Abstract
Functional imaging refers broadly to the visualization of organ or tissue physiology using medical image modalities. In load-bearing tissues of the body, including articular cartilage lining the bony ends of joints, changes in strain, stress, and material properties occur in osteoarthritis (OA), providing an opportunity to probe tissue function through the progression of the disease. Here, biomechanical measures in cartilage and related joint tissues are discussed as key imaging biomarkers in the evaluation of OA. Emphasis will be placed on the a) potential of radiography, ultrasound, and magnetic resonance imaging to assess early tissue pathomechanics in OA, b) relative utility of kinematic, structural, morphological, and biomechanical measures as functional imaging biomarkers, and c) improved diagnostic specificity through the combination of multiple imaging biomarkers with unique contrasts, including elastography and quantitative assessments of tissue biochemistry. In comparison to other modalities, magnetic resonance imaging provides an extensive range of functional measures at the tissue level, with conventional and emerging techniques available to potentially to assess the spectrum of preclinical to advance OA.
Keywords: Cartilage degeneration, magnetic resonance imaging (MRI), radiography, osteoarthritis, elastography, ultrasound, stress and strain, WOMAC, WORMS, PASE
INTRODUCTION
Medical imaging provides a noninvasive means to probe the function of the body. Beyond structural and morphological information commonly derived from imaging modalities, functional imaging further allows two- and three-dimensional visualization of physiological measures, including blood flow [1], tissue motion and deformation [2], neurological activity [3], diffusion and perfusion [4], and metabolism [5]. A well-known example is functional magnetic resonance imaging (fMRI), which indirectly determines brain activity through blood-oxygen-level dependent contrast [3, 6]. Measures of physiology are important, in particular when acquired by noninvasive imaging methods, because the function of organs and tissues is known to change during disease, aging, development, regeneration, and repair (e.g. [7, 8]).
Osteoarthritis (OA), a disease that affects over 20 million people in the United States alone [9], is characterized by pain and functional changes in the joint, including weakening and loss of articular cartilage. OA is often triggered by injury, involving reciprocal actions of joint biomechanics and biochemistry, and advancing through a cascade of degenerative events following inflammation and increased expression of catabolic cytokines and enzymes [10–12]. Advanced OA has been assessed by multiple non-imaging methods, including scoring of pain [13–15], retrieved biopsies [16], and combined (e.g. WOMAC) assessments of pain, joint stiffness and physical function [17–24]. Ideally, the early onset of OA would be identified in pre-clinical (i.e. asymptomatic) individuals, prior to pain and other symptoms that may indicate severe disease progression.
Imaging represents a potentially ideal way to assess early OA by noninvasively probing specific features or functional characteristics of the joint that indicate damage or disease. Joint structures and morphology are commonly assessed in OA using imaging modalities including radiography [25], MRI [26, 27], and ultrasound [28]. Emerging possible imaging biomarkers of early OA include biomechanical measures of strain, stress, and material properties, key measures of mechanical function at the tissue level. Strain measures the normalized deformation of a tissue, and can be readily quantified from image data by visualizing a tissue that is changing shape or size due to mechanical loading over time. Stress, which describes the internal forces of a tissue, and material properties, which relate stress and strain through constitutive equations, can often be determined through the combined use of image data and numerical modeling. Historically, a large number of ex vivo biomechanical studies have clearly identified changes in strain, stress, and material properties associated with degeneration of the joint and cartilage extracellular matrix (ECM) during OA progression [8, 29–33]. An ideal functional imaging modality would therefore provide maximum sensitivity of biomechanical parameters to OA in the earliest stages of the disease, prior to severe cartilage and joint damage, when disease-rectifying therapies would likely be most effective.
This review discusses biomechanical measures of the joint and cartilage as imaging biomarkers in the evaluation of OA. Particular attention is placed on functional imaging of cartilage, in part because wear and loss of this tissue is a hallmark of advanced OA. However, the important role of functional imaging is also discussed for joint kinematics and the study of non-cartilage tissues. Conventional and emerging functional imaging modalities that make use of unique or combined contrasts will be discussed in the context of their potential for diagnosing early disease stages.
TISSUE MECHANICS IN EARLY OA
The potential of functional imaging to diagnose pre-clinical OA depends on the ability to detect disease-associated joint and tissue changes that manifest as aberrant biomechanics. Cartilage defects, ACL tears and reconstruction, and meniscus injuries can all lead to cartilage OA in the long term [34–36], suggesting that local tissue damage and downstream degenerative changes may be useful functional markers to assess a broad range of musculoskeletal joint problems and treatments. Injury often activates the onset of osteoarthritis [37, 38], resulting in direct collagen damage [39], cell death [40], and joint instability following defects or tearing in cartilage, ligaments or menisci [41, 42]. Injury also initiates a cascade of structural (Figure 1) and biochemical changes, exacerbated by mechanical loading, that include inflammation, increased cellular expression of cytokines and enzymes (e.g. IL-1β, aggrecanases, matrix metalloproteinases) [43, 44], degradation of the aggrecan core protein, and increased susceptibility of hyaluronic acid and type II collagen to enzymes through HA oligosaccharides and type II collagen fragments [45, 46].
