Abstract
Introduction
Monotherapy with protease-inhibitors (MPI) may be an alternative to cART for HIV treatment. We assessed the impact of this strategy on immune activation, bacterial translocation and inflammation.
Methods
We performed a cross-sectional study comparing patients on successful MPI (n=40) with patients on cART (n=20). Activation, senescence, exhaustion and differentiation stage in CD4+ and CD8+ T lymphocyte subsets, markers of monocyte activation, microbial translocation, inflammation, coagulation and low-level viremia were assessed.
Results
CD4+ or CD8+ T lymphocyte subset parameters were not significantly different between both groups. Conversely, as compared with triple cART, MPI patients showed a higher proportion of activated monocytes (CD14+ CD16−CD163+ cells, p=0.031), soluble markers of monocyte activation (sCD14 p=0.004, sCD163 p=0.002), microbial translocation (lipopolysaccharide (LPS)-binding protein; LBP p=0.07), inflammation (IL-6 p=0.04) and low-level viremia (p=0.035). In a multivariate model, a higher level of CD14+ CD16−CD163+ cells and sCD14, and presence of very low-level viremia were independently associated with MPI. Monocyte activation was independently associated with markers of inflammation (IL-6, p=0.006), microbial translocation (LBP, p=0.01) and low-level viremia (p=0.01).
Conclusions
Patients on MPI showed a higher level of monocyte activation than patients on standard therapy. Microbial translocation and low-level viremia were associated with the high level of monocyte activation observed in patients on MPI. The long-term clinical consequences of these findings should be assessed.
Keywords: protease inhibitor, monotherapy, immune activation, very low-level viremia, microbial translocation, monocyte
Introduction
The introduction of HIV antiretroviral treatment has completely changed the spectrum of the illness that has become, in the past years, a chronic condition in developed countries. Prompt diagnosis has also permitted to treat patients earlier and the proportion of patients that are first diagnosed with a low number of CD4+ T lymphocytes or with an opportunistic infection has sharply decreased. With stable patients on treatment and living longer, the morbidity associated with medication and cost of treatment has notably increased. Strategies for simplifying treatment and lowering the cost have started to be implemented [1]. Monotherapy with ritonavir-boosted protease inhibitor has been proposed as one of these alternatives.
Several clinical trials have assessed the virological effectiveness of simplification of a successful standard protease inhibitor containing regimen to a protease inhibitor monotherapy. The MONET and the MONOI studies compared ritonavir-boosted darunavir in monotherapy versus the standard triple therapy treatment. Both demonstrated non-inferiority in the per-protocol analysis [2,3]. In the same way, the OK study showed similar rates of viral suppression between the boosted lopinavir monotherapy and the triple therapy group [4].
On the contrary, lower rates of virological suppression were seen in the MONARK study in naïve patients who started monotherapy with boosted lopinavir as compared with standard triple therapy containing regimen [5]. Finally, in the MOST study virologically suppressed patients for at least six months with standard cART were randomized to switch to protease inhibitor monotherapy or to continue with triple therapy. This study was prematurely stopped due to high rates of virologic failure [6].
Overall, monotherapy seems an effective strategy in patients previously suppressed with no prior virologic failure. However, more episodes of transient viremia elevation have been reported in the monotherapy groups included in the studies [3,7], although a higher incidence of PI mutations was not observed [3].
Efficacy in the above-cited clinical trials was determined by viral load (VL) suppression, but no other variables were taken into account. Concerns about the possibility of a higher immune activation in patients on monotherapy, or the possibility of lower penetration in the central nervous system were raised. Few studies were performed in an attempt to answer this question. A retrospective study of the samples in the MONET trial showed no differences in high-sensitive C-reactive protein (hs-CRP) or interleukin-6 (IL-6) between patients in monotherapy or triple therapy [8]. Suppression in CSF has also been studied in patients on boosted lopinavir monotherapy versus triple therapy, with similar findings in both groups [9]. However, some case reports in the literature describe patients on PI monotherapy and with suppressed VL in plasma who showed detectable VL in CSF [10,11]. Moreover, in the MONOI trial, two patients in the monotherapy group that presented neurological symptoms showed VL escape in the CSF when a lumbar puncture was performed [3].
We hypothesized that patients on monotherapy could have a higher level of immune activation than patients on cART and, therefore, were at higher risk of suffering long-term clinical consequences (i.e. non-AIDS events). Here, we perform a study to compare the impact of successful monotherapy, either with boosted darunavir or boosted lopinavir, on immune activation, microbial translocation and other inflammatory markers.
Patients and methods
Patients
Forty patients on antiretroviral treatment who had successfully simplified to monotherapy either with ritonavir-boosted darunavir or ritonavir-boosted lopinavir (MPI group) were recruited between September 2011 and September 2012 in the outpatient HIV clinic in the Hospital Clinic, Barcelona. Patients had to fulfil the following inclusion criteria: age over 18 years, antiretroviral treatment with monotherapy for at least the previous 48 weeks and VL <37 copies/ml in the blood tests performed in the previous 48 weeks. Patients fulfilling the inclusion criteria and willing to participate signed informed consent and an extraction of 60 ml of blood was performed. Twenty patients on triple therapy with a PI-containing regimen (ritonavir-boosted darunavir or lopinavir) (cART group) were recruited as a comparison group. To participate in the study, written informed consent was obtained from all individuals, and the study protocol was evaluated and approved by the Hospital Ethical Committee.
