Abstract
Background
Flow cytometry is the most commonly used technology to measure microvesicles (MVs). Despite reported limitations of this technique, MV levels obtained using conventional flow cytometry have yielded many clinically relevant findings, such as associations with disease severity and ability to predict clinical outcomes. This study aims to determine if MV enumeration by flow cytometry correlates with a measurement of their functional capacity, as this may explain how flow cytometry generates clinically relevant results.
Methods
One hundred samples from healthy individuals and patients with obstructive sleep apnoea were analysed by conventional flow cytometry (FACSCalibur) and by three functional MV assays: Zymuphen MP-activity in which data were given as phosphatidylserine equivalent, STA® Phospholipid Procoag Assay expressed as clotting time and Endogenous Thrombin Potential (ETP) reflecting in vitro thrombin generation. Correlations were determined by Spearman correlation.
Results
Absolute counts of lactadherin+ procoagulant MVs generated by flow cytometry weakly correlated with the results obtained from the Zymuphen MP-activity (r=0.5370, p<0.0001); correlated with ETP (r=0.7444, p<0.0001); negatively correlated with STA® Phospholipid Procoag Assay clotting time (−0.7872, p<0.0001), reflecting a positive correlation between clotting activity and flow cytometry. Levels of Annexin V+ procoagulant and platelet-derived MVs were also associated with functional assays. Absolute counts of MVs derived from other cell types were not correlated with the functional results.
Conclusions
Quantitative results of procoagulant and platelet-derived MVs from conventional flow cytometry are associated with the functional capability of the MVs, as defined by three functional MV assays. Flow cytometry is a valuable technique for the quantification of MVs from different cellular origins; however, a combination of several analytical techniques may give the most comprehensive information on the role of MVs in health and disease.
Keywords: microvesicles, conventional flow cytometry, functional assays, thrombin generation, clotting assays
The last few years have seen a steep increase in the number of investigations of extracellular vesicles within fundamental cell biology and in clinical research. Developments in the size analysis, phenotyping and absolute count determination of microvesicles (MVs) have significantly furthered our understanding of their biological roles in health and diseases. Despite technological developments, the most commonly used method to measure MVs remains conventional flow cytometry, which is acknowledged to have several significant limitations.
The greatest limitation is that conventional flow cytometry is unable to detect small MVs. Depending on the flow cytometer, the lower limit of detection for MVs was estimated to be 0.2–0.5 µm (1–3). This limit of detection has been calculated using size-calibrated polystyrene beads; however, polystyrene beads have a different refractive index to biological MVs and therefore these estimates of flow cytometry resolution may have been generous (4). The difference in refractive index between biological vesicles and polystyrene beads suggests that flow cytometers set-up to gate on particles 0.5–1.0 µm using calibrated beads might actually be selecting vesicles in the range of 0.8–2.4 µm in size (5). Of note, silica beads have a refractive index much closer to that of biological MVs (6).
Technologies, such as nanoparticle tracking analysis (NTA) and atomic force microscopy (AFM), have indicated that the majority of extracellular vesicles are smaller than 0.3 µm in diameter (7,8), suggesting that flow cytometry is only capable of measuring the ‘tip of the iceberg’. However, novel technologies may also be detecting some non-vesicular events.
It is possible that smaller vesicles are measured by flow cytometry, by a process known as swarm detection (6). Due to the small size of the vesicles and the relatively large flow cell, multiple vesicles may pass through the flow cell and be detected at the same time. This would allow for the detection of small-sized vesicles by flow cytometry, but would give a false underestimation of the absolute count coupled with an overestimation of their size range. Nevertheless, it is still possible to determine whether swarming is occurring during measurements by performing serial dilution and linearity analysis of samples (2).
New-generation flow cytometers have improved resolution of small vesicles, potentially down to 0.1 µm (1,9,10), by increasing the light collection angle to facilitate improved resolution. This alters the light collection on scatter, allowing small particle-dedicated instruments to have greater sensitivity. High sensitivity flow cytometry has been found to give improved FSc resolution, lower background noise and detects 8–20 times more MVs than standard flow cytometry in patient samples. The ratio of small to large MVs also varied according to clinical status of the patient, suggesting that the size of MVs may provide additional biological information (11). However, new-generation flow cytometers are still not widely available, particularly in clinical settings.
Despite all of the above mentioned limitations, many studies with quantitative MV results generated using only conventional flow cytometry have revealed clinically relevant findings, such as associations with disease severity and ability to predict clinical outcomes (12–14). It is possible that the ‘tip of the iceberg’ is representative of the whole and a potential way to support this theory would be to determine if the number of MVs measured by flow cytometry is related to their functional capacity which by definition will measure the entire vesicle population.