Figure 1. Noninvasive imaging can probe the pathomechanics of osteoarthritis (OA).
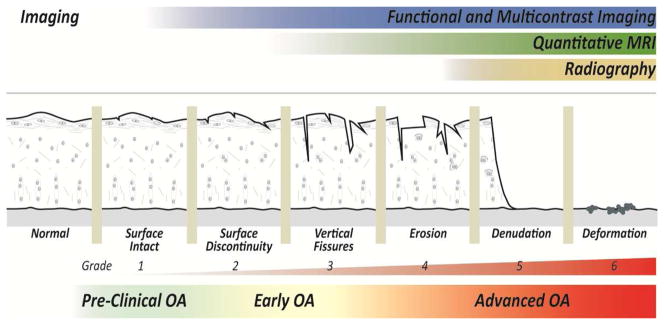
Structural changes in the progression of OA are characterized by cartilage damage and loss, following [156]. Radiography indirectly identifies advanced OA through joint space narrowing. Functional imaging and quantitative MRI show promise to probe early OA, prior to the expression of gross changes in cartilage structure and morphology [143].
The pathomechanics of OA provides multiple possible targets that may be evaluated using functional imaging biomarkers (Figure 1). In the short-term, mechanically-induced damage and tissue tearing may be directly visualized as structural defects or indirectly observed by quantification of strain during passive mechanical loading to the joint. Longer-term changes in the degenerating cartilage ECM and surrounding joint structures may likewise be detected by gross structural changes, including increasing defect size or volume, or indirectly through measures of decreased cartilage stiffness. To detect subtle changes in the cartilage ECM structure and biomechanics, it is critical to develop and use techniques with sufficient precision and accuracy to identify local changes in the internal tissue biomechanics that deviate from normal [47].
Beyond the evaluation of pathomechanics, functional imaging is also needed to noninvasively characterize the biomechanics of damaged musculoskeletal tissues following treatment and therapeutic interventions. Therapies of regenerative medicine (e.g. cell implantation, gene therapy) are emerging as viable repair and management strategies for OA [48–53]. Evaluation of the success of treatments and interventions ultimately depends on the development of imaging tools that can noninvasively assess tissue function in vivo following repair. Quantification of cartilage strain or stiffness using functional imaging represent promising imaging biomarkers considering the important load-bearing function of the tissue [54], which changes following joint injury and disease [7, 8]. Direct evaluation of cartilage by procedures like arthroscopy may be considered a gold standard of assessment, but is highly invasive [55]. Reliable noninvasive methods to probe cartilage and joint function allow for the definition of target properties for engineered tissues, and enable direct evaluation of the tissue and joint following repair.
FUNCTIONAL IMAGING OF BIOMECHANICS IN OA
A basic question emerges when considering the rationale for functional imaging in OA: what is the role of imaging in the evaluation of tissue biomechanics? Because imaging is noninvasive and nondestructive, there is an immediate potential to evaluate pre-clinical OA before the disease manifests as pain or loss of function in patients. Numerous imaging studies directly compare asymptomatic patients with those who display symptomatic OA [26, 28, 41, 49, 56, 57], with asymptomatic patients typically serving as a control group. It is not yet clear whether functional imaging will in the near-term achieve sufficient sensitivity to diagnose pre-clinical OA, or whether routine patient scans will be appropriately justified (with low cost and high specificity) to probe the asymptomatic joint. For example, it is largely unknown how mechanical parameters (e.g. stiffness) of joint tissues vary among people in vivo, and so definitions of population-level baseline values may simply exhibit excessive variability to preclude interpretation of deviations from baseline at the level of the individual patient. However, it may be possible in the near-term to routinely apply functional imaging in animal and clinical trials of therapeutic interventions [22, 58–61], or in cases of (e.g. ACL) injury in patients, where defined steps of disease progression can be known, accelerated, and possibly prevented. While application of functional imaging in the long-term may benefit the asymptomatic patient, short-term applications may better suit efforts in the pharmaceutical industry and researchers working in the fields of regenerative medicine and tissue engineering.
Functional imaging is commonly assessed in OA using radiography [25], MRI [26, 27], and ultrasound [28] (Figure 2, Table 1). Functional imaging data is typically acquired at the tissue-scale, given that the maximum spatial resolution for many common imaging modalities (e.g. MRI, radiography) is on the order of hundreds of microns, or an order of magnitude larger than the typical diameter of single chondrocytes [62]. At the tissue-scale, the knee, hip, and ankle [63] are easily visualized by noninvasive imaging in vivo, as are tissue in the finger joints [64, 65]. For knee OA, imaging can visualize several tissues that are known to exhibit altered biomechanics in disease, including cartilage [66], bone [67–69], and ligament and meniscus [26, 70]. While future imaging studies may better address OA-associated early changes in cell death [40] or molecular targets [71], current functional imaging of biomechanics in OA is focused largely on the analysis of joint kinematics, and the quantification of tissue structure, morphology, and biomechanics.