VL measurement
Plasma HIV-RNA was measured using Versant HIV-1 RNA v3.0 (Siemens, Barcelona, Spain), which has a lower limit of detection and a lower limit of quantification of 37 copies/ml. Patients with a plasma VL that was detectable but below the limit of quantification (<37 copies/ml) were classified as patients with very low-level viremia (VLLV), and those patients with VL reported as not detected were classified as undetectable [13].
Cell samples
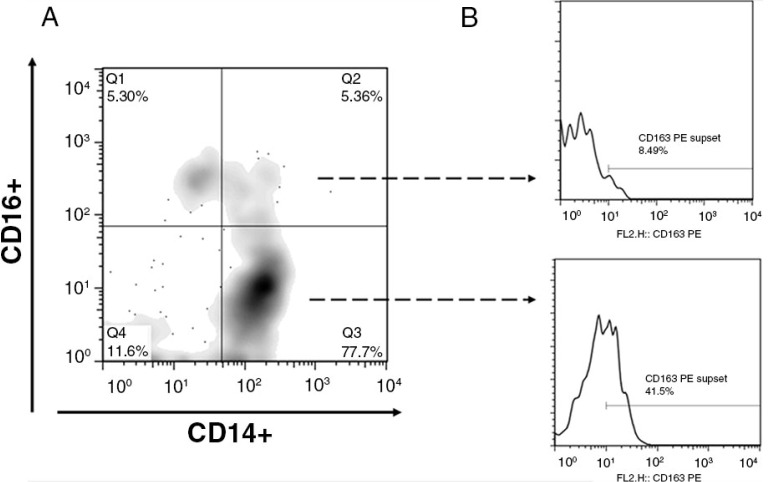
All analyses were done in freshly isolated peripheral blood mononuclear cells (PBMCs). EDTA-anticoagulated blood was obtained by venipuncture; PBMCs were immediately isolated by density gradient centrifugation using Ficoll-Hypaque (Sigma Chemical Co., St. Louis, MO). We used a comprehensive approach of simultaneous measurement of different immunological parameters [12] in CD4+ and CD8+ T lymphocyte: activation (using CD38 and HLADR markers), senescence (using CD28 and CD57 marker), exhaustion (using PD-1 marker), co-receptor expression (CCR5, CXCR4), differentiation stage (using CD45RA and CD45RO markers) and monocyte activation (using CD14, CD16 and CD163 markers). The stained cells were analyzed on a FACSCalibur (Becton Dickinson, San Jose, CA) cytometer. Flow cytometry gating strategy for monocytes is shown in Figure 1. Data were analyzed using FlowJo Software (Tree Star).
Figure 1.
Identification and analysis of monocyte subpopulations. (A) Gated monocytes were subdivided into monocyte subpopulations on the basis of CD14 and CD16 staining characteristics. Subpopulations are defined as CD14+ CD16+ (quadrant 1), CD14++ CD16+ (quadrant 2), CD14++ CD16− (quadrant 3), and CD14-CD16− (quadrant 4). The percentage of cells for each population is depicted in the outer most corner. (B) CD163 activation marker was measured according to the flow cytometry gating strategy indicated above in CD14+CD16– (Q3) and CD14+ CD16+ (Q1+Q2).
Soluble markers of monocyte activation, microbial translocation, inflammation and coagulation markers
Serum was initially frozen at a temperature of −80°C. Soluble markers of monocyte activation [soluble CD14 (sCD14) and soluble CD163 (sCD163)], markers of host response to lipopolysaccharide (LPS) [LPS-binding protein (LBP), endotoxin-core IgM antibody (EndoCAb)], inflammation [high-sensitive C-reactive protein (hs-CRP), interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-alpha)] and pro-coagulation (D-dimer) were assessed.
Soluble CD14 was determined with Quantikine ELISA (R&D systems; limit of detection 125 pg/ml; intra-assay variability is 4.8–6.4%); and soluble CD163 was determined by Macro163 ELISA (IQ products; limit of detection 0.23 ng/ml; intra-assay variability 3–6%) according to manufacturer's instructions. Human LBP and endotoxin-core antibodies were determined by ELISA (Hycult biotech; limit of detection 4.4 ng/ml and 0.05 MMU/ml, respectively). High-sensitive CRP was determined by an immune turbidimetric method (CardioPhase, Siemens Healthcare Diagnostics). A result over 0.5 mg/dl was considered positive. IL-6 and TNF-alpha were determined by ELISA (Diasource Immunoassays, Louvain-la-Neuve, Belgium). Results over 5 and 10 pg/ml, respectively, were considered positive. D-dimer was used as a pro-coagulation marker. It was measured with a turbidimetric method (Innovance, Siemens Diagnostics, Marburg, Germany) in a BCS automated coagulation system XP (Siemens Diagnostics). The sensitivity of the technique allows for the detection of levels as low as 10 ng/ml. The normal cut-off is 500 ng/ml.
Statistical analysis
Characteristics of the study population and the different immunological parameters, microbial translocation and inflammatory markers were recorded as median [interquartile range], and comparisons were made using t-test or the non-parametric tests Mann–Whitney U-test. Correlations between quantitative parameters were explored using the Spearman's rho test. Logistic regression was used to analyze the factors independently associated with the type of therapy (MPI vs. cART). Multiple regression was used to analyze the factors independently associated with monocyte activation. All statistical analyses were performed using the SPSS software version 20 (SPSS Inc., Chicago, IL, USA). All p-values were two-tailed, and were considered significant when lower than 0.05.