Several assays have been developed or adapted, to measure the functional procoagulant capacity of MVs. This study will compare three commercially available functional MVs assays to each other and then with absolute count results generated by flow cytometry, to determine if the quantitative flow cytometry results can be correlated with the functional capacity of MVs.
Methods
Samples
Fifty-three healthy control samples were obtained from hospital staff and from a study investigating sleep and psychomotor performance at altitude (only samples taken at baseline were used). Subjects were eligible if they were healthy, aged between 18 and 70 years. Subjects with a body mass index (BMI) <18 or >30 kg/m2 (mean (SD) BMI=22.5 (2.6)), or any medical condition requiring treatment were excluded. This study was approved by the Oxford Research Ethics Committee (REC No: 11/SC/0183) and by the Zurich ethics committee (KEK ZH 2010-0054/1) and registered (NCT01130948).
Forty-seven obstructive sleep apnoea (OSA) patient samples were used to examine samples with elevated numbers of MVs. Patients were eligible if they were aged between 45 and 75 years, and had proven OSA [mean (SD) BMI=33.4 (6.1)]. Fifty-eight per cent of the OSA patients were on anti-hypertensives, 25% were on cholesterol-lowering medication and 8% were on glucose-lowering medication. The study was approved by the Oxford Research Ethics Committee (REC No: 05/Q1604/159). Written informed consent was obtained from all participants.
For MV analysis, 2.7 ml of blood was drawn into citrated vacutainer tubes containing 0.3 ml of 0.109M (3.2%) buffered sodium citrate (Becton Dickinson, Oxford, UK) using a 19G needle. The first blood tube was discarded to avoid contamination with endothelial-derived MVs generated during venepuncture. Tubes were centrifuged at 1,550 g for 20 min at 20°C, to produce platelet-poor plasma (PPP), within 15 min of the blood being taken. PPP was carefully taken from the top of the tube, without disturbing the buffy coat, frozen immediately and stored at −80°C for approximately 1 year prior to analysis.
Flow cytometry analysis of MVs
Samples were analysed by flow cytometry as previously described (15). Briefly, 250 µl of PPP was thawed at room temperature. Samples were centrifuged twice at 18,000 g for 30 min to pellet and wash the MVs. MVs were ultimately re-suspended in 100 µl of PBS-citrate 0.32%.
Annexin V-fluorescein isothiocyanate (FITC) at 0.10 µg/ml and lactadherin-FITC at 1.38 µg/ml was used to stain procoagulant MVs. CD31-phycoerythrin (PE, clone WM59) at 0.10 µg/ml and CD41-phycoerythrin-Cy5 (PE-Cy5, clone HIP8) at 0.013 µg/ml were used to differentiate between platelet-derived MVs (PMVs, CD31+CD41+) and endothelial-derived MVs (EMVs, CD31+CD41-). CD144-PE (clone 16B1) at 4.17 µg/ml, CD62E-PE-Cy5 (clone 68-5H11) at 0.42 µg/ml and CD106-PE-Cy5 (clone 51-10C9) at 0.21 µg/ml were also used as markers for EMVs. Glycophorin-A (CD235a)-PE (clone GA-R2) at 0.069 µg/ml was used to stain erythrocyte-derived MVs. CD45-allophycocyanin (APC, clone 2D1) at 0.21 µg/ml was used as a marker for total leucocyte-derived MVs (LMVs). CD66B-FITC (clone G10F5) at 0.10 µg/ml was used to stain granulocyte-derived MVs. CD14-PE (clone MϕP9) at 0.42 µg/ml was used to identify monocyte-derived MVs. Annexin V-FITC and all antibodies were supplied by BD (Oxford, UK), except for lactadherin (HTI, Vermont, USA) and CD144 (eBioscience, Hatfield, UK).
Samples were incubated with the appropriate monoclonal antibody for 30 min at room temperature, protected from light, followed by the addition of 900 µl PBS-calcium or 900 µl PBS-citrate in annexin control. Samples were acquired using a BD FACSCalibur® (Becton Dickinson, Oxford, UK). The positivity gates were checked by isotype control and fluorescence minus one (FMO) staining.
Functional assay analysis of microparticles
Zymuphen MP ELISA
The Zymuphen ELISA (HYPHEN BioMed, Quadratech Diagnostics, UK) is a functional assay that measures the procoagulant activity of MVs in PPP. A microtitre plate is pre-coated with annexin V-streptavidin. Phosphatidylserine (PS)+ MVs bind to the plate and expose their phospholipid surface, allowing the pro-thrombinase complex to cleave prothrombin into thrombin. The phospholipid in the sample is the limiting factor, so there is a direct relationship between the MV phospholipid concentration and the thrombin generation. Thrombin generation is measured by a specific chromogenic substrate, giving an indication of the phophatidylserine-expressing MV in the sample (16).
