Abstract
In this study the effect of increasing temperature on photochemical efficiency of PS II in wheat plants has been studied on a hot summer day (9:00 AM (Control)–7:00 PM) by measuring Chl a fluorescence. Increasing temperature for a short period of time (2–4 h), in nature affects the efficiency of PS II complex reversibly and does not cause permanent damage to any of the components of photosystem II. A scheme has been provided to demonstrate the sequence and severity of events which get affected maximum by temperature stress.
Keywords: Chlorophyll a fluorescence, Increasing temperature stress, Wheat, Photosystem II
Introduction
Plants growing in their natural habitats are exposed to a complex of environmental factors. Studies have shown that the projected changes in climate will drastically reduce crop yields (Singh et al. 2010). Wheat is commercially one of the most important cereal crops, especially in tropical, subtropical, temperate countries. High temperature is one of the most important constraints for wheat growth and development (Gupta et al. 2013). Plant productivity is a result of many processes that modulate the photosynthetic machinery in order to maintain functional equilibrium (Tikkanen and Aro 2012). However, it is not only the availability of light but also the metabolic state of the plants that sets the requirements for the photosynthetic machinery.
Photosynthesis is one of the plant functions that is highly sensitive to increasing temperature and light intensity and is often inhibited before other cell functions are impaired. Increasing temperature brings damage to various aspects of photosynthetic functions, including both the photochemical reaction related to Photosystem I (PS I), Photosystem II (PS II) and the dark reactions. However, PS II is one of the most thermolabile components of the photosynthetic apparatus (Allakhverdiev et al. 2008; Chen and Cheng 2009). Heat stress inhibits photosynthetic electron transport activity as well the function of the oxidizing side of PS II resulting in decreased oxygen evolution. High temperature stress also results in an inactivation of PS II reaction centers (Zhao et al. 2008). Many studies have demonstrated that heat stress results in an increase of inactive PS II centers (Wen et al. 2005; Mathur et al. 2011).
Among the various screens used to probe the characteristic features of photosynthetic acclimation, use of chlorophyll (Chl) a fluorescence has been invaluable due to its non-destructive nature and high sensitivity. The JIP-test is a tool to analyze the polyphasic rise of the Chl a fluorescence transient (OJIP labeled phases) (Strasser et al. 2000). It has been found to be very sensitive to stress caused by changes in different environmental conditions, e.g. light intensity, temperature (Kalaji and Loboda 2007).
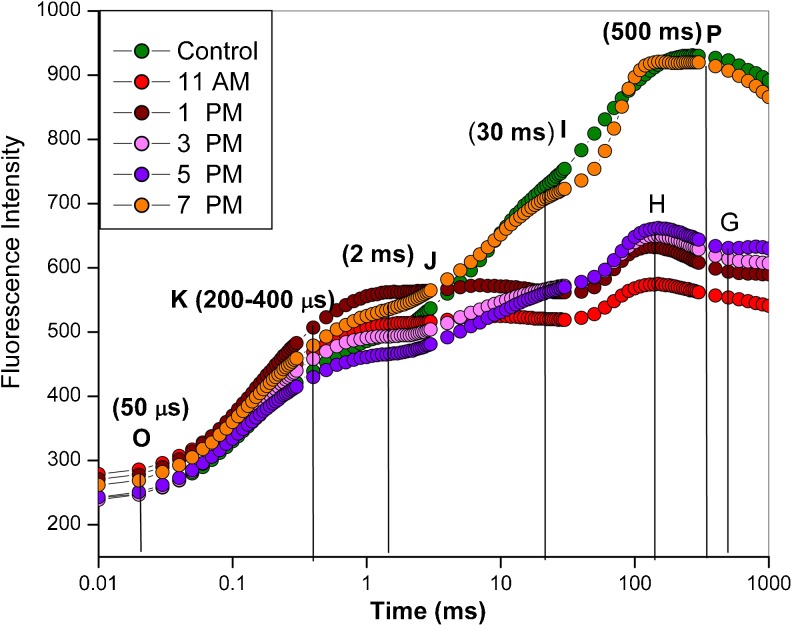
A typical Chl a induction curve is shown in Fig. 1. The plants exhibit a polyphasic rise called O-J-I-P fluorescence transient; the O to J phase (ends at ~2 ms), the J to I phase (ends at ~30 ms) and I to P phase (ends at ~500 ms). The shape of the O-J-I-P fluorescence rise has been related to a major change in the photosynthetic electron transport (Joly and Carpentier 2009; Papageorgiou and Govindjee 2011; Stirbet and Govindjee 2011). According to the recent view, O-J phase is related to the accumulation of QA¯ (Gauthier et al. 2010). The I-P amplitude in the transient has been related to the relative size of the pool of final PS I electron acceptors (Kalachanis and Manetas 2010).