Figure 2. Imaging commonly evaluates joint and tissue function in OA by radiography, MRI and ultrasound over a wide range of spatial resolutions.
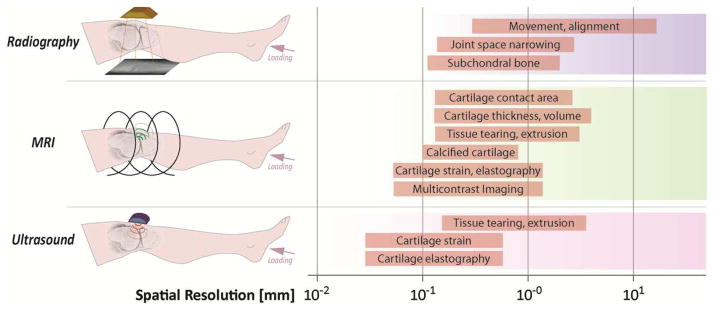
Radiography has historically identified advanced OA through the assessment of joint space narrowing as the cartilage is worn allowing bony surfaces to contact. MRI is a versatile method that can acquire data to assess joint space narrowing, directly visualize cartilage structure and morphology, and reveal internal patterns of strain in tissues active deep within the joint. Multiple contrasts (e.g. relaxivity, internal strains) are a promising new advance in MRI assessment of OA [151, 152]. Ultrasound shows promise for real time assessment of the joint tissues. Various functional measures are depicted over common spatial resolution ranges for each imaging modality.
Table 1. Primary imaging methods utilized for functional assessment of the joint tissues.
Magnetic resonance imaging is used broadly in numerous configurations to assess kinematics, structure, morphology, and mechanics, and provide the best means of directly imaging multiple functional measures in cartilage and related joint tissues. Multicontrast MRI is promising for the early assessment of OA, through the combined acquisition of independent measures that are sensitive to disease. Key references are included for each measure, with ‘+’ to ‘+++’ indicating an increasing degree of technique suitability and significance.
| Radiography | MRI | Ultrasound | |
|---|---|---|---|
|
| |||
| Joint Kinematics | |||
| Movement | +++ [73, 76, 78] | ++ [80, 81] | |
| Alignment | +++ [83] | +++ [83] | |
| Multimodal Imaging (combined use of fluoroscopy and MRI) | +++ [84–86] | +++ [84–86] | |
|
| |||
| Joint Structure and Morphology | |||
| Joint Space Narrowing | +++ [25] | ++ [108] | |
| Cartilage Contact Area | +++ [102–104] | ||
| Cartilage Thickness and Volume | +++ [108] | ||
| Meniscus Tearing and Extrusion | +++ [91] | + [115] | |
| Ligament Tearing | ++ [93] | ||
| Calcified Cartilage and Subchondral Bone | ++ [101] | +++ [94, 111] | |
| Bone Marrow Edema | ++ [95] | ||
|
| |||
| Cartilage Mechanics | |||
| Intratissue Strain | +++ [2, 129, 133] | + [154] | |
| Material Properties and Stress | ++ [122, 125] | + [155] | |
|
| |||
| Multicontrast Imaging | |||
| Relaxation Times | +++ [143] | ||
| Multicontrast Measures (combined use of strain, diffusion, magnetization transfer, or relaxation times) | +++ [151, 152] | ||
Functional Imaging and Joint Kinematics
Gross differences in gait patterns due to OA severity are well documented [57, 72–74]. While the analysis of gait is not strictly a functional imaging method focused on a specific tissue, is reasonable to expect that imaging-assessed gait in patients with moderate or severe OA would differ from asymptomatic individuals due to the likely increase in pain or joint structural alterations that would manifest in macroscopic, whole-body changes. Symptomatic individuals with mild knee OA (Kellegren-Lawrence grade of 2; described subsequently) showed increased medial compartment loading on average compared to asymptomatic individuals with the same radiograph-assessed OA grade [75], suggesting a role of biomechanics to distinguish between symptomatic and asymptomatic OA. Significant improvements in the detail of joint kinematic parameters can be seen in a number of imaging methods based on radiography [76, 77], computed tomography [65, 78, 79], and MRI assessed kinematics [80, 81] and joint alignment [82, 83] (Table 1). Interestingly, multimodal imaging and modeling analyses that integrate real-time acquisition capabilities of biplanar fluoroscopy with high-resolution and three-dimensional MRI allow for the careful in vivo evaluation of joint instability following ligament reconstruction [84, 85] and cartilage contact deformation under body weight [86]. Additionally, dual fluoroscopy alone has successfully differentiated between magnitudes of anterior tibial translation in the healthy knee following functional activities of increasing demand on the quadriceps [87].