The PPP samples and kit controls were diluted 1:20 in the kit sample diluent, which is supplemented with calcium, Factor Xa (FXa) and thrombin. A calibrator curve, diluted controls and diluted samples were added to the ELISA plate and incubated for 1 hour at 37°C. The plate was washed five times, then 100 µl of Reagent A (FXa–FVa) and 50 µl Reagent 2 (prothrombin) were added and incubated for 10 min at 37°C, then 50 µl of Reagent 3 (thrombin substrate) was added, and incubated for 3 min at 37°C. Finally, 50 µl of stop solution was added and the plate was read at 405 nm, subtracting the blank. Results were calculated from the calibrator curve and are expressed as nanomolar (nM) phophatidylserine equivalent.
STA® Phospholipid (PPL) Procoag Assay
STA® PPL Procoag assay (Stago, Berkshire, UK) measures the procoagulant activity of MVs by determining the clotting time (16). The clotting time is dependent on the procoagulant phospholipids of the sample. A shortened clotting time indicates an increase in procoagulant phospholipids (17), which is proportional to the procoagulant MV level in the sample.
PPP samples were tested on the STA® automated analyser (Stago, Berkshire, UK), using the STA® Procoag-PPL kit, according to the manufacturer instructions. Two kit controls, with a known clotting time, were analysed to check the reproducibility of the assay. Samples were loaded and detection of procoagulant phospholipids was automatically carried out. PPP was automatically diluted with phospholipid-depleted plasma and incubated for 120 sec at 37°C. The addition of reagents permits the triggering of the coagulation cascade downstream from FXa. The clot formation is dependent only on the procoagulant phospholipids contained in the plasma sample. A shortened clotting time indicates increased concentration of phospholipids. The final result is expressed as the clotting time in seconds.
Endogenous thrombin potential Assay
Thrombin generation can be assessed using a calibrated automated thrombogram (Stago, Berkshire, UK). PRP reagent containing 1 pM tissue factor (TF) and a minimal amount of phospholipids (PRP reagent, TS42, Thrombinoscope BV) is added initially and acts as a trigger to initiate thrombin generation. At fixed time intervals, a sample is taken from the reaction and the amount of thrombin generated is plotted to give a curve. When using PPP, the thrombin generation is attributable to the procoagulant MV concentration of that sample.
The endogenous thrombin potential (ETP) assay was performed according to the manufacturer instructions. PPP (80 µl) sample was added in duplicate to wells in a 96-well plate. PRP reagent (20 µl; contains TF and a minimal amount of phospholipids) was placed in one set of wells and 20 µl thrombin calibrator was added to the other set of wells. The thrombin calibrator corrects for donor-to-donor differences in colour of plasma and inner filter effects (18). The plate was warmed to 37°C, and then placed on the reader, a Fluoroskan Ascent FL (Thermo Electron Corporation, Helsinki, Finland). Fluca-Kit Reagent (20 µl), which causes the citrated plasma to be re-calcified to start the reaction, was automatically added to each well. The fluorescence intensity was continuously measured by the Fluoroskan and thrombin generation was plotted over 1 hour. The computer software calculates lag time (min), the time to peak (min), the peak of thrombin generation (nM) and the area under the thrombin generation curve (nM × min) or ETP.
Statistics
All statistics were performed using GraphPad Prism 5 Software (GraphPad Software, San Diego). The results from each alternative MV assay were compared to the results from the other assays and correlations were determined by Spearman Correlation, due to the non-parametric nature of the data. A p-value of < 0.05 was considered statistically significant.
Results
Samples
All 100 PPP samples were analysed by flow cytometry and on the Zymuphen MP ELISA. Due to the required sample volume requirement, 69 of the 100 samples could be measured by the PPL assay and 78 of the 100 samples were measured by the ETP assay.
Correlations between functional assays
In order to establish if the three functional assays were giving similar results, correlation analysis was carried out. Table I shows the Spearman r-values and the p-values for correlations between the functional assays.
Table I.
Correlations between functional assays
| Zymuphen ELISA | PPL Procoag assay | ETP assay – ETP | ETP assay – peak | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Spearman r | P | Spearman r | P | Spearman r | P | Spearman r | P | |
| Zymuphen MP ELISA | −0.5308 | <0.0001 | 0.5498 | <0.0001 | 0.5638 | <0.0001 | ||
| PPL Procoag assay | −0.5308 | <0.0001 | −0.7672 | <0.0001 | −0.7244 | <0.0001 | ||
| ETP assay – ETP | 0.5498 | <0.0001 | −0.7672 | <0.0001 | 0.9852 | <0.0001 | ||
| ETP assay – peak | 0.5638 | <0.0001 | −0.7244 | <0.0001 | 0.9852 | <0.0001 | ||
P-value and r-value from Spearman correlation. PPL=phospholipid Procoag assay clotting time; ETP=endogenous thrombin potential; peak=peak thrombin generation.