Results
Clinical characteristics
No differences in age or gender were observed between the two groups (Table 1). Patients with HCV co-infection were equally distributed between groups. Patients on monotherapy had a longer time since HIV diagnosis and had been on antiretroviral treatment longer. The accumulated time on a PI-containing regimen was longer for the patients on monotherapy but without reaching statistical significant difference. The median time on ritonavir-boosted protease inhibitor monotherapy was 37 months (IQR: 13–51 months). CD4+ T lymphocyte count at start of antiretroviral treatment was lower in the MPI group; however, the CD4+ T lymphocyte count at the time of inclusion in the study was similar between the two groups with a median >500 cells/mm3 in both groups. No differences in plasma VL at the start of antiretroviral treatment were seen between groups. All patients had VL <37 copies in the last control performed before the inclusion in the study, but in the blood test performed the day of inclusion, four patients had detectable VL (two patients in the MPI group with values 50 and 52 copies/ml, and two in the cART group, with values of 156 and 39 copies/ml).
Table 1.
Clinical characteristics of patients
| Characteristic | MPI group (n=40) | IP-containing triple therapy (n=20) | P |
|---|---|---|---|
| Age | 49 (45–57) | 44 (40–52) | 0.71 |
| Sex (female). no,% | 11 (27.5) | 4 (20) | 0.753 |
| Time since HIV diagnosis (months) | 184.3 (146.3–223.5) | 96.9 (64.9–222.3) | 0.048 |
| Time on ART (months) | 167.5 (102.7–183.0) | 67.2 (39.8–156.3) | 0.006 |
| Time on a PI-containing regimen (months) | 91.4 (48.2–123.7) | 49.8 (22.0–123.1) | 0.074 |
| VL <37 copies. no, % | 38 (95) | 18 (90) | 0.595 |
| VL at start of treatment (Log) | 5,14 (4,06–5,49) | 4,60 (4,08–5,49) | 0.71 |
| CD4 cell count (cells/mm3) at inclusion | 566 (390–830) | 568 (429–706) | 0.660 |
| CD4 cell count (cells/mm3) at start of treatment | 192 (65–281) | 299 (119–381) | 0.03 |
| CD4 cell count Nadir | 145 (48–227) | 243 (118–319) | 0.16 |
| HCV co-infection. no, % | 9 (22.5) | 5 (25) | 1 |
| Type of PI on the past 12 months (ABT/DRV) | 26/14 | 12/8 | 0.780 |
VL=viral load; ABT=lopinavir/ritonavir; DRV=darunavir/ritonavir; PI=protease inhibitor; HCV=hepatitis C virus; ART=antiretroviral treatment; MPI=Monotherapy with ritonavir-boosted protease inhibitor. cART: triple therapy with a PI-containing regimen (ritonavir-boosted darunavir or lopinavir).
Data are in median [IQR], unless otherwise indicated.
Comparison of immunological parameters between MPI and cART groups
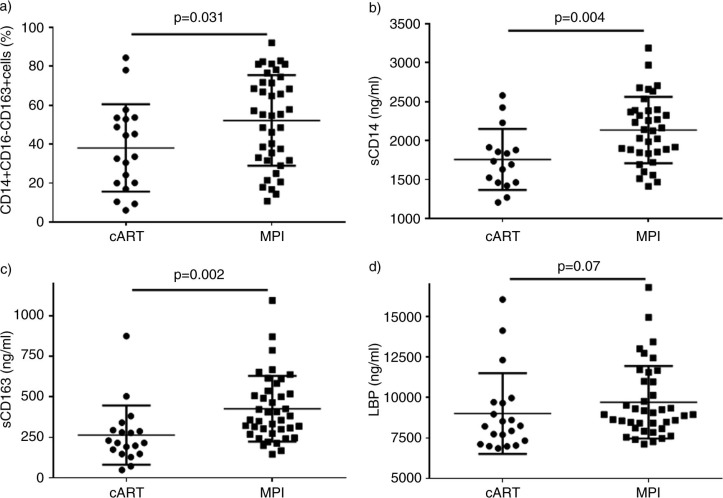
No differences were observed in markers of T lymphocyte activation, senescence, exhaustion, co-receptor expression or differentiation stages between both groups (see Table 2). Conversely, as compared with cART, MPI patients showed a higher proportion of activated monocytes (CD14+CD16+CD163+ cells 9.63% vs. 7.53%, p=0.033; CD14+ CD16− CD163+ cells 55.17% vs. 33.57%, p=0.031 (Figure 2A); and CD14+CD16+ cells 26.85% vs. 19.79%, p=0.029, MPI vs. cART groups)
Table 2.