Functional Imaging of Tissue Structure and Morphology
Imaging of the structure and morphology of joint tissues is a primary means to assess OA severity, with many of the same challenges apparent today that existed decades ago [88]. Quantifying the structure and morphology of cartilage in particular is a reasonable approach to assess OA in light of excessive wear observed in advanced stages of the disease [89]. However, it is doubtful whether pre-clinical OA in cartilage can be directly visualized, since the gross structure (i.e. thickness) remains relatively unchanged from normal (Figure 1), and findings of altered joint structure do not always inevitably lead to degeneration [90]. Alternatively, structural alterations can be visualized in numerous other joint tissues that may lead to degeneration, including meniscal tearing or extrusion [91, 92], ligament tearing [93], calcified cartilage and the osteochondral interface [94], and bone marrow edema [95]. Structure and morphology may therefore directly inform surgical planning and benefit patient-specific models [96], and are commonly assessed by radiography, MRI, and ultrasound.
Radiography
Radiography has been used for many decades to assess joint OA [25, 97]. Because cartilage is radiolucent compared to bone, radiography is best suited to quantify advanced OA as cartilage is lost giving way to excessive joint space narrowing [98]. The scale proposed by Kellgren and Lawrence [25] is a standard for radiological assessment of OA, finding use in multiple studies [75, 99]. Radiographic OA is assessed by evidence of osteophytes and possible joint space narrowing on a (0–4) graded scale, with: 0 (=no evidence), 1 (=questionable, but no direct evidence), 2 (=definite osteophytes, with or without joint space narrowing, or definite joint space narrowing with or without osteophytes), 3 (=at least 50% joint space narrowing), and 4 (=severe joint space narrowing and osteocytes). Modern techniques provide additional functional information that extend the capability of radiography for joint cartilage [100], and additionally focus on non-cartilage tissues such as subchondral bone that is visualized by computed tomography [101].
MRI
Cartilage is easily visualized by standard spin or gradient echo MRI techniques, with excellent soft tissue contrast compared to surrounding joint tissues, including (short T2) ligament and menisci. With the appropriate design of MRI pulse sequences, such as the use of fat-suppression pre-pulses, contrast can be enhanced to facilitate visualization and segmentation of the tissue. Cartilage and joint structure and morphology have been assessed using numerous quantities, including contact area [102–104], thickness and volume [99, 105–109], and diffusion-associated structural changes [4, 110]. For example, in a multi-center MRI study of 145 women, cartilage thickness changes approaching −4% were observed 6 months following baseline analysis in the central medial femorotibial joint of subjects showing Kellegren-Lawrence grades of 3, but not 2, suggesting sensitivity of thickness and joint space narrowing to OA progression [99]. These quantities, especially thickness and volume, can often be determined from standard MRI, making them especially attractive for use in multi-site studies with large patient populations.
The numerous other tissues in the joint are visualized by MRI to provide critical assessment of injury- and osteoarthritis-related structure and morphology. Meniscal tearing and extrusion have been visualized using T2- and intermediate-weighted fast spin echo (FSE) imaging, often with fat-suppression of the marrow space [91]. Tears in double-banded anterior talofibular ligaments were identified as the most common injury in subjects with sprained ankles using proton density-weighted FSE MRI [93]. Morphology of fibrocartilage and the osteochondral interface [94, 111] has been described by ultrashort echo time (UTE) MRI, a powerful method that takes advantage of TEs on the order of microseconds to visualize short T2 tissues. Preparation pulses prior to the UTE acquisition further selectively enable the quantitative analysis of relaxation times T2*, T1, and T1ρ in the zone of calcified cartilage [112], a subchondral mineralized tissue region beneath the tidemark whose mineral content, thickness, and stiffness likely play a role in the pathogenesis of OA [113]. Bone marrow edema-like lesions have been identified in donor tissue from patients scheduled for total knee arthroplasty using T2-weighted FSE images as focal subchondral areas of high intensity, which were associated with overlying cartilage regions of higher T1ρ and T2 [95]. Taken together, the structure, morphology, and relaxation times (discussed subsequently) of non-cartilage tissues can be visualized and quantified by MRI, and support the idea of the role of focal changes among joint tissues in the progression of OA [92]. Mechanical tearing, minearlization, and edema would all be expected to impact joint loading and function, leading to and further compounding cartilage and joint degeneration.
Ultrasound
Compared to the wide-spread use of MRI, fewer studies have quantified cartilage structure and morphology by ultrasound. Joint structures have been characterized in vivo in both the hip [28] and knee [114], and recent studies indicate potential indirect measures of the joint in OA, including meniscal subluxation [115].
FUNCTIONAL IMAGING OF CARTILAGE MECHANICS
While studies of cartilage mechanics using imaging have noninvasively quantified surface deformation (thickness) and volume changes during loading in patient populations [116–119], results from these studies provide limited information on the three-dimensional deformation occurring in the cartilage interior. Surface shape and volume alone does not account for complex internal mechanical behavior, such as deformation between arbitrary tissue locations, which is known to vary over the thickness of the tissue [47] and locally in the progression of OA [66].