The ETP assay gives several readouts for the results, including the ETP (nM×min) and the Peak (nM). As expected, these two results were very closely positively correlated (r=0.9852, p<0.0001, Table I).
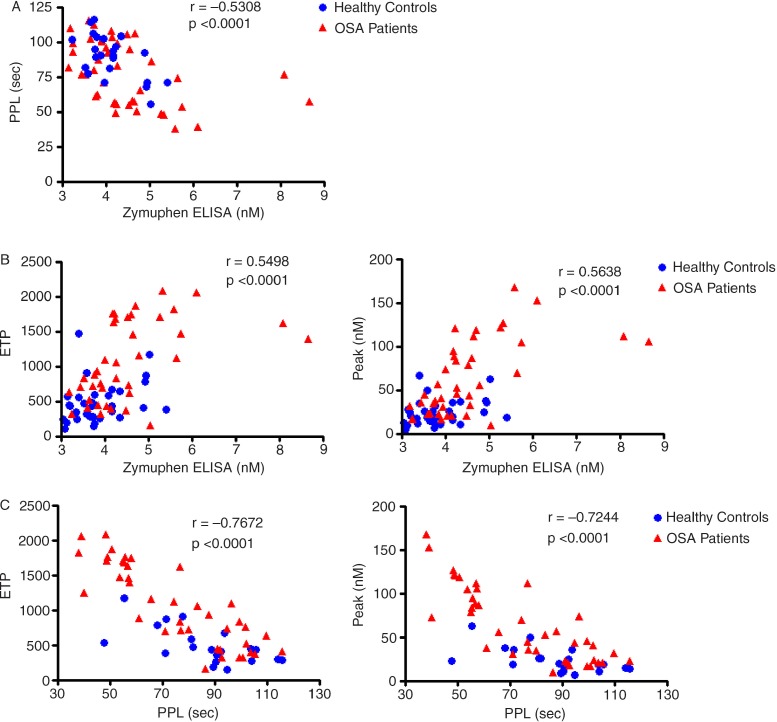
The results from the Zymuphen MP-ELISA were significantly negatively correlated with the STA® PPL Procoag (Fig. 1A) assay and positively associated with the ETP and the Peak results from the ETP assay (Fig. 1B).
Fig. 1.
Correlations between the three functional assays.
A=PPL vs. Zymuphen; B=ETP vs. Zymuphen; C=ETP vs. PPL. P-value and r-value from Spearman correlation. PPL=phospholipid Procoag assay clotting time; ETP=endogenous thrombin potential; peak=peak thrombin generation; sec=seconds.
As expected the clotting times of the STA® PPL Procoag assay were significantly negatively correlated with the ETP and the Peak from the ETP assay (Fig. 1C).
Correlations between functional assays and flow cytometry
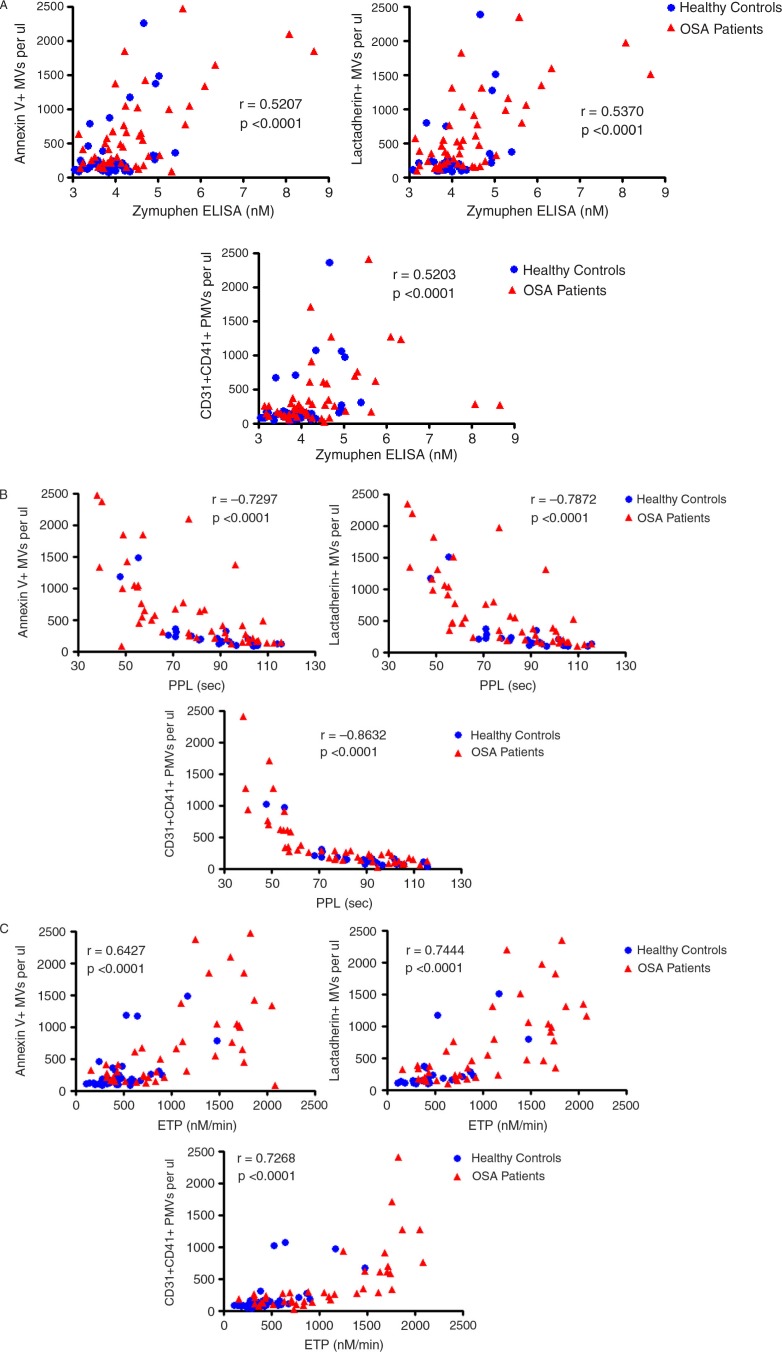
In order to establish if the functional MV results from the commercial assays were associated with the quantitative results from the flow cytometry assay, correlation analysis was carried out. Table II and Fig. 2 show the Spearman r-values and the p-values for correlations between the results from the functional assays and the results determined by flow cytometry.
Table II.
Correlations between functional assays and flow cytometry results
| Zymuphen ELISA | PPL Procoag assay | ETP assay – ETP | ETP assay – peak | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Flow cytometry levels | Spearman r | P | Spearman r | P | Spearman r | P | Spearman r | P |
| Annexin V+ MVs | 0.5207 | <0.0001 | −0.7297 | <0.0001 | 0.6427 | <0.0001 | 0.6550 | <0.0001 |
| Lactadherin+ MVs | 0.5370 | <0.0001 | −0.7872 | <0.0001 | 0.7444 | <0.0001 | 0.7505 | <0.0001 |
| CD31 + CD41+ PMVs | 0.5203 | <0.0001 | −0.8632 | <0.0001 | 0.7268 | <0.0001 | 0.7084 | <0.0001 |
P-value and r-value from Spearman correlation. PPL=phospholipid Procoag assay clotting time; ETP=endogenous thrombin potential; MVs=microvesicles; PMVs=platelet microvesicles.
Fig. 2.
Correlations between functional assays and flow cytometry.
A=flow cytometry vs. Zymuphen; B=flow cytometry vs. PPL; C=flow cytometry vs. ETP. P-value and r-value from Spearman correlation. PPL=phospholipid Procoag assay clotting time; ETP=endogenous thrombin potential; MVs=microvesicles; PMVs=platelet-derived microvesicles.
The results from the Zymuphen ELISA were moderately positively correlated with annexin V+, lactadherin+ and platelet-derived MVs as determined by flow cytometry (Fig. 2A).
The results of the STA® PPL Procoag assay were negatively correlated with annexin V+, lactadherin+ and platelet-derived MVs as measured by flow cytometry (Fig. 2B).
The ETP and Peak results from the ETP assay were positively correlated with annexin V+, lactadherin + and platelet-derived MVs results from the flow cytometry assay (Fig. 2C).
None of the alternative assays were correlated with any other microparticle subtype, including those derived from endothelial cells, erythrocytes, leucocytes, granulocytes or monocytes, as determined by flow cytometry (data not shown).
Discussion
This study compared three functional assays to each other and to the absolute counts of a broad range of MV subtypes identified by flow cytometry in a large number of samples from both healthy controls and patients, allowing for comparisons to be drawn between several assays, which measure different aspects of MVs functional capacity. In general, results from the three functional assays correlated well with each other. Procoagulant MVs (as defined by annexin V and lactadherin) and platelet-derived microparticles also correlate well with these assays. As expected, MVs derived from other cells were not correlated with the results from the functional assays.
The Zymuphen MP ELISA was weakly positively correlated with ETP and Peak from the ETP assay and moderately negatively correlated with the STA® PPL assay. The Zymuphen MP ELISA measures the procoagulant surface and gives results in nM phophatidylserine equivalent, the other functional assays give an indication of clotting and thrombin generation rate, possibly explaining the weaker association. The Zymuphen MP ELISA was only weakly positively correlated with procoagulant MV absolute counts measured by flow cytometry and this association was not as strong as the other two functional assays. These findings are supported by Bohling et al. (19), who noted a moderate correlation between platelet-derived MVs measured by flow cytometry and the results from a chromogenic assay. The weaker association may be due to the phosphatidylserine contribution of small MVs, which are measured by the Zymuphen MP ELISA, but cannot be detected by flow cytometry.
The STA® PPL Procoag assay was negatively correlated with ETP and Peak results from the ETP assay and moderately negatively correlated with the Zymuphen ELISA results. A negative association would be expected, as a shorter PPL clotting time indicates more procoagulant phospholipids in the sample. This assay was also significantly negatively correlated with procoagulant and platelet-derived MVs, suggesting that quantitative results from flow cytometry are related to the clotting potential of MVs. This is supported by the data from two other studies which also found strong negative correlations between platelet-derived MVs detected by flow cytometry and the results from the STA® PPL Procoag assay (19,20).
The ETP and Peak results were both positively correlated with procoagulant and platelet-derived MVs. Both ETP and Peak appear to give a good indication of procoagulant MVs present in a sample, again indicating that quantitative results from flow cytometry are related to the functional capacity of MVs. This is supported by the findings of a study on ageing, where an association between annexin V+ MVs measured by flow cytometry and the peak measured by thrombin generation was found (21). A further study also reported a positive correlation between platelet MV results from flow cytometry and peak thrombin generation (20).
This study has shown that the STA® PPL Procoag assay and the ETP assay correlate better with the absolute counts from flow cytometry than the total phosphatidylserine concentration of MVs. This may suggest that most of the clotting and thrombin generation activity is present in the large MVs, detected by flow cytometry, whereas much of the phosphatidylserine exposure is accounted for by smaller MVs.
Functional assays have been developed to detect procoagulant MVs. As expected they correlate with each other and with annexin V+, lactadherin+ and platelet-derived MVs. No significant correlations were found between the functional assays and other MV subtypes, which included those derived from endothelial cells, erythrocytes, leucocytes, granulocytes or monocytes. Endothelial MVs have previously been found to be mainly annexin V negative (22). Indeed we observed that a significant proportion of MVs derived from endothelial cells and leucocytes (in some cases >50%) do not bind to annexin V or lactadherin, compared to over 90% of platelet-derived MVs which bound annexin V and lactadherin. Also, these vesicles only encompass a small proportion of the total MV pool, being measured by the functional assay, making them less likely to contribute to the overall functional capacity.
Studies have shown that MVs bearing TF on their surface possess the highest prothrombotic potential and these MVs may be predictive of thrombosis in patients with cancer (23–25). Due to this potential clinical utility, functional assays have been developed that specifically measure TF bearing MVs. TF+ MVs were not investigated in the current study due to the expected extremely low levels being present in the populations examined.
Functional assays provide a quick and relatively cheap method of screening large numbers of samples for procoagulant MVs. They provide functional information about the MVs and they can measure the contribution of vesicles too small to be detected by flow cytometry. However, they cannot give an absolute count of MVs and they provide no information about the cellular origin. It is likely that a range of assays is necessary to provide the most comprehensive information about the quantitative levels and functionality of circulating cell-derived MVs.
Interestingly all three functional assays and flow cytometry show some discrimination between the healthy control samples (Fig. 2; blue circles) and the samples from OSA patients (red triangles). It should be noted that the OSA patients in this study had significantly higher BMIs than the healthy controls and some of the OSA patients were on disease modifying medications (including cholesterol lowering treatments) not used by the healthy controls. These differences between the groups may have contributed to the discrimination in MV levels. However, several other studies have demonstrated that procoagulant and platelet MVs are elevated in OSA patients (26,27), and that MV levels are affected by effective treatment of the disease (28,29).
