Abstract
In the present study, we developed an efficient protocol for in vitro plant regeneration and genetically transformed root induction in medicinal plant Artemisia aucheri Boiss. Leaf explants were cultivated in MS medium supplemented by combination of plant growth regulators including α-naphthalene-acetic acid, 6-benzyl-aminopurine, indole-3-acetic acid and 2, 4-dichlorophenoxyaceticacid. The highest frequency of shoot organogenesis occurred on MS medium supplemented with 0.05 mg/l NAA plus 2 mg/l BA (96.3 %) and MS medium supplemented with 0.5 mg/l IAA plus 2 mg/l BA (88.3 %). Root induction was obtained on MS medium supplemented with 0.5 mg/l IBA. This is a simple, reliable, rapid and high efficient regeneration system for A. aucheri Boiss in short period via adventitious shoot induction approach. Also, an efficient genetically transformed root induction for A. aucheri was developed through Agrobacterium rhizogenes-mediated transformation by four bacterial strains, A4, ATCC15834, MSU440, and A13 (MAFF-02-10266). The maximum frequency of hairy root induction was obtained using MSU440 (93 %) and ATCC15834 (89 %) bacterial strains. Hairy root lines were confirmed by PCR using the rolB gene specific primers and Southern blot analysis.
Keywords: Agrobacterium rhizogenes, Artemisia aucheri, Genetic transformation, Regeneration
Introduction
The genus Artemisia (belonging to Astraceae family) includes 34 species which are found wild all over Iran (Hashemi et al. 2007). It has been shown that some Asteraceae species exert their anti-tumor activity due to the presence of flavonoids, sesquiterpene lactones, lignans, acetylenes, triterpenes or glycolipids (Réthy et al. 2006). Artemisinin is an effective antimalarial drug and anticancer agent mainly isolated from A. annua L. (Nadeem et al. 2013; Zanjani et al. 2012).
Artemisia aucheri Boiss (with the common Persian name of ‘Dermane Koohi’) grows on the high lands, mountain foot and high plateaus; It produces some bioactive compounds including verbenone, camphor, 1, 8-cineole, trans-verbenol, chrysanthenone, mesitylene, α-pinene, acyclic monoterpenes, and monoterpen ehydroperoxides (Hashemi et al. 2007; Rustaiyan et al. 1987). Fifty-four components have been identified in the essential oil of A.aucheri (Mahboubi and Bidgoli 2009). The cytotoxic effects of A.aucheri extraction in three human carcinoma cell lines including: MCF-7 (human breast cancer cells), SKNMC (human neuroblastoma) and A2780 (human ovarian cancer cells) was evaluated. It may be considered as a promising chemotherapeutic agent in cancer treatment (Ghazi-Khansaria et al. 2013). These plants have offered many substances that are claimed to be promising for the synthesis of new classes of anti leishmanial chemotherapeutic drugs (Sharif et al. 2006). It revealed that it has the potential of being used for wound healing. In recent years, there is more progress in the molecular regulation of secondary metabolites biosynthesis (Staniek et al. 2013; Siva et al. 2009, 2012). However, efforts in these studies are restricted by the lack of efficient protocols for genetic transformation. Developing transgenic plants with high accumulation of interested compounds can be achieved by introducing genes encoding enzymes regulating the biosynthetic pathway. For successful plant transformation, an efficient protocol for tissue culture and plant regeneration is necessary. There are some successful reports on plant regeneration of A. annua and A. sieberi (Banyai et al. 2010; Sharafi et al. 2014b) but there is no report on tissue culture and plant regeneration of A. aucheri.
Genetically transformed roots (so called hairy roots) mediated with Agrobacterium rhizogenes creates a rapid and simple tool to integrate and express foreign genes in plant cells (Georgiev et al. 2007; Sharafi et al. 2013a, 2014a; Huet et al. 2014). The molecular basis of hairy root induction is a transfer DNA (T-DNA) from root inducing (Ri) plasmid of A. rhizogenes. T-DNA integrated into the genome has rol gene loci (rol A, B, C) which have a major role in promoting phytochemical production in genetically transformed roots (Georgiev et al. 2012).