Lymphocyte and monocyte subpopulations
| Cell subset category | Subpopulations (%) | PI Monotherapy (n=40) | IP-containing triple therapy (n=20) | p |
|---|---|---|---|---|
| Activation of CD4 and CD8 T cells | CD4 DR+38+ | 2.23 (1.59–3.73) | 2.62 (1.57–3.91) | 0.5483 |
| CD8 DR+38+ | 8.3 (5.2–12.62) | 8.33 (6.01–14.73) | 0.8525 | |
| Senescence | CD4 28−57+ | 1.73 (0.64–3.55) | 2.63 (0.83–5.18) | 0.3944 |
| CD8 28−57+ | 28.09 (18.19–39.97) | 31.71 (26.87–43.95) | 0.2363 | |
| Exhaustion | CD4 PD1+ | 20.54 (14.02–30.33) | 18.65 (16.58–27.56) | 0.7212 |
| CD8 PD1+ | 22.72 (18.38–31.35) | 25.30 (21.29–29.52) | 0.4605 | |
| Co-receptors | CD4 CCR5+ | 25.22 (12.59–38.26) | 24.63 (15.13–29.36) | 0.7030 |
| CD8 CCR5+ | 14.66 (7.42–26.77) | 16.85 (6.59–23.24) | 0.8839 | |
| Differentiation stage | CD4 RA+RO− | 31.27 (24.41–48.34) | 31.88 (21.13–40.84) | 0.9032 |
| CD8 RA+RO− | 46.40 (36.09–54.47) | 45.60 (36.38–54.45) | 0.9677 | |
| CD4 RA-RO+ | 57.51 (40.77–69.30) | 57.81 (35.94–63.45) | 0.5008 | |
| CD8 RA-RO+ | 40.06 (28.49–52.23) | 39.69 (25.29–47.85) | 0.5647 | |
| Monocyte activation | CD14+16+163+ | 9.63 (3.65–15.62) | 7.53 (3.23–9.09) | 0.033 |
| CD14+16−163+ | 55.17 (31.58–71.76) | 33.57 (20.00–53.53) | 0.031 | |
| CD14+16+163− | 13.51 (10.81–21.71) | 12.04 (9.88–15.97) | 0.1518 | |
| CD14+16+ | 26.85 (18.99–41.06) | 19.79 (14.94–25.88) | 0.029 |
Figure 2.
Comparison of the levels of monocyte activation and markers of microbial translocation between patients on triple protease inhibitor regimen vs. patients on protease inhibitor monotherapy.
Comparison of soluble markers of monocyte activation, markers of microbial translocation, inflammation, pro-coagulation and VLLV between MPI and cART groups
Levels of soluble markers of monocyte activation were higher in MPI vs. cART patients [sCD14: 2133 vs. 1714 ng/ml, p=0.004 (Figure 2B); sCD163: 369 vs. 215 ng/ml, p=0.002 (Figure 2C)] (Table 3). LBP levels, but not EndoCab were also higher in MPI patients [LBP: 8948 vs. 8237 ng/ml, p=0.07 (Figure 2D)]. Regarding inflammatory markers, only IL-6 levels were significantly higher in the MPI group as compared with cART (IL-6: 34.5 vs. 8 pg/ml, p=0.04). D-dimer levels were similar between both groups. Given that patients on monotherapy had a significantly longer time of known HIV infection and a lower CD4+ T lymphocyte count before starting cART and these variables could mean a bias to compare both groups, we repeated the analysis controlling both variables, excluding those patients on monotherapy with longer follow-up (more than 12 years, lower IQR of MPI group) and lower CD4+ T lymphocyte count previous to treatment (below 200 cells/mm3, median value of MPI group). The differences in monocyte activation, microbial translocation and inflammatory markers between both groups were confirmed (data not shown).
Table 3.
Markers of bacterial translocation, inflammation, pro-coagulation and very low-level viremia
| PI Monotherapy (n=40) | IP-containing triple therapy (n=20) | p | |
|---|---|---|---|
| Bacterial translocation | |||
| LBP (ng/ml) | 8948 (8115–10,999) | 8237 (7171–9693) | 0.07 |
| sCD14 (ng/ml) | 2133.18 (1847.13–2387.14) | 1714.36 (1460.53–1904.20) | 0.004 |
| EndoCAb (MMU/ml) | 68 (44–99) | 74 (34–105) | 0.47 |
| sCD163 (ng/ml) | 368 (279.76–530.90) | 215.38 (147.18–292.23) | 0.002 |
| Inflammation | |||
| hs-CRP (mg/dl) | 0.2 (0.08–0.46) | 0.18 (0.09–0.33) | 0.72 |
| IL-6 (pg/ml) | 34.5 (18–258) | 8 (8–27.5) | 0.04 |
| TNFα (pg/ml) | 5 (4–7) | 4.5 (4–7) | 0.27 |
| Pro-coagulation | |||
| D-dimer (ng/ml) | 201 (90–320) | 176 (111–269) | 0.23 |
| Viremia | |||
| Detection of VLLV. n (%) | 15/38 (39.4) | 2/18 (11) | 0.03 |
LBP=Lipopolysaccharide-binding protein; sCD14=soluble CD14; EndoCAb=endotoxin core IgM antibody; sCD163=soluble CD163; hs-CRP=high sensitive C-reactive protein; IL-6=interleukin-6; VLLV=very low-level viremia. Data are in median (IQR) unless otherwise indicated.
Finally, we analyzed the presence of VLLV, defined as viremia below limit of detection (37 copies) but qualitatively detectable. Fifteen out of 38 (39%) patients in the monotherapy group presented positive signal under the limit detection versus two out of 18 (11%) in the triple therapy one (p=0.035).