Limitations
Correlations between the Zymuphen MP ELISA and the flow cytometry results were examined in all 100 samples. However, sample volume requirements meant that fewer samples could be analysed by the STA® PPL Procoag assay and the ETP assay. Ideally all 100 samples would have been measured in all assays. Platelet-poor-plasma was used to compare the assays, as opposed to platelet-free plasma, therefore, some ex vivo generation of PMVs may have occurred during the freeze–thaw process. Citrate blood samples were used, not corn trypsin inhibitor samples, so contact activation may contribute to the thrombin generation assays. Samples were stored at −80°C for approximately 1 year prior to analysis. Long-term storage of samples at −80°C can impact on the absolute counts of MVs detected (15) and may have had differential impacts on the functional assays. Despite these limitations, we show good correlations between the four assays examined.
Conclusions
The absolute counts of procoagulant and platelet-derived MVs obtained from conventional flow cytometry correlate with functional assay results, suggesting that the quantitative results given by flow cytometry are associated with the functional capability of the MVs, perhaps explaining how conventional flow cytometry is able to generate clinically relevant results. Functional assays are useful in screening large populations for procoagulant MVs; however, flow cytometry is still necessary for obtaining information on other MV subtypes. Therefore, the combination of several analytical techniques will give the most comprehensive information of the role of MVs in health and disease. Development of Fluorescent NTA and dedicated flow cytometers for measuring all size ranges of vesicles is required to phenotype the entire size range of vesicles encountered in biological samples.
References
- 1.Shantsila E, Kamphuisen PW, Lip GY. Circulating microparticles in cardiovascular disease: implications for atherogenesis and atherothrombosis. J Thromb Haemost. 2010;8:2358–68. doi: 10.1111/j.1538-7836.2010.04007.x. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen MH, Beck-Nielsen H, Andersen MN, Handberg A. A flow cytometric method for characterization of circulating cell-derived microparticles in plasma. J Extracell Vesicles. 2014;3:20795. doi: 10.3402/jev.v3.20795. http://dx.doi.org/10.3402/jev.v3.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert S, Poncelet P, Lacroix R, Arnaud L, Giraudo L, Hauchard A, et al. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies? J Thromb Haemost. 2009;7:190–7. doi: 10.1111/j.1538-7836.2008.03200.x. [DOI] [PubMed] [Google Scholar]
- 4.Chandler WL, Yeung W, Tait JF. A new microparticle size calibration standard for use in measuring smaller microparticles using a new flow cytometer. J Thromb Haemost. 2011;9:1216–24. doi: 10.1111/j.1538-7836.2011.04283.x. [DOI] [PubMed] [Google Scholar]
- 5.Harrison P, Gardiner C. Invisible vesicles swarm within the iceberg. J Thromb Haemost. 2012;10:916–18. doi: 10.1111/j.1538-7836.2012.04711.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Der Pol E, Van Gemert MJ, Sturk A, Nieuwland R, Van Leeuwen TG. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J Thromb Haemost. 2012;10:919–30. doi: 10.1111/j.1538-7836.2012.04683.x. [DOI] [PubMed] [Google Scholar]
- 7.Freyssinet JM, Berndt MC, Dignat-George F, Griffin JH, Newman PJ, editors. 55th Annual Scientific and Standardisation Committee Meeting 2009. USA: Boston; Vascular biology. [Google Scholar]
- 8.Yuana Y, Oosterkamp TH, Bahatyrova S, Ashcroft B, Garcia Rodriguez P, Bertina RM, et al. Atomic force microscopy: a novel approach to the detection of nanosized blood microparticles. J Thromb Haemost. 2010;8:315–23. doi: 10.1111/j.1538-7836.2009.03654.x. [DOI] [PubMed] [Google Scholar]
- 9.Montoro-Garcia S, Shantsila E, Tapp LD, Lopez-Cuenca A, Romero AI, Hernandez-Romero D, et al. Small-size circulating microparticles in acute coronary syndromes: relevance to fibrinolytic status, reparative markers and outcomes. Atherosclerosis. 2013;227:313–22. doi: 10.1016/j.atherosclerosis.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Nolte-‘t Hoen EN, van der Vlist EJ, Aalberts M, Mertens HC, Bosch BJ, Bartelink W, et al. Quantitative and qualitative flow cytometric analysis of nanosized cell-derived membrane vesicles. Nanomedicine. 2012;8:712–20. doi: 10.1016/j.nano.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert S, Lacroix R, Poncelet P, Harhouri K, Bouriche T, Judicone C, et al. High-sensitivity flow cytometry provides access to standardized measurement of small-size microparticles – brief report. Arterioscler Thromb Vasc Biol. 2012;32:1054–8. doi: 10.1161/ATVBAHA.111.244616. [DOI] [PubMed] [Google Scholar]