Fig. 1.
The Chl a fluorescence induction curves in wheat leaves recorded at different times of the day
In this study, we have focused on the changes in the photochemical events experienced by a plant facing stress from morning till evening. The purpose is to evaluate efficiency and adaptability of crop plants to increasing temperature during a hot day and thus to assess the vitality of a plant in vivo. The sequence of the processes of photosynthesis getting damaged with increasing temperature in wheat plant has been elucidated.
Materials and method
Plant material, growth conditions and increasing temperature treatment
Lok-1 cultivar of wheat (Triticum aestivum) was used.
Wheat plants were grown under normal soil conditions in pots. Experiments were performed in 2nd and 3rd week of May at Indore (22° 44′ N) India when the temperature is maximum. The environmental conditions were very stable with temperature variations (±0.2 °C), humidity (±16 %). Plants were watered sufficiently to avoid any kind of water stress. 9 AM was considered as control and temperature at 9 AM was 33 °C, 41 °C at 11 AM, 43 °C at 1 PM, 40 °C at 3 PM, 37 °C at 5 PM and 30 °C at 7 PM. After every 2 h 10–15 recordings of Chl a fluorescence induction kinetics were made after dark adaptation (15 min). Measurements were performed 2 inches away from the tip and the base, i.e. in the middle portion of leaves. The whole set of experiment was performed 5–6 times.
Chlorophyll a fluorescence measurements
Chl a fluorescence was studied using plant efficiency analyzer (PEA, Hansatech, King’s Lynn, Norfolk, UK). Details regarding the measurements can be seen in Mathur et al. 2011.
Results and discussion
Chl a fluorescence induction kinetics was measured from 9 AM (control) to 7 PM in a hot summer day. In contrast to control (9:00 AM), a dramatic change in Chl a fluorescence was observed in stressed plants (Fig. 1). The K step (at 300-400 μs ), which is suggested to be characteristic of high temperature stress (Guisse et al. 1995; Srivastava et al. 1997; Strasser 1997; Mathur et al. 2011; Mathur et al. 2012), was found to be diminished in the present data. It was probably due to the fact that this work was done under natural conditions where the plant was getting sunlight continuously. This explanation finds support from earlier observations in which a K step is observed in leaves exposed to continuous illumination (Toth et al. 2011; Brestic et al. 2012). An additional H-G step was observed in the heat treated plants (Fig. 1). The P step measured at high intensity of excitation light splits into two steps (H and G) in the fluorescence curve (Tsimilli-Michael et al. 1998). The fluorescence decrease from H step to a dip between H and G steps is caused by a removal of limitation on the acceptor side of PS I (Ilik et al. 2006).
As the temperature increased, a noticeable difference was observed between control and stressed plants. The parameters that decreased at 11 AM and 1 PM were Fv/Fm (φpo), Fv/F0 (φpo/1-φpo), φRo, Ψo/1-Ψo (Appendix 1) (Table 1).
Table 1.