In this paper we describe a simple, efficient and reliable regeneration protocol for the medicinal plant A. aucheri as the first report. Also, the present study focused on development of an efficient protocol for induction of genetically transformed roots from A. aucheri by different strains of A. rhizogenes.
Materials and methods
Plant materials and tissue culture conditions
Seeds of A. aucheri were procured from Pakanbazr Company (Esfahan, Iran) and the seeds were surface-sterilized with 70 % (v/v) ethanol for 1 min and 3 % (v/v) sodium hypochlorite for 12 mins and then rinsed five times with sterilized water. The seeds were placed on agar solidified MS (Murashige and Skoog 1962) medium. The medium was adjusted to pH 5.8 before adding agar and then sterilized by autoclave. The seeds were incubated in a growth chamber under a 16/8-h (light/dark) photoperiod under cool-white light at 25 °C. The leaves from the 4 weeks old plants were cut and used as explant.
Tissue culture and regeneration media
The leaves from 4 weeks old plants were cut and cultured on solidified MS medium supplemented with different combination of BA, IAA, NAA and 2, 4-D as follows: BA (1, 2, 3 mg/l) plus IAA (0, 0.1, 0.5 mg/l); BA (0, 1, 2 mg/l) plus NAA (0.05, 0.1 mg/l); BA (0, 1, 2 mg/l) plus 2, 4-D (0.5, 1, 1.5 mg/l). The petri dishes were incubated in a growth chamber. Explants were transferred to fresh medium every 2 weeks. After callus induction, explants were transferred to MS medium without hormones or supplemented with 0.5 or 1 mg/l BA for adventitious shoot induction. For root induction, obtained shoots were cut and transferred to MS medium supplemented with 0.5 mg/l IBA. Finally, the obtained plantlets were acclimatized in greenhouse.
Preparation of A. rhizogenes strains
Four strains of A. rhizogenes were used: A4, ATCC15834, MSU440 and A13 (MAFF-02-10266). All the strains were provided by the bank of microbes at the National Institute of Genetic Engineering and Biotechnology in Tehran. The A. rhizogenes strains were cultured in liquid LB medium containing 50 mg/l rifampicin, pH 7.2 to an optical density of 0.6, at 28°C, 120 rpm on a shaker incubator. The bacteria were pelleted by centrifugation for 12 min at 3,500 rpm and re- suspended at a cell density of OD600 0.7 in inoculation medium which consisted ½ MS salts and vitamins along with 150 μM acetocyringone.
Co-cultivation and induction of hairy roots
Leaf explants from 4-week old A. aucheri seedlings were used for co-cultivation with different strains of A. rhizogenes. The explants were inoculated with different bacterial strains for 5 min. The explants were briefly dried on sterile filter paper and transferred to co-cultivation medium which was the same as the inoculation medium but solidified by agar. The explants transferred to MS media supplemented with 350 mg/l cefotaxime to eliminate the bacteria after 2 days of co-cultivation. Control explants were treated similarly but without inoculation with bacteria.
PCR analysis and Southern hybridization
Total genomic DNA was isolated from hairy root samples and control roots using genomic plant DNA extraction kit (iNtRON Biotech. Co.). Isolated genomic DNA was used in PCR analysis for detecting the rolB gene. The primers designed to amplify rolB were 5′ GCTCTTGCAGTGCTAGATTT′ (forward primer) and 5′ GAAGGTGCAAGCTACCTCTC3′ (reverse primer). PCR products were separated by electrophoresis on 0.8 % agarose gel in 0.5 × TBE buffer, stained with ethidium bromide and visualized under UV trans-illuminator.
Genomic DNA (10–15 μg) of hairy roots and control (normal root) were digested using XbaI restriction enzyme separated on 0.8 % agarose gel and subsequently transferred to a nylon membrane. Probe was designed on the basis of rolB sequence, using the Dig DNA labeling Kit (Roche Co., Germany). Hybridization, washing and detection were performed according to the instruction manual of the DIG labeling and detection system.
Statistical analysis
The experiments were laid on a completely randomized design (CRD) with 4 replications and 25 explants cultured in each petri dish. The data collected were subjected to analysis of variance test. The means were compared using Duncan’s multiple range tests at the 5 % probability level (P ≤ 0.05). The data expressed as percentage were subjected to arc sine (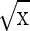 ) transformation before the statistical analysis.
) transformation before the statistical analysis.
Results and discussion
Tissue culture and regeneration of A. aucheri
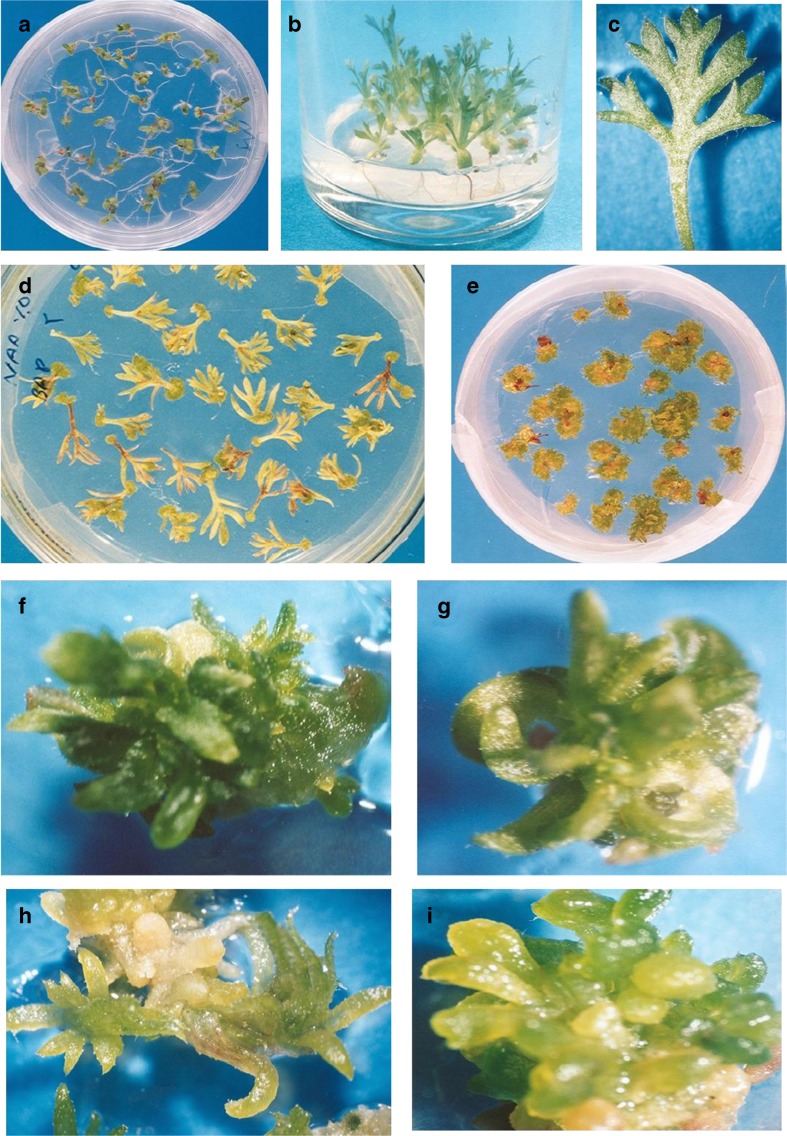
The seeds were germinated for 3 days (Fig. 1a). The leaves from 4 weeks old seedlings (Fig. 1b) were cut and used as explants (Fig. 1c) to culture on callus induction media. To avoid browning of explants were transferred to fresh medium every 2 weeks. Callus appeared on most of explants after two sub-cultures (Fig. 1d). Explants were transferred to MS medium without hormone or supplemented with 0.5 or 1 mg/l BA for shoot induction. In these media, regeneration appeared after 3 weeks (Fig. 1e). High frequency of shoot organogenesis was observed in MS medium containing 2 mg/l BA and 0.05 mg/l NAA (96.3 % regeneration) (Fig. 1f). Also, the calli originated from combination of 2 mg/l BA and 0.5 mg/l IAA showed high frequency of shoot induction (88.3 % regeneration) (Fig. 1g). A low rate of shoot organogenesis was observed in medium supplemented with 1 mg/l BA and 0.05 mg/l NAA and medium supplemented with combination of BA and 2, 4-D (Fig. 1h, i).
Fig. 1.
Adventitious shoot induction from Artemisia aucheri leaf explants; a Seed germination on MS medium; b Seedlings growing; c leaf explant; d callus induction from the leaf explants in MS medium containing 2 mg/l BA and 0.05 mg/l NAA; e adventitious shoots induction from calli in MS medium containing 2 mg/l BA and 0.05 mg/l NAA; f adventitious shoots induction in MS medium containing 2 mg/l BA and 0.05 mg/l NAA; g adventitious shoots induction in MS medium containing 2 mg/l BA and 0.5 mg/l IAA; h adventitious shoots induction in MS medium containing 1 mg/l BA and 0.05 mg/l NAA; i adventitious shoots induction in MS medium containing 2 mg/l BA and 0.5 mg/l 2, 4- D
The characters of shoot induction in present study were different from those in our study on A. annua (data not shown). While, callus and shoot organogenesis were induced from leaf stalks in A. aucheri (Fig. 1d), whole leaf blades produced callus and shoot organogenesis in A. annua. On the other hand, wounded leaf stalks of A. aucheri produced higher shoot induction in comparison with whole leaf blade.
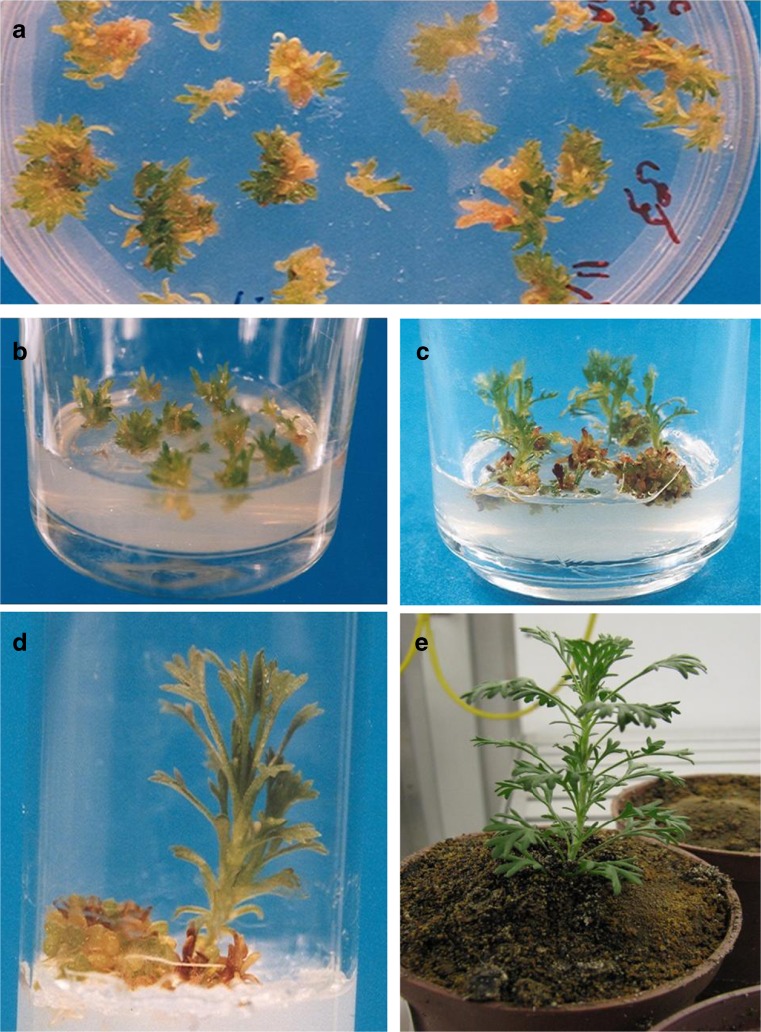
Obtained adventitious shoots (Fig. 2a) were transferred in MS medium supplemented with 0.5 mg/l GA3 for shoot elongation and avoiding vitrification (Fig. 2b). The elongated shoots were transferred to root induction medium containing 0.5 mg/l IBA for 10 days (Fig. 2c). The plantlets were transferred to MS medium without hormone for more growth (Fig. 2d). Finally, the plantlets were acclimatized in greenhouse (Fig. 2e).
Fig. 2.
Development of a regeneration system for Artemisia aucheri; a high frequency of shoot organogenesis from leaf explants; b transferring obtained adventitious shoots to MS medium for elongation; c adventitious shoots elongation; d root development following subculture on MS medium containing 0.5 mg/l IBA; e transferring regenerated plant to soil
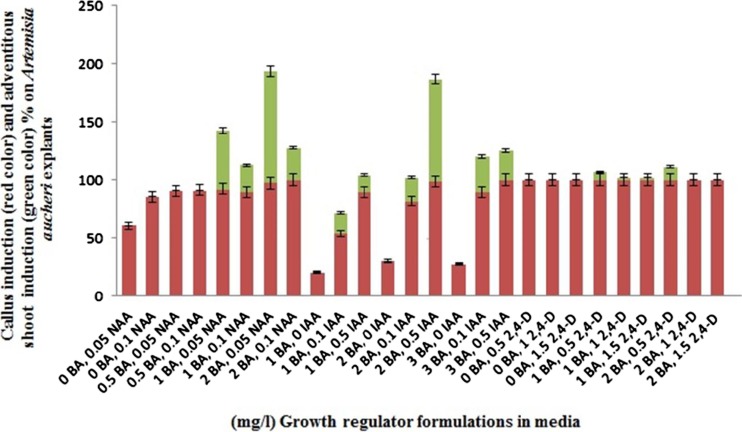
Media containing 2 mg/l BAP plus 0.05 mg/l NAA and 2 mg/l BAP plus 0.5 mg/l IAA showed the highest frequency of regeneration with 96.3 % and 88.3 % respectively (Fig. 3). Sharafi et al. (2014b) reported the highest frequency of shoot organogenesis from A. sieberi explants on MS medium supplemented with 2 mg/l BA plus 0.05 mg/l NAA. Liu et al. (2003) reported the stimulation of shoot induction by addition of BA in the Egyptian plant A. judaica L. Our results suggest that BA in combination with low concentrations of NAA or IAA is suitable for shoot organogenesis in A. aucheri Boiss.
Fig. 3.
Frequency of callus and adventitious shoot induction from A. aucheri leaf explants using different growth regulator formulations in MS media
This protocol is introduced as a simple and rapid tissue culture system for high frequency of A. aucheri Boiss regeneration in short period via adventitious shoot induction. In vitro regeneration can be used as a strong tool in conservation to produce high number of genetically alike plants (Sharafi et al. 2014b).
Hairy roots induction
Four different Agrobacterium rhizogenes strains: ATCC15834, A4, A13, MSU440 were evaluated for A. aucheri transformation. It is reported that macro elements in inoculation and co-cultivation media have inhibitory effects on A. rhizogenes mediated transformation, as the transformation efficiency was drastically increased by removing some major mineral components (Sharafi et al. 2013b, c); so, in the present study, ½ MS was used for inoculation and co- cultivation medium instead of full strength MS medium.
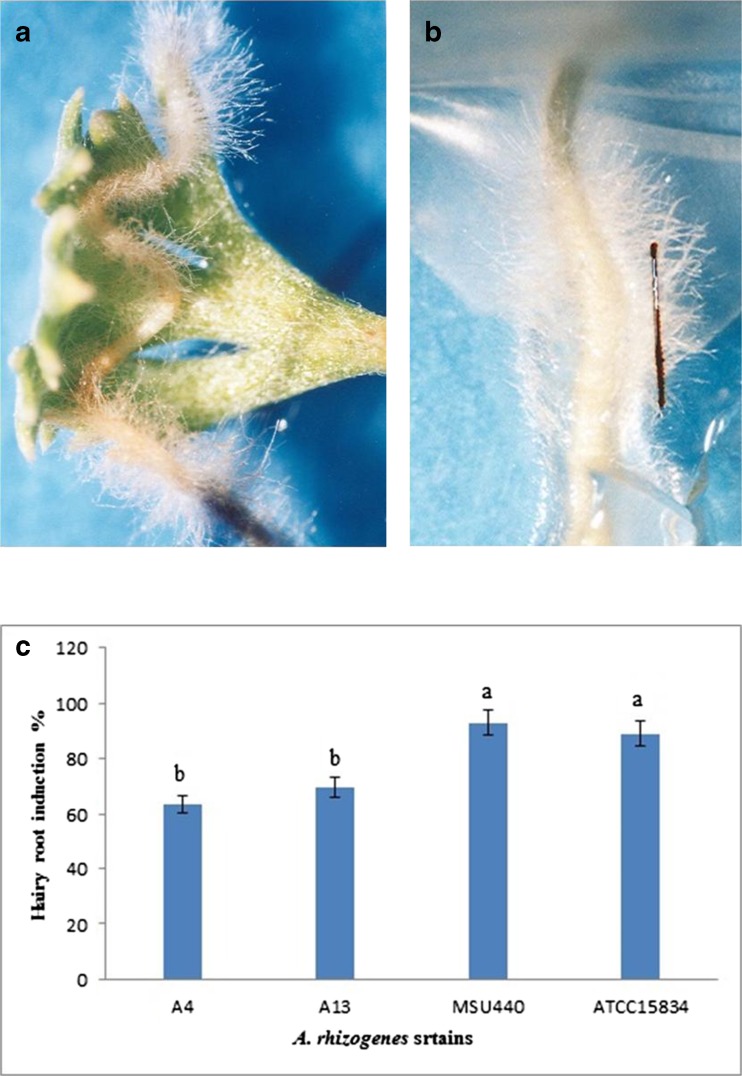
Hairy roots of A. aucheri were initiated from leaf explants after 2 to 3 weeks (Fig. 4a). They were cut and transferred to MS medium for more growth. As Nin et al. (1997) reported on A. absintium L., they were covered with numerous hairs and highly branched (Fig. 4b). A. aucheri leaves were susceptible to infection by all A. rhizogenes strains, as shown by the percentage of hairy roots emerged. Valimehr et al. (2014) reported that stem explants were the best explants for A. rhizogenes mediated transformation in Nepeta pogonosperma while leaf explants showed a low rate of hairy root induction. The highest rate of hairy root induction was obtained using strain ATCC15834 and MSU440 (Fig. 4c). All of the A. rhizogenes strains led to hairy root induction, but MSU440 and ATCC15834 were significantly effective than A4 and A13. It is revealed that the ability of A. rhizogenes to plant transformation is strain dependent (Sudha et al. 2012).
Fig. 4.
A. rhizogenes mediated transformation of A. aucheri; a hairy root induction on leaf explant after 3 weeks using strain ATCC 15834; b hairy root induction on leaf explant after 3 weeks using strain A4; c effect of different A. rhizogenes strains on frequency of hairy root induction in A. aucheri; The data were obtained as a mean of three replications. The different letters denote a statistically significant difference at P ≤ 0.05, as determined by Duncan’s multiple range test. Vertical lines represent standard errors
PCR and Southern bolting analysis
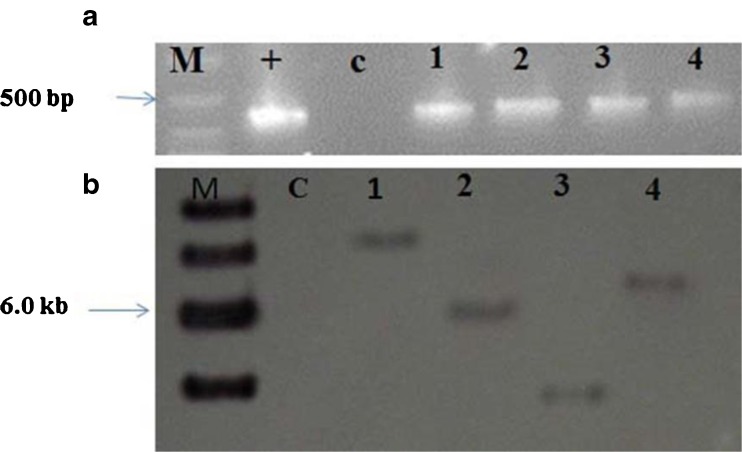
To investigate presence of the rolB gene transferred from Ri plasmid, PCR analysis was performed. PCR led to amplification of rolB gene in all transgenic hairy root lines. Fig. 5a shows PCR analysis for detection of the rolB gene in four obtained hairy root lines of A. aucheri. T-DNA copy number in transgenic hairy root lines was determined by Southern blot analysis on nylon membranes and the results showed that one T-DNA copy number was inserted into the tested lines, while no hybridization signal was observed in the untransformed root (Fig 5b). This protocol has a high potential to be used in genetic transformation of this important medicinal plant.
Fig. 5.
Molecular analysis of hairy roots; a PCR analysis for detection of the rolB gene in hairy root lines of A. aucheri; M: Molecular size marker (1 kb ladder); 1–4: hairy root lines, C: negative control (non-transformed root); +: positive control (Ri plasmid). b Southern blot analysis of hairy root lines (1–4) and an untransformed root (C); DNA samples were digested with XbaI and hybridized to rolB probe. Molecular size markers are indicated on the left
This study, for the first time, established a simple, rapid and reliable protocol for plant regeneration and hairy root induction of the medicinal plant A. aucheri. The use of fast-growing hairy roots could have immense potential in investigating the molecular regulation of genes encoding biosynthetic enzymes of important medicinal compounds in this plant.
Acknowledgments
This work was supported by Novin Giti Gene Biotech. Co. (NGene Biotech. Co.), Biotechnology Incubator Center of NIGEB, Tehran, Iran.
References
- Banyai W, Kirdmanee C, Mii M, Supaibulwatana K. Overexpression of farnesyl pyrophosphate synthase (FPS) gene affected artemisinin content and growth of Artemisia annua L. Plant Cell Tissue Organ Cult. 2010;103:255–265. doi: 10.1007/s11240-010-9775-8. [DOI] [Google Scholar]
- Georgiev M, Pavlov A, Bley T. Hairy root type plant in vitro systems as sources of bioactive substances. Appl Microbiol Biotechnol. 2007;74:1175–1185. doi: 10.1007/s00253-007-0856-5. [DOI] [PubMed] [Google Scholar]
- Georgiev MI, Agostini E, Ludwig-Mu J, Xu J. Genetically transformed roots: from plant disease to biotechnological resource. Trends Biotechnol. 2012;30:528–537. doi: 10.1016/j.tibtech.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Ghazi-Khansaria M, Mojarrabb M, Ahmadian F, Hosseinzadeh L. The antiproliferative effects of petroleum ether extract of Artemisia aucheri on human cancerous cell lines. J Rep Pharm Sci. 2013;2:61–66. [Google Scholar]
- Hashemi P, Abolghasemi MM, Fakhari AR, Ebrahimi SN, Ahmadi S. Hydrodistillation–Solvent Microextraction and GC–MS Identification of Volatile Components of Artemisia aucheri. Chromatographia. 2007;66:283–286. doi: 10.1365/s10337-007-0289-4. [DOI] [Google Scholar]
- Huet Y, Ekouna JP, Caron A, Mezreb K, Boitel-Conti M, Guerineau F. Production and secretion of a heterologous protein by turnip hairy roots with superiority over tobacco hairy roots. Biotechnol Lett. 2014;36:181–190. doi: 10.1007/s10529-013-1335-y. [DOI] [PubMed] [Google Scholar]
- Liu CZ, Murch SJ, EL-Demerdash M, Saxena PK. Regeneration of the Egyptian medicinal plant Artemisia judaica L. Plant Cell Rep. 2003;21:525–530. doi: 10.1007/s00299-002-0561-x. [DOI] [PubMed] [Google Scholar]
- Mahboubi M, Bidgoli FG (2009) Iran: Farhang Moaser. Biological activity of essential oil from aerial parts of Artemisia aucheri Boiss from Iran. Herba Polonica 55(4)
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. J Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nadeem M, Shinwari ZK, Qaiser M. Screening of folk remedies by genus Artemisia based on ethnomedicinal surveys and traditional knowledge of native communities of Pakistan. Pak J Bot. 2013;45(S1):111–117. [Google Scholar]
- Nin S, Bennici A, Roselli G, Mariotti D, Schiff S, Magherini R. Agrobacterium-mediated transformation of Artemisia absinthium L. (wormwood) and production of secondary metabolites. Plant Cell Rep. 1997;16:725–730. doi: 10.1007/s002990050310. [DOI] [PubMed] [Google Scholar]
- Réthy B, Kovàcs A, Zupk I, Forgo P, Vasas A, Falkay G, Hohmann J. Cytotoxic phenanthrenes from the rhizomes of Tamus communis. Planta Med. 2006;72:767–770. doi: 10.1055/s-2006-941505. [DOI] [PubMed] [Google Scholar]
- Rustaiyan A, Bamoniri A, Raffatrad M, Jakupovic J, Bohlman F. Eudesmanederivatives and highly oxygenatedmonoterpenes from Iranian Artemisia species. Phytochemistry. 1987;26:2307–2310. doi: 10.1016/S0031-9422(00)84708-1. [DOI] [Google Scholar]
- Sharafi A, Sohi HH, Mousavi A, Azadi P, Dehsara B, Khalifani BH. Increasing morphinan alkaloid production by over-expressing salutaridinol 7- o—acetyltransferase in Iranian poppy hairy roots. World J Microbiol Biotechnol. 2013;29:2125–2131. doi: 10.1007/s11274-013-1377-2. [DOI] [PubMed] [Google Scholar]
- Sharafi A, Sohi HH, Mousavi A, Azadi P, Khalifani BH, Razavi K. Metabolic engineering of morphinane alkaloids by over expression of Codeinine reductase in transgenic hairy root of Papaver bracteatum. Biotechnol Lett. 2013;35:445–453. doi: 10.1007/s10529-012-1080-7. [DOI] [PubMed] [Google Scholar]
- Sharafi A, Sohi HH, Mousavi A, Azadi P, Razavi K, Ntui VO. A reliable and efficient protocol for inducing hairy roots in Papaver bracteatum. Plant Cell Tissue Org Cult. 2013;113:1–9. doi: 10.1007/s11240-012-0246-2. [DOI] [Google Scholar]
- Sharafi A, Sohi HH, Azadi P, Sharafi AA. Hairy root induction and plant regeneration of medicinal plant Dracocephalum kotschyi. Physiol Mol Biol Plants. 2014 doi: 10.1007/s12298-013-0217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharafi A, Sohi HH, Sharafi AA, Azadi P (2014b) Tissue culture and regeneration of new antimalarial plant, Artemisia sieberi. Res J Pharmacogn
- Sharif M, Ziaei H, Azadbakht M, Daryani A, Ebadattalab A, Rostami M. Effect of methanolic extracts of Artemisia aucheri and Camellia sinensis on Leishmania major (in vitro) Turk J Med Sci. 2006;36(6):365–369. [Google Scholar]
- Siva R, Rajasekaran C, Mudgal Induction of somatic embryogenesis and organogenesis in Oldenlandia umbellata L., a dye yielding medicinal plant. J Plant Cell Tissue Org Cult. 2009;98:205–211. doi: 10.1007/s11240-009-9553-7. [DOI] [Google Scholar]
- Siva R, Mayes S, Behera SK, Rajasekaran C. Anthroquinones dye production using root cultures of Oldenlandia umbellata L. Ind Crop Prod. 2012;37(1):415–419. doi: 10.1016/j.indcrop.2011.12.027. [DOI] [Google Scholar]
- Staniek A, Bouwmeester H, Fraser PD, Kayser O, Martens S, Tissier A, Krol S, Wessjohann L, Warzecha H. Natural products—modifying metabolite pathways in plants. Biotechnol J. 2013 doi: 10.1002/biot.201300224. [DOI] [PubMed] [Google Scholar]
- Sudha CG, Sherina TV, Anand VP, Reji JV, Padmesh P, Sonia EV. Agrobacterium rhizogenes mediated transformation of the medicinal plant Decalepis arayalpathra and production of 2-hydroxy-4-methoxy benzaldehyde. Plant Cell Tissue Organ Cult. 2012 [Google Scholar]
- Valimehr S, Sanjarian F, Sohi HH, Sharafi A, Sabouni F. A reliable and efficient protocol for inducing genetically transformed roots in medicinal plant Nepeta pogonosperma. Physiol Mol Biol Plants. 2014 doi: 10.1007/s12298-014-0235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanjani KE, Rad ASH, Bitarafan Z, Aghdam AM, Taherkhani T, Khalili P (2012) Physiological response of sweet wormwood to salt stress under salicylic acid application and non-application conditions. Life Sci J 9(4): ISSN: 1097–8135