- 12.Amabile N, Guerin AP, Tedgui A, Boulanger CM, London GM. Predictive value of circulating endothelial microparticles for cardiovascular mortality in end-stage renal failure: a pilot study. Nephrol Dial Transplant. 2012;27:1873–80. doi: 10.1093/ndt/gfr573. [DOI] [PubMed] [Google Scholar]
- 13.Amabile N, Heiss C, Real WM, Minasi P, McGlothlin D, Rame EJ, et al. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1268–75. doi: 10.1164/rccm.200710-1458OC. [DOI] [PubMed] [Google Scholar]
- 14.Lee ST, Chu K, Jung KH, Kim JM, Moon HJ, Bahn JJ, et al. Circulating CD62E+ microparticles and cardiovascular outcomes. PLoS One. 2012;7:e35713. doi: 10.1371/journal.pone.0035713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayers L, Kohler M, Harrison P, Sargent I, Dragovic R, Schaap M, et al. Measurement of circulating cell-derived microparticles by flow cytometry: sources of variability within the assay. Thromb Res. 2011;127:370–7. doi: 10.1016/j.thromres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Owens AP, 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–97. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dreden P, Rousseau A, Fontaine S, Woodhams BJ, Exner T. Clinical evaluation of a new functional test for detection of plasma procoagulant phospholipids. Blood Coagul Fibrinolysis. 2009;20:494–502. doi: 10.1097/MBC.0b013e32832c5e51. [DOI] [PubMed] [Google Scholar]
- 18.Owen BA, Xue A, Heit JA, Owen WG. Procoagulant activity, but not number, of microparticles increases with age and in individuals after a single venous thromboembolism. Thromb Res. 2011;127:39–46. doi: 10.1016/j.thromres.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohling SD, Pagano MB, Stitzel MR, Ferrell C, Yeung W, Chandler WL. Comparison of clot-based vs chromogenic factor Xa procoagulant phospholipid activity assays. Am J Clin Pathol. 2012;137:185–92. doi: 10.1309/AJCPGSJ4NHFQMX9W. [DOI] [PubMed] [Google Scholar]
- 20.Stagnara J, Garnache Ottou F, Angelot F, Mourey G, Seilles E, Biichle S, et al. Correlation between platelet-derived microparticle enumeration by flow cytometry and phospholipid-dependent procoagulant activity in microparticles: the centrifugation step matters! Thromb Haemost. 2012;107:1185–7. doi: 10.1160/TH11-07-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emmerechts J, Loyen S, Hoylaerts MF. Microparticle number or procoagulant activity are not upregulated in healthy elderly persons. Thromb Res. 2012;129:98–100. doi: 10.1016/j.thromres.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Ahn YS, Jy W, Jimenez JJ, Horstman LL. More on: cellular microparticles: what are they bad or good for? J Thromb Haemost. 2004;2:1215–16. doi: 10.1111/j.1538-7836.2004.00797.x. [DOI] [PubMed] [Google Scholar]
- 23.Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122:1873–80. doi: 10.1182/blood-2013-04-460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gheldof D, Mullier F, Bailly N, Devalet B, Dogne JM, Chatelain B, et al. Microparticle bearing tissue factor: a link between promyelocytic cells and hypercoagulable state. Thromb Res. 2014;133:433–9. doi: 10.1016/j.thromres.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Thaler J, Pabinger I, Sperr WR, Ay C. Clinical evidence for a link between microparticle-associated tissue factor activity and overt disseminated intravascular coagulation in patients with acute myelocytic leukemia. Thromb Res. 2014;133:303–5. doi: 10.1016/j.thromres.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 26.Ayers L, Ferry B, Craig S, Nicoll D, Stradling JR, Kohler M. Circulating cell-derived microparticles in patients with minimally symptomatic obstructive sleep apnoea. Eur Respir J. 2009;33:574–80. doi: 10.1183/09031936.00107408. [DOI] [PubMed] [Google Scholar]
- 27.Maruyama K, Morishita E, Sekiya A, Omote M, Kadono T, Asakura H, et al. Plasma levels of platelet-derived microparticles in patients with obstructive sleep apnea syndrome. J Atheroscler Thromb. 2012;19:98–104. doi: 10.5551/jat.8565. [DOI] [PubMed] [Google Scholar]
- 28.Ayers L, Stoewhas AC, Ferry B, Stradling J, Kohler M. Elevated levels of endothelial cell-derived microparticles following short-term withdrawal of continuous positive airway pressure in patients with obstructive sleep apnea: data from a randomized controlled trial. Respiration. 2013;85:478–85. doi: 10.1159/000342877. [DOI] [PubMed] [Google Scholar]
- 29.Yun CH, Jung KH, Chu K, Kim SH, Ji KH, Park HK, et al. Increased circulating endothelial microparticles and carotid atherosclerosis in obstructive sleep apnea. J Clin Neurol. 2010;6:89–98. doi: 10.3988/jcn.2010.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]