Various parameters of OJIP which decreased or increased at different time interval during a hot summer day. The temperature at 9 AM (Control) was (33 °C), 11 AM (41 °C), 1 PM (43 °C), 3 PM (40 °C), 5 PM (37 °C), 7 PM (30 °C)
| 9 AM (control) | 11 AM | 1 PM | 3PM | 5 PM | 7 PM | |
|---|---|---|---|---|---|---|
| ΦEo | 0.452 ± 0.004 | 0.106 ± 0.007 | 0.109 ± 0.008 | 0.238 ± 0.005 | 0.289 ± 0.005 | 0.406 ± 0.006 |
| Fv/Fm | 0.743 ± 0.001 | 0.576 ± 0.001 | 0.578 ± 0.005 | 0.626 ± 0.007 | 0.634 ± 0.001 | 0.717 ± 0.001 |
| Fv/F0 | 2.88 ± 0.002 | 1.359 ± 0.002 | 1.367 ± 0.004 | 1.676 ± 0.003 | 1.731 ± 0.007 | 2.529 ± 0.026 |
| ΦRo | 0.240 ± 0.01 | 0.097 ± 0.001 | 0.111 ± 0.008 | 0.119 ± 0.003 | 0.137 ± 0.001 | 0.230 ± 0.005 |
| Ψo/1-Ψo | 1.829 ± 0.001 | 0.309 ± 0.01 | 0.277 ± 0.01 | 0.731 ± 0.01 | 1.032 ± 0.00 | 1.503 ± 0.001 |
| Increased Parameter | ||||||
| F0 | 235 ± 2 | 280 ± 1 | 267 ± 2 | 247 ± 2 | 238 ± 1 | 235 ± 1 |
| M0 | 1.233 ± 0.003 | 2.077 ± 0.008 | 2.126 ± 0.002 | 1.712 ± 0.001 | 1.383 ± 0.016 | 1.056 ± 0.003 |
| ΦDo | 0.309 ± 0.001 | 0.496 ± 0.001 | 0.551 ± 0.001 | 0.435 ± 0.001 | 0.431 ± 0.001 | 0.322 ± 0.001 |
The declined ratio of Fv/Fm (φpo) indicated a decrease in the quantum efficiency of PS II photochemistry due to either a decrease in the rate of primary charge separation; or due to disconnection of some minor antenna from PS II (Briantais et al. 1996). In the noon time when the sunlight was maximum changes took place in the primary photochemistry of wheat which was evident through a decrease in the Fv/F0 (φpo/1-φpo). The value of φRo decreased at 11 AM (Table 1) which indicated that the quantum yield of electron transport decreased from QA─ to PS I end electron acceptor (Chen and Cheng 2009) with increasing temperature. Increased day temperature caused damage to PS II and decreased the conversion of excitation energy to electron transport indicated by a decreased ratio of Ψo/1-Ψo. A decrease was also observed in the linear electron transport rate (also expressed as probability that an absorbed photon moves an electron further than QA─) which was depicted by a decreased φEo value.
At 11 AM and 1 PM when the day temperature raised near to 43 °C, parameters like F0, M0, F0/Fm(φDo) (Appendix 1) (Table 1) increased as compared to the control. An increase in F0 (and a decrease in Fv/Fm) has been attributed to the physical separation of PS II RC from their associated pigment antenna resulting in blocked energy transfer of the PS II traps, although a part of this phenomenon could possibly reflect the accumulation of the reduced form of QA¯. In this study F0 was used as indicator for damage in PS II, associated to LHC II dissociation and blocking the electron transfer in the reductant side of PS II (Costa et al. 2002). M0 showed its maximal rate at 11 AM and 1 PM when QA¯ reoxidation is inhibited. F0/Fm (φDo) (Strasser and Tsimilli-Michael 2001; Christen et al. 2007) ratio was largely increased indicating that high temperature in the mid-day caused more energy dissipation and thus the quantum yield of non photochemical de-excitation also increased resulting in an increased ratio of F0/Fm. This is also in corroboration with an increased value of M0 and a decreased value of φEo.
Recovery
The processes which decreased during the noon (11 AM to 1 PM) started to recover slightly at 3 PM. The parameters like Fv/Fm, Fv/F0, φRo, increased slightly but could not reach the values near to control. The value of Ψo/1-Ψo also increased as compared to 11 AM and 1 PM but it could not reach upto control values.
With the onset of evening at 5 PM when the temperature was between 37 °C many parameters started to recover. The parameters like Fv/F0, Ψo/1- Ψo, and the flux ratios increased indicating that the plant was trying to recover again from the extreme increasing temperature of hot summer. At 5 PM, all the parameters appear to be in the process of recovery.
At 7 PM the plant almost totally recovered from stress. The value of parameters like Fv/Fm, Fv/F0, φRo, Ψo/1-Ψo reached close to the control values. The values of other parameters like F0, M0, F0/Fm also reached almost close to the control values. This indicated that the whole plant acclimatized itself to the increasing temperature and has several mechanisms by which it can resist extreme temperature of hot summer day and can survive.
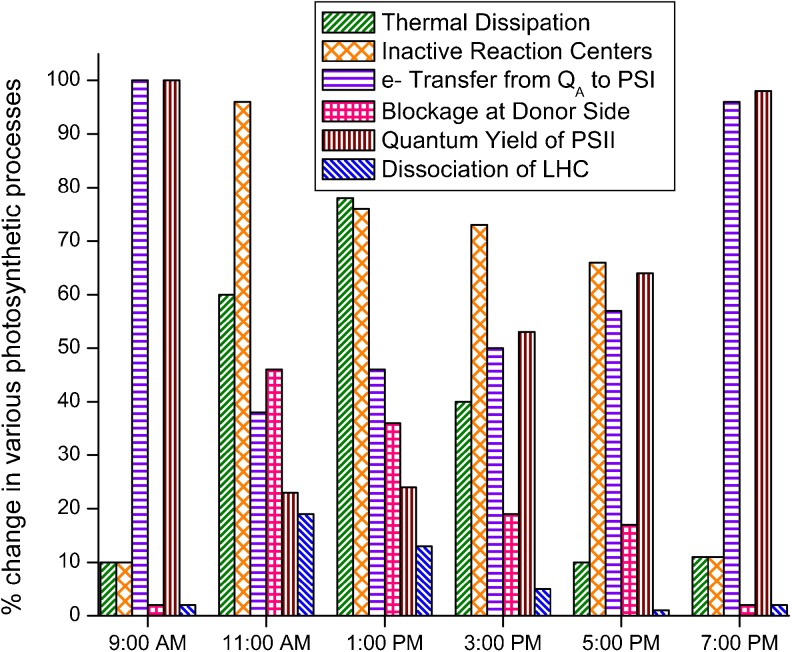
To summarize our observations and results, a scheme is presented (Fig. 2) in which the damage and recovery of various photosynthetic processes during whole day is shown. It demonstrates the sequential events taking place in the plant facing high temperature under natural conditions. As soon as the plant faces high temperature stress, PS II reaction centers are closed and most of the energy absorbed starts to be dissipated as heat or thermal energy. At the same time, to cope up with the stress, reactions taking place at the donor and acceptor side are inhibited (Fv/Fm). It results in the decrease in the quantum yield of PS II (φRo). Dissociation of LHC II (F0) seems to play a minor role in adaptation to high temperature. The severity of events increase a little more at 1 PM and at 3 PM the recovery processes start. Thermal energy dissipation (φDo) leading to down-regulation of PS II activity is most pronounced during the periods of increasing temperature and may be associated with daily variations in the accumulation of zeaxanthin which plays a central role in thermal energy dissipation and in protecting thylakoid membranes against photooxidative damage (Haldiman et al. 2008).
Fig. 2.
The photochemical events in PS II which are affected by high temperature stress and their recovery
Conclusion
The results depicted that increasing temperature caused down regulation of PSII activity. Chl a fluorescence measurements revealed that performance of PSII was mainly down regulated via slowing down of the reduction of QA, decrease in electron transport beyond QA− in the day time. However PS II recovered from the damage caused by increasing temperature stress when the normal temperature was restored in the evening. However, further research is required to differentiate between light intensity induced changes and temperature induced changes.
Acknowledgments
SM thanks Council of Scientific and Industrial Research (CSIR), India for the Senior Research Fellowship Extended (09/301/(0125)/2013/EMR-I). AJ thanks DST-RFBR for the project (INT/RFBR/P-173). We are also thankful to Prof. Reto J. Strasser and Ronaldo Maldonado-Rodriguez for gifting Biolyzer HP 3 Software.
Appendix 1
Table 2.
Derivation of parameters directly obtained from the recorded fluorescence transients
| Parameters | Calculation | Description |
|---|---|---|
| Technical and derived parameters | ||
| O | Fluorescence intensity at 50 μs | Fluorescence intensity when all RCs are open |
| K | Fluorescence intensity at 200-400 μs | |
| J | Fluorescence intensity at 2 ms | |
| I | Fluorescence intensity at 30 ms | |
| M0 | 4(F300-Fo)/(Fm-F0) | Slope of the normalised curve at the origin of the fluorescence rise. Net rate of closed reaction centres accumulation |
| Quantum efficiencies | ||
| φPo | Fv/Fm =1- F0/Fm=TRo/ABS | Maximum quantum yield of primary photochemistry of PSII. Probability that an absorbed photon will be trapped by the PSII RC with the resulting reduction of QA |
| (φPo/1- φPo) | (Fm- F0)/F0=Fv/F0 | Proportional to the activity of the water-splitting complex on the donor side of the PSII |
| ΦEo | ETo/ABS=[1- (F0/Fm)] ψo | Quantum yield of electron transport |
| ΦRo | REo/ABS | Quantum yield of electron transport from QA ─ |
| ΦDo | DIo/ABS = 1 − ϕPo = F0/Fm | Maximum quantum yield of non-photochemical deexcitation |
| ψo /(1 − ψo) | ETo/(dQ A −/dt0) | Conformation term for the thermal reactions (non light-depending reaction beyond QA ─) |
References
- Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P. Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res. 2008;98:541–550. doi: 10.1007/s11120-008-9331-0. [DOI] [PubMed] [Google Scholar]
- Brestic M, Zivcak M, Kalaji HM, Carpentier R, Allakhverdiev SI. Photosystem II thermostability in situ: environmentally induced acclimation and genotype-specific reactions in Triticum aestivum L. Plant Physiol Biochem. 2012;57:93–105. doi: 10.1016/j.plaphy.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Briantais JM, Dacosta JG, Ducruet JM, Moya I. Heat stress induces in leaves an increase of the minimum level of chlorophyll fluorescence Fo: A time-resolved analysis. Photosynth Res. 1996;48:189–196. doi: 10.1007/BF00041008. [DOI] [PubMed] [Google Scholar]
- Chen LS, Cheng L. Photosystem 2 is more tolerant to high temperature in apple (Malus domestica Borkh) leaves than in fruit peel. Photosynthetica. 2009;47(1):112–120. doi: 10.1007/s11099-009-0017-4. [DOI] [Google Scholar]
- Christen D, Schőnmanna S, Jermini M, Strasser RJ, Defago G. Characterization and early detection of grapevine (Vitis vinifera) stress responses to esca disease by in situ chlorophyll fluorescence and comparison with drought stress. Environ Exp Bot. 2007;60:504–514. doi: 10.1016/j.envexpbot.2007.02.003. [DOI] [Google Scholar]
- Costa ES, Smith RB, Oliveira JG, Campostrini E, Pimentel C. Photochemical efficiency in bean plants (Phaseolus vulgaris L. and Vigna unguiculata L. Walp) during recovery from high temperature stress. Braz J Plant Physiol. 2002;14(2):105–110. doi: 10.1590/S1677-04202002000200004. [DOI] [Google Scholar]
- Gauthier A, Joly D, Boisvert S, Carpentier R. Period-four Modulation of photosystem II primary quinone acceptor (QA) reduction/oxidation kinetics in thylakoid membranes. Photochem Photobiol. 2010;86:1064–1070. doi: 10.1111/j.1751-1097.2010.00765.x. [DOI] [PubMed] [Google Scholar]
- Guisse B, Srivastava A, Strasser RJ. The polyphasic rise of the Chlorophyll a fluorescence (O-K-J-I-P) in heat-stressed leaves. Arch Sci. 1995;48:147–160. [Google Scholar]
- Gupta NK, Agarwal S, Agarwal VP, Nathawat NS, Gupta S, Singh G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol Plant. 2013;35:1837–1842. doi: 10.1007/s11738-013-1221-1. [DOI] [Google Scholar]
- Haldiman P, Galle A, Feller U. Impact of an exceptionally hot summer day on photosynthetic traits in Oak (Quercus pubscens) leave. Tree Physiol. 2008;28:785–795. doi: 10.1093/treephys/28.5.785. [DOI] [PubMed] [Google Scholar]
- Ilik P, Schansker G, Kotabova E, Vaczi P, Strasser RJ, Bartak M. A dip in the chlorophyll fluorescence induction at 0.2-2 s in Trebouxia- possessing lichens reflects a fast reoxidation of photosystem I, a comparision with higher plants. Biochim Biophys Acta. 2006;1757(1):12–20. doi: 10.1016/j.bbabio.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Joly D, Carpentier R. Sigmoidal reduction kinetics of the photosystem II acceptor side in intact photosynthetic materials during fluorescence induction. Photochem Photobiol Sci. 2009;8:167–173. doi: 10.1039/b815070b. [DOI] [PubMed] [Google Scholar]
- Kalachanis D, Manetas Y. Analysis of fast chlorophyll fluorescence rise (O-K-J-I-P) curves in green fruits indicates electron flow limitations at the donor side of PS II and the acceptor sides of both photosystems. Physiol Plant. 2010;139:313–323. doi: 10.1111/j.1399-3054.2010.01362.x. [DOI] [PubMed] [Google Scholar]
- Kalaji HM, Loboda T. Photosystem II of barley seedlings under cadmium and lead stress. Plant Soil Environ. 2007;53(12):511–516. [Google Scholar]
- Mathur S, Jajoo A, Mehta P, Bharti S. Analysis of elevated temperature-induced inhibition of photosystem II by using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum) Plant Biol. 2011;13:1–6. doi: 10.1111/j.1438-8677.2009.00319.x. [DOI] [PubMed] [Google Scholar]
- Mathur S, Mehta P, Jajoo A. Effects of dual stress (high salt and high temperature) on the photochemical efficiency of wheat leaves (Triticum aestivum) Physiol Mol Biol Plants. 2012 doi: 10.1007/s12298-012-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou GC, Govindjee Phtosystem II fluorescence: slow changes-scaling from the past. J Photochem Photobiol B Biol. 2011;104:258–270. doi: 10.1016/j.jphotobiol.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Singh SK, Kakani VG, Surabhi GK, Reddy KR. Cowpea (Vigna unguiculata (L.) Walp.) genotypes response to multiple abiotic stresses. J Photochem Photobiol B Biol. 2010;100:135–146. doi: 10.1016/j.jphotobiol.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Guisse B, Greppin H, Strasser RJ. Regulation of antenna structure and electron transport in photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim Biophys Acta. 1997;1320:95–106. doi: 10.1016/S0005-2728(97)00017-0. [DOI] [Google Scholar]
- Stirbet A, Govindjee On the relation between the kautsky effect (Chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient. J Photochem Photobiol B Biol. 2011;104:236–257. doi: 10.1016/j.jphotobiol.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Strasser BJ. Donor side capacity of Photosystem II probed by chlorophyll a fluorescence transients. Photosynth Res. 1997;52:147–155. doi: 10.1023/A:1005896029778. [DOI] [Google Scholar]
- Strasser RJ, Tsimilli-Michael M. Stress in plants from daily rhythm to global changes, detected and quantified by the JIP-test. Chim Nouv. 2001;75:3321–3326. [Google Scholar]
- Strasser RJ, Srivastava A, Tsimilli-Michael M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P, editors. Probing photosynthesis: mechanisms, regulation and adaptation. London: Taylor & Francis; 2000. [Google Scholar]
- Tikkanen M, Aro EM. Thylakoid protein phosphorylation in dynamic regulation of photosystem II in higher plants. Biochim Biophys Acta. 2012;1817:232–238. doi: 10.1016/j.bbabio.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Toth SZ, Nagy V, Puthur JT, Kovács L, Garab G. The physiological role of ascorbate as photosystem ii electron donor: protection against photoinactivation in heat-stressed leaves. Plant Physiol. 2011;156:382–392. doi: 10.1104/pp.110.171918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsimilli-Michael M, Pecheux M, Strasser RJ. Biomonitoring of coral reef and temperate foraminifers by the Chl a fluorescence rise O-J-I-P of their symbionts. In: Garab G, editor. Photosynthesis: mechanisms and effects. Dordrecht: Kluwer Academic Publishers; 1998. pp. 4113–4116. [Google Scholar]
- Wen X, Gong H, Lu C. Heat stress induces a reversible inhibition of electron transport at the acceptor side of photosystem II in a cyanobacterium Spirulina platensis. Plant Sci. 2005;168:1471–1476. doi: 10.1016/j.plantsci.2005.01.015. [DOI] [Google Scholar]
- Zhao B, Wang J, Gong H, Wen X, Ren H, Lu C. Effects of heat stress on PS II photochemistry in a cyanobacterium Spirulina platensis. Plant Sci. 2008;175:556–564. doi: 10.1016/j.plantsci.2008.06.003. [DOI] [Google Scholar]