Moreover, it is still unclear whether small structural changes in cartilage during the very earliest stages of OA can be detected by medical imaging. For example, the practical spatial resolution limit of clinical MRI for cartilage applications approaches pixel dimensions of ~200–300 μm. Not considering interpolation schemes [118], the change in the cartilage nominal (surface-to-bone) thickness by only 300 μm (i.e. a single pixel) may indicate complete loss of the superficial zone, a thin layer critical for low friction and normal joint sliding [71, 120]. It is further very challenging to reliably measure small changes in cartilage thickness of large patient populations, noting that the viscoelastic nature of the tissue can mean small deviations in tissue thickness develop even as the patient walks from waiting room to MRI scanner. Care must therefore be placed on the development of rigorous protocols that account for the history of loading in individual patients [119]. These challenges also suggest the importance of looking beyond structural and morphological measures alone to consider functional imaging methods that enhance sensitivity and specificity to disease severity (e.g. [64, 66, 121]).
An important consideration in the functional imaging of cartilage is whether measures of strain, stress, or material properties provide the best diagnostic utility for OA. A large number of MRI- and ultrasound-based techniques are available to map displacements and strain at high resolution, which are then coupled to material models to estimate stress [122] or material properties [123–126], most often classified as elastography [127]. Because the material properties of cartilage change in OA [8], controlled magnitudes of ex vivo or in vivo mechanical loading would likely result in aberrant deformation for progressively diseased tissue, suggesting that displacement or strain alone may be a sufficiently unique functional measure. On the other hand, calculation of stress and material properties potentially includes more information (e.g. boundary conditions, tissue morphology), revealing more subtle changes in the tissue. Unfortunately, conventional MR elastography for cartilage [125] is not yet easily adapted for in vivo imaging because of challenges in visualizing small, high frequency loading in thin (<3 mm thick) cartilage that is deeply embedded within the joint. As a result, high resolution measures of strain have instead been coupled to materials models for joint imaging [122].
Direct, high-resolution measures of cartilage deformation have been accomplished using two MRI-based displacement-encoding methods: 1) tag line registration, termed Cartilage Deformation by Tag Registration (CDTR) [47, 128–132], and 2) phase contrast, termed displacements under applied loading by MRI (dualMRI) [2, 133] (Figure 3). CDTR used preparatory pulses to generate a high-density grid pattern of thin tag lines that deform with the cartilage during mechanical loading, enabling the tracking of individual material points. Using CDTR, depth-dependent and three-dimensional strains were noninvasively documented and shown to be finite (i.e. large), especially in directions transverse to the loading direction, during physiologically-relevant compressive loading to articular cartilage explants [47]. However, one limitation to CDTR, especially considering its potential for translation to clinical imaging in vivo, is the difficulty in locating a large number of tag lines that are needed to track local tissue changes in the thin cartilage. A natural consequence of this limitation was the need to extrapolate deformation near the tissue boundaries, a problem that would be exacerbated using fewer tag lines or clinical MRI systems that typically acquire images with lower spatial resolution compared to dedicated animal systems [128, 134].
Figure 3. Multicontrast imaging of human joint cartilage is possible through complementary MRI techniques.
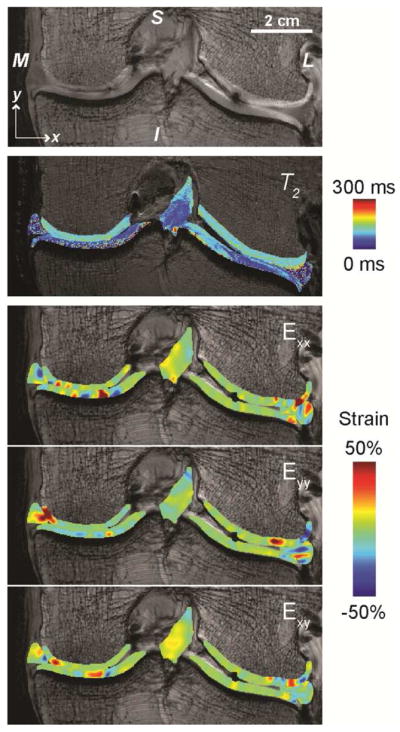
Standard MRI (e.g. fast spin echo pulse sequence) depicts morphology of the left knee joint in the coronal plane (M=medial, L=lateral, S=superior, I=inferior). Spatial maps of relaxivity (e.g. T2) can be visualized in cartilage, ligament, and meniscus tissues. Compressive loading to the joint in the inferior to superior direction, determined by dualMRI, results in complex internal strain patterns that can be related to disease severity, material properties of the tissues, and contact conditions in the loaded joint. The data is adapted from [2].
dualMRI is a phase contrast method that integrates actions of an MRI-compatible mechanical loading device with specialized MRI code (i.e. pulse sequences) and post-processing algorithms to reveal internal cartilage deformation [2]. dualMRI can acquire strain data at each three-dimensional pixel location depicting the image, a distinct advantage over CDTR, in either ramp-and-hold configurations or with high spatiotemporal resolution, approaching 100 μm pixel dimensions with a temporal resolution better than 3 ms [2]. Mechanical loading is typically applied to mimic the walking cycle (i.e. ~0.1–0.5 Hz) with physiologically-relevant load magnitudes (~0.5–1× body weight) resulting in large deformation in the cartilage [2, 133]. The high displacement and strain precision of dualMRI (on the order of 11 μm and 0.1%, respectively [2]) suggests that the technique is highly sensitive to measure small local changes in cartilage strain during OA that indicate altered stiffness and degenerative changes. The deformation data has permitted the description of cartilage strain in OA [66], repair models [135], intact joints [136], and in relation to stress and material properties [122]. Moreover, the fast acquisition of the dualMRI data has permitted direct measurement of cartilage strain in vivo on clinical MRI systems for the first time [137], hinting at the future possibility for human studies of joint disease and repair.
BIOMECHANICS AND MULTICONTRAST IMAGING
There are a growing number of imaging biomarkers that show great potential and promise for the diagnosis of early OA. Within the last decade alone, data from a number of longitudinal (e.g. Osteoarthritis Initiative) studies in large patient populations is emerging [138, 139], which incorporate new and advanced pulse sequences and techniques [140]. Biomarkers that provide information beyond cartilage structure and morphology alone [141, 142], to now include quantitative measures of the extracellular matrix (e.g. relaxivity mapping of T1ρ, T1 and T2), indicate an increasing promise for the reliable diagnosis of early OA. For a more complete review of the relative potential of individual quantitative MRI methods, please refer to [143].
Quantitative MRI techniques have been correlated to cartilage biomechanics, and used to complement structural and morphological information in studies of patient populations. The cartilage ECM is generally associated with measures of gadolinium-enhanced T1 (proteoglycan [144]), T1ρ (water, proteoglycan, collagen [145]), and T2 (collagen [146]). Glycosaminoglycan content, assessed by gadolinium-enhanced imaging (i.e. dGEMRIC), has been correlated to surface indentation measures of cartilage mechanical properties [147], suggesting the possibility that cartilage function can be indirectly determined by imaging the spatial location of ECM molecules (e.g. proteoglycans) that contribute to compressive stiffness. T1ρ has assessed symptomatic osteoarthritis in subjects [148] and biphasic material properties (aggregate modulus and hydraulic permeability) of cartilage in a cytokine-induced model of degeneration [149]. T2 and cartilage thickness decreased in superficial zone cartilage following running [106], and T2 correlated with body mass index in patients [150], demonstrating complementary combinations of relaxivity and structural measures. Importantly, conventional quantitative MRI techniques detect mid-stage to advanced OA where large changes in the cartilage ECM occur during degeneration. It is not clear whether quantitative MRI can be sufficiently sensitive and specific to detect early OA, especially at the level of individual patients, though tremendous promise still remains for early OA diagnosis for a wide range of techniques [143].
Recent exciting approaches that combine complementary biomarkers, often termed as ‘multicontrast’ or ‘multiparametric’ methods, show increased sensitivity and specificity for cartilage function and OA. Interestingly, the use of multiple independent assessment of disease severity is not unlike the development of WOMAC and related scoring systems [18, 19, 22, 49]. In one study, authors combined dualMRI, relaxivity measures (e.g. T1ρ, T1 and T2; Figure 3), and standard MRI (e.g. thickness) in a statistical model to quantify the ability to detect OA severity in human cartilage explants from donors undergoing total knee replacement surgery [151]. From this study, two-dimensional finite and Von Mises strains from dualMRI were strong predictors of histologically-assessed OA severity compared to relaxivity or standard MRI, but the combine approach of multicontrast MRI, inclusive of all markers, was the strongest predictor overall. In another study [152], authors combined the apparent diffusion coefficient, T1, T2, and magnetization transfer rate in a multiparametric analysis to evaluate control and degraded bovine nasal cartilage. Sensitivity was improved through the multiparametric analysis, with potential to expand to other biomaterials and the analysis of human tissues. These studies, along with contrast-enhanced MRI that identifies specific chemical targets [153], show exciting potential and promise for future functional imaging approaches aimed as diagnosing early OA.
CONCLUSIONS
Functional imaging of cartilage encompasses a growing number of outstanding techniques that show potential to evaluate biomechanics in early OA. Imaging modalities are available to researchers to quantify detailed structure, morphology, motion, and material properties of joint tissues, and MRI-based techniques currently provide the greatest potential to assess multiple measures of joint function noninvasively compared to techniques based on radiography or ultrasound. Internal strains, imaged by techniques such as dualMRI, may represent the most sensitive current measure of OA severity, which is only enhanced when combined with other independent measures. Given the slow and natural degradation of cartilage matrix molecules over the time course of the disease, combined imaging modalities that provide multiple contrasts, reflecting the deterioration of specific cartilage macromolecules and biomechanical parameters, likely will provide the best sensitivity and specificity to early OA. Toward this end, researchers should continue to place a premium effort on 1) the development of functional imaging modalities that provide robust data at the level of the individual patient, 2) advancement and validation of biomechanics and multicontrast techniques in human populations, and 3) the appropriate refinement and optimization of the functional imaging to evaluate asymptomatic versus symptomatic patients, as well as time-course changes following administered therapeutic agents in defined animal and human trials.
Acknowledgments
This work was supported in part by grants from NSF (CMMI 1100554) and NIH (R01 AR063712 and R21 AR064178).
Footnotes
CONFLICT OF INTERESTS STATEMENT
There are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Corey P. Neu is the sole author of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Muir ER, Watts LT, Tiwari YV, Bresnen A, Shen Q, Duong TQ. Quantitative cerebral blood flow measurements using MRI. Methods Mol Biol. 2014;1135:205–211. doi: 10.1007/978-1-4939-0320-7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan DD, Neu CP. Transient and microscale deformations and strains measured under exogenous loading by noninvasive magnetic resonance. PloS one. 2012;7:e33463. doi: 10.1371/journal.pone.0033463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burstein D, Gray ML, Hartman AL, Gipe R, Foy BD. Diffusion of small solutes in cartilage as measured by nuclear magnetic resonance (NMR) spectroscopy and imaging. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1993;11:465–478. doi: 10.1002/jor.1100110402. [DOI] [PubMed] [Google Scholar]
- 5.Haris M, Singh A, Cai K, Kogan F, McGarvey J, Debrosse C, et al. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nat Med. 2014;20:209–214. doi: 10.1038/nm.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Setton LA, Elliott DM, Mow VC. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 1999;7:2–14. doi: 10.1053/joca.1998.0170. [DOI] [PubMed] [Google Scholar]
- 8.Setton LA, Mow VC, Muller FJ, Pita JC, Howell DS. Mechanical properties of canine articular cartilage are significantly altered following transection of the anterior cruciate ligament. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1994;12:451–463. doi: 10.1002/jor.1100120402. [DOI] [PubMed] [Google Scholar]
- 9.Praemer A, Furner S, Rice DP. American Academy of Orthopaedic Surgeons. 1999. Musculoskeletal conditions in the United States. [Google Scholar]
- 10.Moos V, Fickert S, Muller B, Weber U, Sieper J. Immunohistological analysis of cytokine expression in human osteoarthritic and healthy cartilage. J Rheumatol. 1999;26:870–879. [PubMed] [Google Scholar]
- 11.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.van den Berg WB, Joosten LA, Kollias G, van De Loo FA. Role of tumour necrosis factor alpha in experimental arthritis: separate activity of interleukin 1beta in chronicity and cartilage destruction. Annals of the rheumatic diseases. 1999;58 (Suppl 1):I40–48. doi: 10.1136/ard.58.2008.i40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki E, Tsuda E, Yamamoto Y, Maeda S, Inoue R, Chiba D, et al. Nocturnal knee pain increases with the severity of knee osteoarthritis, disturbing patient sleep quality. Arthritis care & research. 2014 doi: 10.1002/acr.22258. [DOI] [PubMed] [Google Scholar]
- 14.Bellamy N, Campbell J, Haraoui B, Buchbinder R, Hobby K, Roth JH, et al. Dimensionality and clinical importance of pain and disability in hand osteoarthritis: Development of the Australian/Canadian (AUSCAN) Osteoarthritis Hand Index. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2002;10:855–862. doi: 10.1053/joca.2002.0837. [DOI] [PubMed] [Google Scholar]
- 15.Lanyon P, O’Reilly S, Jones A, Doherty M. Radiographic assessment of symptomatic knee osteoarthritis in the community: definitions and normal joint space. Annals of the rheumatic diseases. 1998;57:595–601. doi: 10.1136/ard.57.10.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson M, Jones BS. Evaluation of needle biopsy of synovial membrane. Annals of the rheumatic diseases. 1963;22:100–105. doi: 10.1136/ard.22.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawker G, Melfi C, Paul J, Green R, Bombardier C. Comparison of a generic (SF-36) and a disease specific (WOMAC) (Western Ontario and McMaster Universities Osteoarthritis Index) instrument in the measurement of outcomes after knee replacement surgery. The Journal of rheumatology. 1995;22:1193–1196. [PubMed] [Google Scholar]
- 18.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of rheumatology. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 19.Bellamy N, Wells G, Campbell J. Relationship between severity and clinical importance of symptoms in osteoarthritis. Clinical rheumatology. 1991;10:138–143. doi: 10.1007/BF02207652. [DOI] [PubMed] [Google Scholar]
- 20.Konstantinidis GA, Aletras VH, Kanakari KA, Natsis K, Bellamy N, Niakas D. Comparative validation of the WOMAC osteoarthritis and Lequesne algofunctional indices in Greek patients with hip or knee osteoarthritis. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2013 doi: 10.1007/s11136-013-0490-x. [DOI] [PubMed] [Google Scholar]
- 21.Bellamy N, Wilson C, Hendrikz J. Population-based normative values for the Western Ontario and McMaster (WOMAC) Osteoarthritis Index: part I. Seminars in arthritis and rheumatism. 2011;41:139–148. doi: 10.1016/j.semarthrit.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Theiler R, Ghosh P, Brooks P. Clinical, biochemical and imaging methods of assessing osteoarthritis and clinical trials with agents claiming ‘chondromodulating’ activity. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 1994;2:1–23. doi: 10.1016/s1063-4584(05)80002-0. [DOI] [PubMed] [Google Scholar]
- 23.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. Journal of clinical epidemiology. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 24.Martin KA, Rejeski WJ, Miller ME, James MK, Ettinger WH, Jr, Messier SP. Validation of the PASE in older adults with knee pain and physical disability. Medicine and science in sports and exercise. 1999;31:627–633. doi: 10.1097/00005768-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Annals of the rheumatic diseases. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:776–786. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Iagnocco A, Filippucci E, Riente L, Meenagh G, Delle Sedie A, Sakellariu G, et al. Ultrasound imaging for the rheumatologist XLI. Sonographic assessment of the hip in OA patients Clinical and experimental rheumatology. 2012;30:652–657. [PubMed] [Google Scholar]
- 29.Gofton JP. Studies in osteoarthritis of the hip. IV. Biomechanics and clinical considerations. Canadian Medical Association journal. 1971;104:1007–1011. [PMC free article] [PubMed] [Google Scholar]
- 30.Frankel VH, Burstein AH, Brooks DB. Biomechanics of internal derangement of the knee. Pathomechanics as determined by analysis of the instant centers of motion. The Journal of bone and joint surgery. 1971;53:945–962. American volume. [PubMed] [Google Scholar]
- 31.Maquet P. The biomechanics of the knee and surgical possibilities of healing osteoarthritic knee joints. Clinical orthopaedics and related research. 1980:102–110. [PubMed] [Google Scholar]
- 32.Malemud CJ, Martel-Pelletier J, Pelletier JP. Degradation of extracellular matrix in osteoarthritis: 4 fundamental questions. The Journal of rheumatology. 1987;14(Spec No):20–22. [PubMed] [Google Scholar]
- 33.Adams ME. Cartilage hypertrophy following canine anterior cruciate ligament transection differs among different areas of the joint. The Journal of rheumatology. 1989;16:818–824. [PubMed] [Google Scholar]
- 34.Friel NA, Chu CR. The role of ACL injury in the development of posttraumatic knee osteoarthritis. Clinics in sports medicine. 2013;32:1–12. doi: 10.1016/j.csm.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang YZ, Zhang SF, Qi YY, Wang LL, Ouyang HW. Cell transplantation for articular cartilage defects: principles of past, present, and future practice. Cell transplantation. 2011;20:593–607. doi: 10.3727/096368910X532738. [DOI] [PubMed] [Google Scholar]
- 36.Petty CA, Lubowitz JH. Does arthroscopic partial meniscectomy result in knee osteoarthritis? A systematic review with a minimum of 8 years’ follow-up. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2011;27:419–424. doi: 10.1016/j.arthro.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Felson DT. Osteoarthritis as a disease of mechanics. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:10–15. doi: 10.1016/j.joca.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muthuri SG, Hui M, Doherty M, Zhang W. What if we prevent obesity? Risk reduction in knee osteoarthritis estimated through a meta-analysis of observational studies. Arthritis care & research. 2011;63:982–990. doi: 10.1002/acr.20464. [DOI] [PubMed] [Google Scholar]
- 39.Wilson W, van Burken C, van Donkelaar C, Buma P, van Rietbergen B, Huiskes R. Causes of mechanically induced collagen damage in articular cartilage. J Orthop Res. 2006;24:220–228. doi: 10.1002/jor.20027. [DOI] [PubMed] [Google Scholar]
- 40.Phillips DM, Haut RC. The use of a non-ionic surfactant (P188) to save chondrocytes from necrosis following impact loading of chondral explants. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2004;22:1135–1142. doi: 10.1016/j.orthres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale ME, McLaughlin S, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. The Journal of bone and joint surgery. 2003;85-A:4–9. doi: 10.2106/00004623-200301000-00002. American volume. [DOI] [PubMed] [Google Scholar]
- 42.Masrouha KZ, Anderson DD, Thomas TP, Kuhl LL, Brown TD, Marsh JL. Acute articular fracture severity and chronic cartilage stress challenge as quantitative risk factors for post-traumatic osteoarthritis: illustrative cases. Iowa Orthop J. 2010;30:47–54. [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 2005;52:2386–2395. doi: 10.1002/art.21215. [DOI] [PubMed] [Google Scholar]
- 44.Stevens AL, Wishnok JS, White FM, Grodzinsky AJ, Tannenbaum SR. Mechanical injury and cytokines cause loss of cartilage integrity and upregulate proteins associated with catabolism, immunity, inflammation, and repair. Mol Cell Proteomics. 2009;8:1475–1489. doi: 10.1074/mcp.M800181-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klatt AR, Paul-Klausch B, Klinger G, