reagent 5-bromo-4-chloro-3-indolyl-b- D-glucuronate (X-Gluc; Biosynth AG) was added to the medium at 20 μg mL−1, and the blue colonies were selected for further analysis.
Root elongation and colonization assay by isolate REN1
The plant root elongation-promoting activity and endophytic colonization of P. fluorescence REN1 marked with gusA were determined with a gnotobiotic system, using axenic rice plantlets. Dehulled seeds were surface-sterilized in 70 % ethanol for 5 min, followed by 0.2 % mercuric chloride for 30 s, and washed five times with sterile water. The seeds were germinated on nutrient agar plates (1 % agar) and uncontaminated seedlings were used for axenic experiments. Uniform-sized uncontaminated seedlings were transferred to an eppendorf plastic tube of which the tip had been excised. The plastic tube was placed in a 20 × 200 mm test tube on top of 20 mL of Hoagland solution including different treatments. The isolate was grown on NB medium for 2 days at 30 °C and then, the bacterial cell pellet of the strain was washed and re-suspended in 0.5 mL sterile 0.03 M MgSO4, subsequently 100 μL of this suspension (5 × 108 cells mL−1) were added in sterile Hoagland’s medium including different treatments. For treatments with chemical inhibitor of ethylene biosynthesis, the nutrient solution was supplemented with 2 μM CoCl2 (final concentration) and for treatments with N source, the nutrient solution was supplemented with 5 mM (NH4)2SO4. This test was performed by factorial tests in a randomized complete block design with four replications within laboratory tubes and repeated twice. The tubes were maintained in a growth chamber (28 ± 2 °C; 75 % relative humidity; 14 h light intensity of 60 mlux m−2; 10 h dark). All manipulations were performed under sterile conditions. The effects of different treatments on the root length and endophytic colonization were measured and registered after 20 days. For enumerating of P. fluorescence REN1 colonizing rice roots, rice plants in culture tubes were gently uprooted 3, 7, 14 and 20 days after inoculation, and then excised at the stem base. Roots were surface-sterilized and then isolated using the same procedures as described above. Viable plate counts of the rice endophyte population were made 3 days after incubation of diluted root macerates plated on MinA agar amended with X-gluc (20 μg mL−1) as an indicator of bacterial invasion capacity (bacterial colonies showing blue coloration) and considered as endophytic population.
Statistical analysis
Analysis of variance (ANOVA) was performed on all experimental data and means were compared by Tukey’s test at 5 % probability level using the Statistical Analysis System (V. 8) software package (SAS Institute, Cary, NC, USA). All results presented are the means of three independent replicates.
Results
Isolation of endophytic bacteria
Eighty bacterial strains were isolated from the surface-sterilized roots of rice plants grown in the farmers’ fields in Guilan, Iran. Colony and cell morphology, and gram-staining tests were performed on the isolates. Fifty-five percent of the isolates were gram positive and the rest were gram negative. All of the 80 bacterial isolates were selected for this study. The population sizes of these isolates were from 3.3 to 6.8 × 106 CFU g−1 FW.
In vitro PGP traits of isolates
The potential of the isolates for plant growth promotion was evaluated by screening for PGP characteristics. Forty-seven out of 80 endophytic isolates produced IAA whereas two produced HCN. Eighteen strains formed an orange halo surrounding bacterial colonies on CAS agar. This occurred because the iron was removed from the blue CAS-Fe (III) complex during siderophores production. Ten isolates were also able to solubilize phosphate and 28 isolate was able to utilize ACC as sole nitrogen source, a trait that is a consequence of the ACC deaminase activity (Table 1). Of 80 isolates, 15 isolates showed multiple plant growth promoting traits and were potent in terms of PGP activities. The studied isolates produced different amounts of siderophores, IAA and ACC deaminase, and also had a different ability at solubilizing phosphate (Table 1). The results showed that the isolates produced IAA ranging from 2.24 ± 0.22 to 30.32 ± 0.58 μg mL−1 in the presence of L-tryptophan (100 μg mL−1). These results denote that an important proportion of the isolates had the ability to produce IAA and ACC deaminase. Among 47 IAA-producing isolates, 32 isolates produced IAA between 2.24 and 15 μg ml−1 and the rest produced IAA more than 15 μg mL−1. In addition, 68.7 % of ACC deaminase producing isolates produced ACC deaminase more than OD > 0.3 (Table 1). In general, the percentage of five PGP traits among the isolates decreased in the order; IAA producing > ACC deaminase producing > siderophore producing > phosphate solubilizing > HCN producing.
Table 1.
Frequency and production amount of Plant growth-promoting activities of eighty endophytic isolates obtained from rice roots
| No. of studied isolates | No. of isolates showing production of: | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IAA (μg mL−1) | ACC deaminase | Siderophore | Phosphate solubilization | HCN | Multiple PGP traits | |||||||
| 80 | 47 | 28 | 18 | 10 | 2 | 15 | ||||||
| IAA > 15 | IAA < 15 | OD > 0.3 | OD < 0.3 | + | ++ | +++ | + | ++ | +++ | |||
| 15 | 32 | 19 | 9 | 4 | 12 | 2 | 4 | 3 | 3 | |||
Symbols: +, 1 to 3 mm; ++, 4 to 6 mm; +++, more than 6 mm diameter of halo on agar plates in assaying siderophore production and phosphate solubilization
Colonization of rice seedlings by isolated strains
To determine which of the endophytic isolates had the ability to colonize and persist at high levels in rice plants and find competent endophytes, we performed studies with a controlled system, using axenic rice plantlets. Only twelve endophytes were re-isolated from rice seedlings after 20 days. Plating experiments of macerates from surface-sterilized roots of the inoculated plants indicated substantial populations of the endophytic bacteria (Table 2). The isolates inoculated on rice seedlings colonized the plants at levels ranging from 3.5 to 5.1 log10 CFU g−1 FW at 20 days post-inoculation (Table 2). Throughout the study, both control and inoculated plants were without any visible disease symptoms.
Table 2.
Bacterial endophyte recovery from rice seedlings grown in gnotobiotic tube culture for 20 days in Hoagland liquid by batch inoculations put on four mixtures of 20 strains each
| No. of isolates tested | No. of isolates recovered | Colonization level (avg ± SD) |
|---|---|---|
| 20 | 4 | 5.1 ± 0.3 |
| 20 | 3 | 4.45 ± 1.2 |
| 20 | 3 | 3. 8 ± 0.3 |
| 20 | 2 | 3.5 ± 2.2 |
± shows standard deviation (SD)
PGP activity of recovered isolates
We recovered 12 isolates of rice seedlings grown in the growth medium (Table 3). To re-screen the recovered isolates from rice seedlings for PGP traits, we studied all of the 12 recovered isolates from rice seedlings for all PGP traits. When the bacterial isolates were evaluated for their PGP traits, all of the isolates produced IAA and ACC deaminase, whereas the production ability of other PGP traits was not observed among all of the isolates (Table 3). All of the isolates produced IAA between 8 and 17 μg mL−1. Most of the re-isolated strains from seedlings produced IAA less than 15 μg mL−1 and ACC deaminase with OD more than 0.3 (Table 3). The results show that IAA production and ACC deaminase activity may be main factors in colonizing rice seedlings roots compared to other PGP traits (siderophore production and phosphate solubilization) tested in this study. In general, the total number of the isolates producing five PGP traits recovered from rice plantlets also decreased in the order; IAA producing > ACC deaminase producing > siderophore producing > phosphate solubilizing > HCN producing (Table 3). For confirming the probable role of the isolates in colonizing the rice seedlings by utilizing ACC as a nutrient substance under studied conditions, we selected the isolate REN1 (the highest in ACC deaminase production) for further study (Table 3).
Table 3.
Plant growth promoting activities of twelve rice endophytic strains re-isolated from the root interior of rice seedlings grown in gnotobiotic tube culture in Hoagland liquid
| Bacterial isolates | IAA production (μg mL−1) | Siderophore production | ACC deaminase activity (OD 540 nm) | Phosphate solubilization | HCN production |
|---|---|---|---|---|---|
| REN1 | 13.28 ± 0.3 | ++ | 0.73 ± 0.03 | − | + |
| REN10 | 13.19 ± 0.2 | + | 0.43 ± 0.05 | − | − |
| REN11 | 12.72 ± 1.8 | +++ | 0.52 ± 0.06 | − | − |
| REN13 | 16.06 ± 0.3 | +++ | 0.46 ± 0.04 | − | − |
| REN21 | 11.65 ± 0.7 | − | 0.51 ± 0.02 | − | − |
| REN42 | 14.60 ± 0.5 | ++ | 0.43 ± 0.02 | + | − |
| REN50 | 17.86 ± 0.3 | − | 0.65 ± 0.01 | − | − |
| REN52 | 10.88 ± 0.2 | − | 0.53 ± 0.04 | + | − |
| REN33 | 14.04 ± 0.8 | − | 0.49 ± 0.05 | − | − |
| REN14 | 12.34 ± 0.3 | ++ | 0.42 ± 0.03 | − | − |
| REN19 | 13.32 ± 0.6 | − | 0.54 ± 0.02 | − | − |
| REN44 | 8.69 ± 0.3 | − | 0.61 ± 0.01 | − | − |
± shows standard deviation. The presence of an activity is indicated by + and the absence is indicated by - . Symbols: +, 1 to 3 mm; ++, 4 to 6 mm; +++, more than 6 mm diameter of halo on agar plates
16S rDNA sequence analysis and morphological and biochemical features of isolate REN1
The best bacterial isolate REN1 was identified, based on its ability to produce ACC deaminase. The strain REN1 is a gram-negative, catalase-positive, oxidase positive, motile rod-shaped bacterium, fluorescent pigment positive, starch hydrolysis negative and gelatin liquefaction positive. Color of the colony and type of growth were yellowish and fast, respectively. Databases in GenBank were searched for sequences similar to the 16S rDNA gene sequence. The 16S rDNA sequence of the isolate, REN1 revealed that the endophyte is closely related to Pseudomonas fluorescens. The 16S rDNA gene sequences (974 bp) of the strain REN1 showed 99 % similarity with P. fluorescens IAM12022 (accession number NR043420) by nucleotide Basic Local Alignment Search Tool (BLAST) program at the National Center for Biotechnology Information (NCBI) BLAST server (http://www.ncbi.nlm.nih.gov/BLAST). A neighbour-joining dendrogram was generated using the sequence from REN1 (974 bp) and representative sequences from databases. Phylogenetic tree showed that the strain is closely related to P. fluorescens IAM12022 (Fig. 1). The nucleotide sequence determined in this work has been deposited in GenBank database with accession number KF731833.
Fig. 1.
Phylogenetic tree constructed using 16S rDNA gene sequences, available in the GenBank database, employing the Neighbour-joining method. Bootstrap values based on 1,000 replications were listed as percentages at the nodes. The scale bar indicates genetic distance. The GenBank accession number is given in parentheses for each organism
Colonization of rice seedlings by gusA-marked P. fluorescens REN1
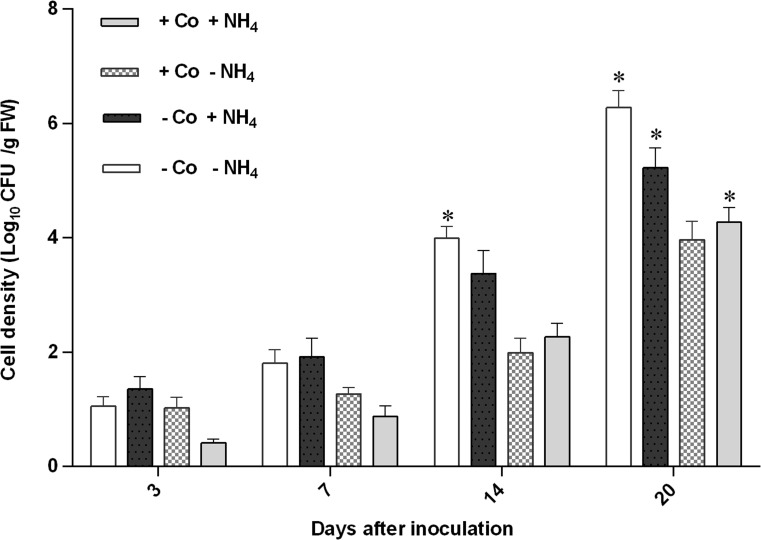
To determine whether the endophyte (isolate REN1) also has the ability to colonize and persist in rice seedings under constant flooded conditions in addition to ability to use ACC, we carried out studies with a gnotobiotic system, using different treatments. In addition, the experiment was conducted to test the hypothesis that rice seedlings under stressful conditions may preferentially select ACC deaminase-producing endophytic bacterial population compared to ACC deaminase non-producing endophytes. This isolate had the utilization ability of both ACC and (NH4)2 SO4 as N source and its growth rate on ACC and (NH4)2 SO4 was closely the same (data not shown). This isolate colonized interior roots of rice, as suggested by re-isolation of the inoculated strain from different treatments (Fig. 2). The population of the isolate in roots increased up to 20 days after inoculation (DAI). The population density of this strain re-isolated was different at different treatments ranging between 0.4 and 6 log10 CFU g−1 root (FW). Initially, population densities of bacteria within roots in different treatments were very low (i.e., <log10 2.0 CFU g−1 root FW). However, after 7 DAI, population densities within roots increased significantly (log10 2–6 CFU/g root FW) and significant treatment differences were found (p < 0.05) (Fig. 2). The higher population size of bacteria were re-isolated from inside roots detected on MinA agar medium, without cobalt (Co) as a chemical inhibitor of ethylene biosynthesis (−Co) and N source (−NH+4). No bacteria could be isolated from the control plants under the test conditions. Uninoculated control plants did not show any blue coloration with X-Gluc. Throughout the study, both control and inoculated plants were without any visible disease symptoms.
Fig. 2.
Population levels of P. fluorescens REN1 marked with gusA colonizing internal parts of roots (endophytic) of the rice seedlings (Cv, Gohar) grown in tube culture 3, 7, 14 and 20 days after inoculation in Hoagland liquid media under gnotobiotic conditions. Legend (treatments) shows: NH4, 5 mM (NH4)2SO4; Co, 2 μM CoCl2. (+) and (−) stand for presence and absence of treatments in sterile nutrient solution respectively. Initial bacterial concentration was 108 cell mL−1. Error bars indicate standard error of the mean. *; Significant at P <0.05
Root length promotion
The effects of strain REN1 on root elongation of rice seedlings in the presence of different treatments are shown in Fig. 3. Following addition of 2 μM Co to the Hoagland’s plant growth medium, the root elongation of uninoculated seedlings increased by 31 % in comparison with control (without Co and strain) (P < 0.05; Tukey’s test). Inoculation with strain REN1 in the absence of Co also significantly increased the root length of rice seedlings by 43 % as compared with control (strain and Co-untreated plants). The maximum root length-promoting effect on rice seedlings was observed after inoculation with strain REN1, in the absence of Co with NH+4 (+P−Co+NH+4). Treatments with chemical inhibitor of ethylene biosynthesis (+Co) and with NH+4 as N source also stimulated root elongation to a similar extent as treatments with chemical inhibitor of ethylene biosynthesis (+Co) and without NH+4. There was no significant difference between these treatments (Fig. 3). No contamination of roots with extraneous microorganisms was detected. Stimulation of root elongation by the isolate was more pronounced with Co-untreated plants as compared to Co-treated plants. The root elongation of Co-treated seedlings was not significantly affected by NH+4. However, the root elongation of seedlings treated with NH+4 increased compared to that of untreated with NH+4. Seedlings inoculation of rice by the individual isolate REN1 had a considerable impact on different growth parameters (data not shown) including root elongation that was positively correlated with ACC deaminase activity and IAA production.
Fig. 3.
Effect of P. fluorescence strain REN1 marked with gusA and chemical ethylene inhibitor (Co) on Rice cv. Gohar root elongation. Treatments: P, P. fluorescence strain REN1; NH4, 5 mM (NH4)2SO4; Co, 2 μM CoCl2. (+) and (−) stand for presence and absence of treatments in sterile nutrient solution respectively. Initial bacterial concentration was 108 cell mL−1. Vertical bars represent the standard error. The data are means of at least two experiments for each treatment. Values followed by different letters are significantly different (P < 0.05; Tukey’s test)
Discussion
This study provided an initial assessment of the potential of endophytic bacteria associated with rice plants within the studied area in Iran to evaluate PGP traits and the probable role of ACC deaminase- producing isolates in colonizing rice seedling roots under controlled conditions. Among all PGP traits evaluated in this study, IAA production and ACC deaminase activity appeared to be the most promising PGP traits in endophytic isolates. The use of beneficial bacteria as agricultural inputs for increasing crop production needs the selection of competent PGPR with plant growth promoting traits. We performed a colonization assay to find competent endophytes, since colonization is essential for PGP effects produced by bacteria and the interaction of them with plants (Compant et al. 2011). Rice is grown under constant flooded conditions in Iran. Therefore, we assayed this test under constant flooding conditions. A number of studies on PGPR have shown the growth promotion of plant but only under controlled conditions (Glick et al. 1995; Shaharoona et al. 2006) where these bacteria do not compete with the normal array of microbes. For finding competent isolates in this study, we inoculated all 80 isolates as mixtures of isolates on rice seedling, because competition should possibly imitate the situation in raw soil, which contains approximately 108 bacteria g−1 (Lugtenberg and Dekkers 1999). Twelve out of 80 inoculated isolates colonized within the roots of rice seedlings under axenic conditions. Since aseptic techniques were used throughout, and the surface-sterilization procedure was effective, we may confirm that the same isolates inoculated to sterile rice seedlings could be re-isolated from these seedlings (to fulfill Koch’s postulate). The results of re-screening the isolates (12 isolates) for PGP traits showed the isolates with high ability in the production of ACC deaminase were able to colonize rice roots than other isolates (Table 3). In accordance with previous studies, our results confirm the wide distribution of ACC deaminase activity in bacteria (Shah et al. 1998). It may be suggested that, rice plant may shape its own associated microbial community and act as filters for endophyte communities with ACC deaminase activity under flooded conditions ameliorate the probable production of ethylene. For verifying these results, we studied the effects of Co as a chemical inhibitor of ethylene biosynthesis, a superior isolate (REN1) in production ACC deaminase and NH4 as an N source on colonization size and elongation of rice roots under the same conditions done for 80 endophytic isolates. The results of this study showed that inoculation with the selected isolate increased the root length and colonization of rice seedlings, in comparison to uninoculated control (Figs. 2 and 3). The higher population size of isolate in treatments without Co and NH4 may be due to the higher levels of ethylene in the rhizosphere and plant under stress conditions (like our conditions). In higher plants, ethylene is produced from ACC (Yang and Hoffman 1984). ACC deaminase-producing isolate REN1 may hydrolyze ACC, the immediate precursor of the plant hormone ethylene, by ACC deaminase to NH3 and α-ketobutyrate (Penrose et al. 2001). The isolate uses the NH+4 evolved from ACC as a source of N and thereby decrease ACC within the plant (Penrose and Glick 2001) with the accompanying reduction of plant ethylene (Belimov et al. 2001; Grichko and Glick 2001). Previous studies showed addition of ACC reduced the endophytic colonization of parental Medicago sativa by K. pneumoniae 342 (Iniguez et al. 2005). It is seemed that N source as NH+4 was more preferable for this strain than ACC. In other words, ACC deminase-producing strain REN1 has not used ACC, since N source as NH+4 existed in culture medium (Hoagland solution). The population size of the isolate in treatment without Co and with NH+4 was lower than treatment without Co and NH+4 (−CO−NH+4). Because this strain could use NH+4 present in culture medium (Hoagland solution) and few has entered rice roots to use ACC. In addition, under nitrogen-limiting conditions, the production of stress ethylene is increased and plants will need to attract more bacteria to decrease this ethylene. In this study, strain REN1 as a PGPR was able to increase root length in comparison to uninoculated plant in all treatments (Fig. 3). Chemical inhibitor Co also increased root elongation in comparison to Co-untreated and strain uninoculated plants in this study. These results are in agreement with the findings of Belimov et al. (2007). Treatments without Co enhanced the root length of seedlings than Co-treated plants and treatments with NH+4 showed more increased in root length than NH+4-untreated plant in both root elongation and colonization assay. Because nitrogen can alter the physiological state of the plant, and this subsequently affect its association with the bacterial population (Prakamhang et al. 2009). Nitrogen also plays a role in plant root system and can increase root length. Plants that are inoculated with rhizobacteria having ACC-deaminase are more resistant to the damaging effects of stress ethylene that is produced as a result of stressed environments such as flooding (Grichko and Glick 2001), drought (Zahir et al. 2007) and high salt concentration (Kausar and Shahzad 2006; Nadeem et al. 2007). In the present work, higher population size of endophytic ACC deaminase and IAA-producing bacteria were recovered from roots. It has been postulated that enough buildup of ACC deaminase-possessing bacterial populations in plant root establish a sink for ACC thereby lowering the endogenous ethylene levels resulting in stress tolerance and enhanced root elongation (Shah et al. 1998). These results indicate that ethylene is a key regulator of the colonization of plant tissue by bacteria and that this regulation is most likely mediated by its effect on the plant signaling pathways. In addition, the regularly occurring flooding conditions in Iranian rice fields may have given rise to rice root communities that are rich in ACC deaminase-producing isolates. Therefore, preferential selection by rice plants of bacteria with high ACC-deaminase activity (instead of those with low or no activity) could confer benefits to the plant. In addition, IAA and ACC deaminase production is being deployed as tools for identification and screening of endophytes (Khalid et al. 2005; Shaharoona et al. 2006). We identified the best ACC deaminase-producing isolate as Pseudomonas fluorescens. The isolate REN1 exhibited multiple PGP activities including production of IAA, siderophore, phosphate solubilization and HCN. It is seemed the utilization ability of ACC was the most mechanism involved in root elongation and endophytic colonization by this isolate. These results are in agreement with the findings of other researchers (Glick et al. 1995; Mayak et al. 2004; Shaharoona et al. 2006). A direct correlation has been found between in vitro bacterial ACC-deaminase activity and root growth (Mayak et al. 2004; Shaharoona et al. 2006). P. fluorescens strain REN1 could well promote rice growth and probably by lowering the ACC deaminase activity affected its colonization in rice seedlings. By contrary, Belimov et al. (2007) showed lowering the ACC deaminase activity by Pseudomonas brassicacearum strain Am3 did not have any effect colonization. This results shows interaction between bacteria and plant is very complex and sometimes the effect of a particular bacterium may vary as its growth conditions change (Lynch 1990). The results obtained here confirm previous conclusions about the importance of bacterial ACC deaminase in plant growth promotion (Glick et al. 1998). For our knowledge, this is the first report showing utilization of ACC by bacteria in more colonization of rice seedlings under constant flooded conditions.
Conclusions
It is concluded that the presence of ACC-deaminase enzyme activity could be a useful tool for screening effective endophyes to promote seedling growth of rice under controlled conditions before testing their effectiveness under natural environment. The root length-promoting effect of the selected P. fluorescens REN1 is most probably due to its ability to act as a sink for ACC. The results suggest that ACC deaminase-producing isolates, particularly strain REN1, offers promise as a bacterial inoculant for improvement of root growth of rice plants in the presence of constant flooded conditions. Although the P. fluorescens REN1 showed significant increase in root length and endophytic colonization, further studies will be needed to evaluate the efficacy of this strain on rice plants under field conditions before it can be considered for agricultural practices.
Acknowledgments
The authors wish to thank the head of the Department of Soil Science, University of Tehran, Iran, and Iran National Science Foundation (INSF) for providing the necessary facilities for this study. We also would like to thank Prof. Dr. Beattie and Dr. Chen and Liang, Department of Plant Pathology and Microbiology, Iowa State University, for valuable helps and guidances in doing some parts of the manuscript.
References
- Ahmad F, Ahmad I, Khan MS. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res. 2008;163:173–181. doi: 10.1016/j.micres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Belimov A, Safronova V, Sergeyeva T, Egorova T, Matveyeva V, Tsyganov V, Borisov A, Tikhonovich I, Kluge C, Preisfeld A, Dietz K, Stepanok V. Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol. 2001;47:642–652. doi: 10.1139/w01-062. [DOI] [PubMed] [Google Scholar]
- Belimov AA, Safronova VI, Mimura T. Response of spring rape to inoculation with plant growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase depends on nutrient status of the plant. Can J Microbiol. 2002;48:189–199. doi: 10.1139/w02-007. [DOI] [PubMed] [Google Scholar]
- Belimov AA, Dodd IC, Safronova VI, Hontzeas N, Davies WJ. Pseudomonas brassicacearum strain Am3 containing 1-aminocyclopropane-1-carboxylate deaminase can show both pathogenic and growth-promoting properties in its interaction with tomato. J Exp Bot. 2007;58:1485–1495. doi: 10.1093/jxb/erm010. [DOI] [PubMed] [Google Scholar]
- Burd GI, Dixon DG, Glick BR. Plant growthpromoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol. 2000;4:237–245. doi: 10.1139/w99-143. [DOI] [PubMed] [Google Scholar]
- Chen C, Malek AA, Wargo MJ, Hogan DA, Beattie GA. The ATP-binding cassette transporter Cbc (choline/betaine/carnitine) recruits multiple substrate-binding proteins with strong specificityfor distinct quaternary ammonium compounds. Mol Microbiol. 2010;75:29–45. doi: 10.1111/j.1365-2958.2009.06962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compant S, Mitter B, Colli-Mull JG, Gangl H, Sessitsch A. Endophytes of grapevine flowers, berries, and seeds: identification of cultivable bacteria, comparison with other plant parts, and visualization of niches of colonization. Microb Ecol. 2011;62:188–197. doi: 10.1007/s00248-011-9883-y. [DOI] [PubMed] [Google Scholar]
- Dimkpa C, Svatos A, Dabrowska P, Schmidt A, Boland W, Kothe E. Involvement of siderophores in reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere. 2008;74:19–25. doi: 10.1016/j.chemosphere.2008.09.079. [DOI] [PubMed] [Google Scholar]
- Ditta G, Stanfield S, Corbin D, Helinski DR. Broad host range DNA cloning system for Gram-negative bacteria–construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980;77:7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etesami H, Mirsyed Hosseini H, Alikhani HA, Mohammadi L (2014) Bacterial biosynthesis of 1-aminocyclopropane-1-carboxylate (ACC) deaminase and indole-3-acetic acid (IAA) as endophytic preferential selection traits by rice plant seedlings. J Plant Growth Regul:1–17. doi:10.1007/s00344-014-9415-3
- Gerhardt P, Murray RGE, Costilow RN, Wester EW. Methods for general bacteriology. New York: Amer Soc Microbiol; 1994. [Google Scholar]
- Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 2013;169(1):30–3915. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Glick BR, Karaturovic DM, Newell PC. A novel procedure for rapid isolation of plant growth promoting Pseudomonads. Can J Microbiol. 1995;41:533–536. doi: 10.1139/m95-070. [DOI] [Google Scholar]
- Glick BR, Penrose DM, Li J. A model for lowering plant ethylene concentrations by plant growth promoting rhizobacteria. J Theor Biol. 1998;190:63–68. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- Glickmann E, Dessaux Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61:793–796. doi: 10.1128/aem.61.2.793-796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichko VP, Glick BR. Amelioration of flooding stress by ACC-deaminase containing plant growth promoting bacteria. Plant Physiol Biochem. 2001;39:11–17. doi: 10.1016/S0981-9428(00)01212-2. [DOI] [Google Scholar]
- Iniguez AL, Dong Y, Carter HD, Ahmer BMM, Stone JM, Triplett EW. Regulation of enteric endophytic bacterial colonization by plant defenses. Mol Plant-Microbe Interact. 2005;18:169–178. doi: 10.1094/MPMI-18-0169. [DOI] [PubMed] [Google Scholar]
- Kaga H, Mano H, Tanaka F, Watanabe A, Kaneko S. Rice seeds as sources of endophytic bacteria. Microbes Environ. 2009;24:154–162. doi: 10.1264/jsme2.ME09113. [DOI] [PubMed] [Google Scholar]
- Kausar R, Shahzad SM. Effect of ACC-deaminase containing rhizobacteria on growth promotion of maize under salinity stress. J Agric Soc Res. 2006;2:216–218. [Google Scholar]
- Kennedy IR, Choudhury ATMA, Kecskes ML. Non-symbiotic bacterial diazotrophs in crop-farming system: can their potential for plant growth promotion be better exploited? Soil Biol Biochem. 2004;36:1229–1244. doi: 10.1016/j.soilbio.2004.04.006. [DOI] [Google Scholar]
- Khalid A, Arshad M, Zahir ZA. Screening plant growth promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol. 2004;96:473–480. doi: 10.1046/j.1365-2672.2003.02161.x. [DOI] [PubMed] [Google Scholar]
- Khalid A, Arshad M, Zahir Z. Screening plant growth promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol. 2005;96:473–480. doi: 10.1046/j.1365-2672.2003.02161.x. [DOI] [PubMed] [Google Scholar]
- Laslo E, György E, Mara G, Tamás E. Screening of plant growth promoting rhizobacteria as potential microbial inoculants. Crop Prot. 2012;40:43–48. doi: 10.1016/j.cropro.2012.05.002. [DOI] [Google Scholar]
- Lorck H. Production of hydrocyanic acid by bacteria. Physiol Plant. 1948;1:142–146. doi: 10.1111/j.1399-3054.1948.tb07118.x. [DOI] [Google Scholar]
- Lugtenberg BJJ, Dekkers LC. What makes Pseudomonas bacteria rhizosphere competent? Environ Microbiol. 1999;1(1):9–13. doi: 10.1046/j.1462-2920.1999.00005.x. [DOI] [PubMed] [Google Scholar]
- Lynch JM. The rhizosphere. Chichester: Wiley-Interscience; 1990. [Google Scholar]
- Malhotra M, Srivastava S. Stress-responsive indole-3-acetic acid biosynthesis by Azospirillum brasilense SM and its ability to modulate plant growth. Eur J Soil Biol. 2009;45:73–80. doi: 10.1016/j.ejsobi.2008.05.006. [DOI] [Google Scholar]
- Mayak S, Tirosh T, Glick BR. Plant growth promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem. 2004;42:565–572. doi: 10.1016/j.plaphy.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Miller JH. Experiments in molecular genetics. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- Nadeem SM, Zahir ZA, Naveed M, Arshad M. Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC-deaminase activity. Can J Microbiol. 2007;53:1141–1149. doi: 10.1139/W07-081. [DOI] [PubMed] [Google Scholar]
- Pedraza RO, Ramirez-Marta A, Xiqui ML, Baca BE. Aromatic amino acid aminotransferase activity and indole-3-acetic acid production by associative nitrogen-fixing bacteria. FEMS Microbiol Lett. 2004;233:15–21. doi: 10.1016/j.femsle.2004.01.047. [DOI] [PubMed] [Google Scholar]
- Penrose DM, Glick BR. Levels of ACC and related compounds in exudate and extracts of canola seeds treated with ACC deaminase-containing plant growth-promoting bacteria. Can J Microbiol. 2001;47(4):368–372. doi: 10.1139/w01-014. [DOI] [PubMed] [Google Scholar]
- Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase-containing plant growth promoting rhizobacteria. Physiol Plant. 2003;118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- Penrose DM, Moffatt BA, Glick BR. Determination of 1- aminocyclopropane-1-carboxylic acid (ACC) to assess the effects of ACC deaminase-containing bacteria on roots of canola seedlings. Can J Microbiol. 2001;47:77–80. doi: 10.1139/w00-128. [DOI] [PubMed] [Google Scholar]
- Pikovskaya RI. Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiologia. 1948;17:362–370. [Google Scholar]
- Prakamhang J, Minamisawa K, Teamtaisong K, Boonkerd N, Teaumroong N. The communities of endophytic diazotrophic bacteria in cultivated rice (Oryza sativa L.) Appl Soil Ecol. 2009;142(2):141–149. doi: 10.1016/j.apsoil.2009.02.008. [DOI] [Google Scholar]
- Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil. 2009;321:305–339. doi: 10.1007/s11104-009-9895-2. [DOI] [Google Scholar]
- Safronova VI, Stepanok VV, Engqvist GL, Alekseyev YV, Belimov AA. Root-associated bacteria containing 1-aminocyclopropane-1-carboxylate deaminase improve growth and nutrient uptake by pea genotypes cultivated in cadmium supplemented soil. Biol Fertil Soils. 2006;42:267–272. doi: 10.1007/s00374-005-0024-y. [DOI] [Google Scholar]
- Sandhu A, Halverson LJ, Beattie GA. Identification and genetic characterization of phenol-degrading bacteria from leaf microbial communities. Microb Ecol. 2009;57:276–285. doi: 10.1007/s00248-008-9473-9. [DOI] [PubMed] [Google Scholar]
- Schwyn B, Neilands J. Universal chemical assays for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Shah S, Li J, Moffatt BA, Glick BR. Isolation and characterization of ACC deaminase genes from two different plant growth-promoting rhizobacteria. Can J Microbiol. 1998;44:833–843. doi: 10.1139/w98-074. [DOI] [PubMed] [Google Scholar]
- Shaharoona B, Arshad M, Zahir Z. Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.) Lett Appl Microbiol. 2006;42:155–159. doi: 10.1111/j.1472-765X.2005.01827.x. [DOI] [PubMed] [Google Scholar]
- Wang C, Knill E, Glick BR, Defago G. Effect of transferring 1-amino-cyclopropane-1-carboxylic acid (ACC) deaminase genes into Pseudomonas fluorescens strain CHA0 and its gacA derivative CHA96 on their growth-promoting and disease-suppressive capacities. Can J Microbiol. 2000;46:898–907. doi: 10.1139/cjm-46-10-898. [DOI] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. doi: 10.1146/annurev.pp.35.060184.001103. [DOI] [Google Scholar]
- Yanni YG, Rizk RY, Corich V, Squartini A, Ninke K, Philip-Hollingsworth S, Orgambide G, deBruijn F, Stoltzfus J, Buckley D, Schmidt TM, Mateos PF, Ladha JK, Dazzo FB. Natural endophytic association between Rhizobium leguminosarum bv. trifolii and rice roots and assessment of its potential to promote rice growth. Plant Soil. 1997;194:99–114. doi: 10.1023/A:1004269902246. [DOI] [Google Scholar]
- Zahir ZA, Munir A, Asghar HN, Shaharoona B, Arshad M. Effectiveness of rhizobacteria containing ACC-deaminase for growth promotion of pea (Pisum sativum) under drought conditions. J Microbiol Biotechnol. 2007;18:958–963. [PubMed] [Google Scholar]