A multivariate analysis was performed to assess the factors independently associated with the type of therapy (MPI vs. cART). Variables with statistically significant differences in univariate analysis were included in the model. A higher level of CD14+ CD16− CD163+ cells (p=0.023) and sCD14 (p=0.013), and presence of VLLV (p=0.027) were independently associated with MPI. We repeated the multivariate model controlling for CD4+ T lymphocyte at the start of the antiretroviral treatment, CD4+ T lymphocyte at inclusion, nadir CD4+ T lymphocyte and time of HIV infection. The results showed that CD14+ CD16− CD163+ cells and sCD14, but not VLLV, remained independently associated with MPI.
Correlations among monocyte activation levels, markers of microbial translocation and inflammatory markers
Patients with higher levels of monocyte activation showed the higher levels of microbial translocation and inflammatory markers. The levels of CD14+CD16+CD163+ cells were correlated with sCD14 (rho=0.29, p=0.039), sCD163 (rho=0.26, p=0.047), hsPCR (rho=0.29, p=0.02); the levels of CD14+CD16+ cells were correlated with IL-6 (rho=0.70, p=0.003) and TNF-alpha (rho=0.36, p=0.006); and the levels of sCD14 with LBP (rho=0.54, p<0.0001), hsPCR (rho=0.29, p=0.038) and TNF-alpha (rho=0.41, p=0.003). Markers of monocyte activation were not correlated with CD4+ T cell at start of antiretroviral treatment or nadir CD4+ T lymphocyte.
In a multivariate analysis (using in the model as dependent variable either CD14+CD16+CD163+ cells or CD14+CD16+ cells), monocyte activation was independently associated with markers of inflammation (IL-6, p=0.006) and low-level viremia (p=0.01). In addition, in a multivariate model using as dependent variable sCD14, this soluble marker of monocyte activation was independently associated with the marker of microbial translocation LBP (p=0.028) and low-level viremia (p=0.05).
In addition to sCD14, the marker of microbial translocation LBP was directly correlated with sCD163 (rho=0.28, p=0.034) and inflammatory markers hs-PCR (rho=0.45, p<0.0001) and TNF-alpha (rho=0.37, p=0.004).
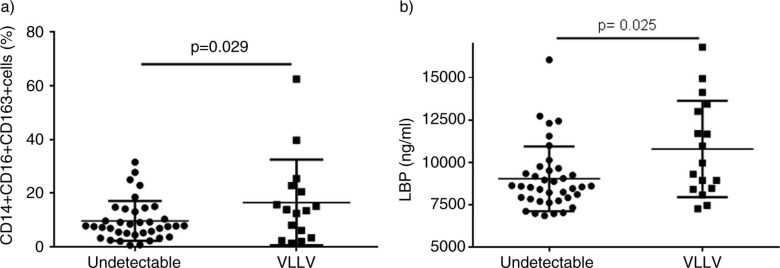
Finally, as compared with patients with undetectable level of VL, patients with VLLV showed a higher percentage of CD14+CD16+CD163+ cells [median 13.65, IQR (4.02–22.17) vs. 7.65 (4.21–13.31), p=0.029] and higher levels of LBP [median 9964 ng/ml, IQR(8452–13,213) vs. 8574 (7820–9548), p=0.025] (Figure 3).
Figure 3.
Comparison of the levels of monocyte activation and markers of microbial translocation between patients with undetectable viral load and patients with very low-level of viremia.
Discussion
The results of our study show a higher level of monocyte activation in patients on successful monotherapy with a boosted PI as compared with protease inhibitor standard regimen. The level of monocyte activation was associated with markers of host response to microbial translocation, inflammation and low-level viremia. These data might suggest that microbial translocation and VLLV drive the high level of monocyte activation observed in these patients on protease inhibitor monotherapy.
The first objective of our study was to assess if the simplification to a successful monotherapy had an impact on activation of the immune system. Although no differences were observed in the percentage of CD4+ and CD8+ T lymphocytes subpopulations (measuring activation, senescence, exhaustion and differentiation stage), patients on monotherapy with a boosted PI showed a higher level of monocyte activation as measured by the expression of CD16 and CD163 in CD14 cells, and the levels in plasma of soluble CD14 and CD163. Recent studies describe higher percentage of markers of monocyte activation in HIV viremic patients and, also in HIV-treated patients who, despite effective antiretroviral treatment, still express higher monocyte activation than age matched uninfected patients [14,15]. The marker CD16 is known to be expressed in more mature monocytes with a distinct pattern of cytokine production and they are considered to be proinflammatory monocytes [16]. CD163 is a monocyte/macrophage haemoglobin scavenger receptor involved in the anti-inflammatory response of monocytes [17]. CD163 is predominantly expressed in CD14+ CD16− monocytes in the general population and in those who are HIV positive [17,18]. Surface expression of CD163 also accounts for activation and can be co-expressed altogether with CD16 [19]. Both CD163 and CD14 are shed upon monocyte activation and the soluble form can be detected in plasma. The increase of all these markers of monocyte activation has been associated with clinical end-points. The frequency of inflammatory CD16+ monocytes is associated with risk of coronary artery progression [20]. Soluble CD14 was reported to be an independent predictor of mortality in HIV [21] and has been related to the increase in the yearly rate of carotid intima-media thickness [22], whereas soluble CD163 has been associated with increased risk of coronary artery inflammation and atherosclerosis and with subclinical atherosclerosis [23]. In addition, a study that assessed neurocognitive impairment in virologically suppressed HIV patients found higher levels of soluble CD163 in patients with a higher global deficit score, suggesting persistent monocyte activation in neuropsychological impaired patients [24]. All these data suggest that the long-term consequences of elevated monocyte activation in patients on PI monotherapy should be further assessed in longitudinal studies.
The second objective of the study was to investigate the factors associated with monocyte activation in this cohort. We found that patients with higher monocyte activation had a higher level of IL-6, LBP and low-level of viremia. These data might suggest that microbial translocation and VLLV drive the monocyte activation in patients on protease inhibitor monotherapy. We found that IL-6 was significantly higher in the monotherapy group. It has been reported that increases in concentrations of interleukin-6 are strongly associated with all-cause mortality [25]. If the higher level of IL-6 would be associated with a worse long-term clinical outcome in these patients deserves further investigation.
Early gut mucosal destruction in HIV infection results in microbial translocation and higher microbial products in the circulation that are supposed to be partially responsible of HIV-associated immune activation that persists despite the introduction of the antiretroviral treatment [26]. A recent study has shown an increase in mucosal macrophages in HIV naïve patients due to enhanced trafficking of blood monocytes in the gut but with lower phagocytic activity [27]. In addition, products of microbial translocation, such as LPS, bind to LBP and cause monocyte activation via toll-like receptor 4 [28]. Higher microbial translocation is then closely related to monocyte activation and has been related to disease progression [29], neurocognitive impairment [30], subclinical atherosclerosis [22] and other pathogenic conditions as non-Hodgkin lymphoma [31] in HIV patients. Our data support these findings, since we observed not only that microbial translocation was associated with monocyte activation, but that both microbial translocation and monocyte activation were associated with an increase of inflammatory markers (hsPCR, IL-6 and TNF-alpha).
Finally, we observed that a higher number of patients included in the MPI group presented with VLLV. The importance of VLLV has been a recent issue of interest. Some studies have related VLLV to a higher risk of virologic failure, and have suggested that low-level viral replication is a cause of VLLV [32,33]. In our study, when we compared patients with VLLV with patients with undetectable VL, we observed a higher percentage of activated monocytes (CD14+CD16+CD163+) and levels of LBP in the first group, suggesting a relation between VLLV and monocyte activation. Traditionally, monocytes were considered to be non-permissive for HIV infection. However, replication competent virus can be detected following activation of these cells [34]. A study that characterized HIV-1 RNA in treated patients with low-level viremia (<48 copies) and examined the sources of residual plasma viremia compared to that expressed in CD4+ T lymphocytes and in CD14+ CD16+ monocytes observed that plasma sequences were more related to that sequenced in activated monocytes, suggesting that residual viremia found in cART-suppressed patients could have its origin from cells from the myeloid lineage [35]. We could hypothesize that monotherapy with a boosted PI regimen is less able to control viral replication in reservoirs as monocyte subsets, leading to higher monocyte activation, inflammation and microbial translocation.
We are aware that our study has other limitations. First of all, it is a transversal study and a low number of patients are included. Second, regarding baseline characteristics, the CD4+ T lymphocyte count at the start of treatment was lower in the MPI group, although no significant differences were observed in VL, nadir of CD4+ or CD4+ T lymphocyte count at the time of inclusion. In fact, it has been reported that clinically important CD4+ T lymphocyte count responses are likely to be better defined in terms of absolute postcART CD4+ T lymphocyte counts, rather than change from baseline [36]. In addition, we have recently reported that differences in CD4+ T lymphocyte gain with different cART regimen are not immunologically meaningful [37]. Moreover, we have repeated the analysis controlling by time of known HIV infection and CD4+ T lymphocyte count previous to cART and the results were confirmed. Finally, VLLV as defined by plasma VL that was detectable but below the limit of quantification could not be a good measurement of the reservoir or residual viremia. In fact, other studies [38,39] did not find an increase of the level of persistent viremia as measured by Roche Amplicor HIV-1 RNA assay with a quantification limit of three copies/ml or by single-copy assay. Apart from the technique used for the measurement, the main difference with our study is that in these studies residual viremia was assessed during the first 48 weeks of simplification, while in our study the median time on monotherapy was three years and all of the patients were on monotherapy for at least the previous 48 weeks before the inclusion and had a VL <37 copies/ml in all the blood tests performed in the previous 48 weeks. We know that the best option for assessing persistent viremia was to measure residual viremia directly; regretfully, we did not have enough sample availability to measure it by other techniques.
In summary, the higher monocyte activation observed in the monotherapy group raises concern about this strategy and the possible association with a higher mortality and long-term cardiovascular and neurocognitive deleterious effects. Larger clinical trials should be performed in order to confirm these results.
Acknowledgements
This study was partially supported by grants: SAF 2012-39075, EC10-153, TRA-094, PS09/01297, FIS PI10/02984 and by the RD12/0017/00XX project as part of the Plan Nacional R+D+I and cofinanced by ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER).
Dr. Montserrat Plana is a researcher from the Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and is supported by the Spanish Health Institute Carlos III (ISCIII) and the Health Department of the Catalan Government (Generalitat de Catalunya.
This study has been presented in part at the 20th Conference on Retroviruses and Opportunistic Infections, 3–6 March 2013 Atlanta, USA (Abstract 795).
Competing interests
The following authors have received research funding, consultancy fees or lecture sponsorships, or served on advisory boards: F García – Abbott, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline and MSD. JM Gatell – Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Pfizer, Theratechnologies and Tibotec. Other authors have no financial conflict of interest.
Authors' contributions
BT, ACG, JMG, MP and FG contributed to the design of the clinical study. BT, LL, AL, CL, MMR, AGC and FG contributed to the implementation of the clinical protocol. BT, ACG, MJAM, MM, JV, MP and FG contributed to the immune analysis and interpretation of the immune data. BT, ACG, JMG, MP and FG contributed to the manuscript writing. All authors revised and approved the final manuscript.
References
- 1.Llibre JM, Cardona G, Santos JR, Andreu A, Estrada JO, Ara J, et al. Antiretroviral treatment switch strategies for lowering the costs of antiretroviral therapy in subjects with suppressed HIV-1 viremia in Spain. Clinicoecon Outcomes Res. 2013;5:215–21. doi: 10.2147/CEOR.S43662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arribas JR, Horban A, Gerstoft J, Fatkenheuer G, Nelson M, Clumeck N, et al. The MONET trial: darunavir/ritonavir with or without nucleoside analogues, for patients with HIV RNA below 50 copies/ml. AIDS. 2010;24(2):223–30. doi: 10.1097/QAD.0b013e3283348944. [DOI] [PubMed] [Google Scholar]
- 3.Katlama C, Valantin MA, Algarte-Genin M, Duvivier C, Lambert-Niclot S, Girard PM, et al. Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV-1 viral suppression: a randomized open-label, noninferiority trial, MONOI-ANRS 136. AIDS. 2010;24(15):2365–74. doi: 10.1097/QAD.0b013e32833dec20. [DOI] [PubMed] [Google Scholar]
- 4.Arribas JR, Pulido F, Delgado R, Lorenzo A, Miralles P, Arranz A, et al. Lopinavir/ritonavir as single-drug therapy for maintenance of HIV-1 viral suppression: 48-week results of a randomized, controlled, open-label, proof-of-concept pilot clinical trial (OK Study) J Acquir Immune Defic Syndr. 2005;40(3):280–7. doi: 10.1097/01.qai.0000180077.59159.f4. [DOI] [PubMed] [Google Scholar]
- 5.Delfraissy JF, Flandre P, Delaugerre C, Ghosn J, Horban A, Girard PM, et al. Lopinavir/ritonavir monotherapy or plus zidovudine and lamivudine in antiretroviral-naive HIV-infected patients. AIDS. 2008;22(3):385–93. doi: 10.1097/QAD.0b013e3282f3f16d. [DOI] [PubMed] [Google Scholar]
- 6.Gutmann C, Cusini A, Gunthard HF, Fux C, Hirschel B, Decosterd LA, et al. Randomized controlled study demonstrating failure of LPV/r monotherapy in HIV: the role of compartment and CD4-nadir. AIDS. 2010;24(15):2347–54. doi: 10.1097/QAD.0b013e32833db9a1. [DOI] [PubMed] [Google Scholar]
- 7.Arribas JR, Clumeck N, Nelson M, Hill A, van Delft Y, Moecklinghoff C. The MONET trial: week 144 analysis of the efficacy of darunavir/ritonavir (DRV/r) monotherapy versus DRV/r plus two nucleoside reverse transcriptase inhibitors, for patients with viral load <50 HIV-1 RNA copies/mL at baseline. HIV Med. 2012;13(7):398–405. doi: 10.1111/j.1468-1293.2012.00989.x. [DOI] [PubMed] [Google Scholar]
- 8.Arribas J, Hill A, Xi N, van Delft Y, Moecklinghoff C. Interleukin-6 and C-reactive protein levels after 3 years of treatment with darunavir/ritonavir monotherapy or darunavir/ritonavir+two nucleoside reverse transcriptase inhibitors in the MONET trial. J Antimicrob Chemother. 2012;67(7):1804–6. doi: 10.1093/jac/dks102. [DOI] [PubMed] [Google Scholar]
- 9.Santos JR, Munoz-Moreno JA, Molto J, Prats A, Curran A, Domingo P, et al. Virological efficacy in cerebrospinal fluid and neurocognitive status in patients with long-term monotherapy based on lopinavir/ritonavir: an exploratory study. PLoS One. 2013;8(7):e70201. doi: 10.1371/journal.pone.0070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gisslen M, Fuchs D, Hagberg L, Svennerholm B, Zetterberg H. Cerebrospinal fluid viral breakthrough in two HIV-infected subjects on darunavir/ritonavir monotherapy. Scand J Infect Dis. 2012;44(12):997–1000. doi: 10.3109/00365548.2012.690526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bierhoff M, Boucher CA, Fibriani A, Ten Kate RW. Ongoing HIV replication in cerebrospinal fluid under successful monotherapy. Antivir Ther. 2013;18(4):641–3. doi: 10.3851/IMP2530. [DOI] [PubMed] [Google Scholar]
- 12.Guardo AC, Alvarez-Fernandez C, Arberas H, Garcia-Perez J, Garcia F, Bargallo ME, et al. Use of RT-defective HIV virions: new tool to evaluate specific response in chronic asymptomatic HIV-infected individuals. PLoS One. 2013;8(3):e58927. doi: 10.1371/journal.pone.0058927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henrich TJ, Wood BR, Kuritzkes DR. Increased risk of virologic rebound in patients on antiviral therapy with a detectable HIV load <48 copies/mL. PLoS One. 2012;7(11):e50065. doi: 10.1371/journal.pone.0050065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndromes. Blood. 2012;120(23):4599–608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hearps AC, Maisa A, Cheng WJ, Angelovich TA, Lichtfuss GF, Palmer CS, et al. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS. 2012;26(7):843–53. doi: 10.1097/QAD.0b013e328351f756. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81(3):584–92. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 17.Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67(1):97–103. [PubMed] [Google Scholar]
- 18.Tippett E, Cheng WJ, Westhorpe C, Cameron PU, Brew BJ, Lewin SR, et al. Differential expression of CD163 on monocyte subsets in healthy and HIV-1 infected individuals. PLoS One. 2011;6(5):e19968. doi: 10.1371/journal.pone.0019968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer-Smith T, Tedaldi EM, Rappaport J. CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res Hum Retrovir. 2008;24(3):417–21. doi: 10.1089/aid.2007.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker JV, Hullsiek KH, Singh A, Wilson E, Henry K, Lichtenstein K, et al. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS. 2014;28(6):831–40. doi: 10.1097/QAD.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206(10):1558–67. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204(8):1227–36. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS. 2013;27(9):1387–95. doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 27.Allers K, Fehr M, Conrad K, Epple HJ, Schurmann D, Geelhaar-Karsch A, et al. Macrophages accumulate in the Gut Mucosa of untreated HIV-infected patients. J Infect Dis. 2014;209(5):739–48. doi: 10.1093/infdis/jit547. [DOI] [PubMed] [Google Scholar]
- 28.Dunzendorfer S, Lee HK, Soldau K, Tobias PS. Toll-like receptor 4 functions intracellularly in human coronary artery endothelial cells: roles of LBP and sCD14 in mediating LPS responses. FASEB J. 2004;18(10):1117–19. doi: 10.1096/fj.03-1263fje. [DOI] [PubMed] [Google Scholar]
- 29.Marchetti G, Cozzi-Lepri A, Merlini E, Bellistri GM, Castagna A, Galli M, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS. 2011;25(11):1385–94. doi: 10.1097/QAD.0b013e3283471d10. [DOI] [PubMed] [Google Scholar]
- 30.Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3(6):e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marks MA, Rabkin CS, Engels EA, Busch E, Kopp W, Rager H, et al. Markers of microbial translocation and risk of AIDS-related lymphoma. AIDS. 2013;27(3):469–74. doi: 10.1097/QAD.0b013e32835c1333. [DOI] [PubMed] [Google Scholar]
- 32.Doyle T, Smith C, Vitiello P, Cambiano V, Johnson M, Owen A, et al. Plasma HIV-1 RNA detection below 50 copies/ml and risk of virologic rebound in patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2012;54(5):724–32. doi: 10.1093/cid/cir936. [DOI] [PubMed] [Google Scholar]
- 33.Maggiolo F, Callegaro A, Cologni G, Bernardini C, Velenti D, Gregis G, et al. Ultrasensitive assessment of residual low-level HIV viremia in HAART-treated patients and risk of virological failure. J Acquir Immune Defic Syndr. 2012;60(5):473–82. doi: 10.1097/QAI.0b013e3182567a57. [DOI] [PubMed] [Google Scholar]
- 34.Sonza S, Mutimer HP, Oelrichs R, Jardine D, Harvey K, Dunne A, et al. Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy. AIDS. 2001;15(1):17–22. doi: 10.1097/00002030-200101050-00005. [DOI] [PubMed] [Google Scholar]
- 35.Lopez CA, Vazquez M, Hill MD, Colon Mdel C, Porrata-Doria T, Johnston IC, et al. Characterization of HIV-1 RNA forms in the plasma of patients undergoing successful HAART. Arch Virol. 2010;155(6):895–903. doi: 10.1007/s00705-010-0659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore DM, Harris R, Lima V, Hogg B, May M, Yip B, et al. Effect of baseline CD4 cell counts on the clinical significance of short-term immunologic response to antiretroviral therapy in individuals with virologic suppression. J Acquir Immune Defic Syndr. 2009;52(3):357–63. doi: 10.1097/QAI.0b013e3181b62933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres B, Rallon N, Lonca M, Diaz A, Alos L, Martinez E, et al. Immunological function restoration with Lopinavir/ritonavir vs Efavirenz containing regimens in HIV infected patients: a randomized clinical trial. AIDS Res Hum Retroviruses. 2014;30(5):425–33. doi: 10.1089/aid.2013.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKinnon JE, Arribas JR, Pulido F, Delgado R, Mellors JW. The level of persistent HIV viremia does not increase after successful simplification of maintenance therapy to lopinavir/ritonavir alone. AIDS. 2006;20(18):2331–5. doi: 10.1097/QAD.0b013e32801189f6. [DOI] [PubMed] [Google Scholar]
- 39.Wilkin TJ, McKinnon JE, DiRienzo AG, Mollan K, Fletcher CV, Margolis DM, et al. Regimen simplification to atazanavir-ritonavir alone as maintenance antiretroviral therapy: final 48-week clinical and virologic outcomes. J Infect Dis. 2009;199(6):866–71. doi: 10.1086/597119. [DOI] [PMC free article] [PubMed] [Google Scholar]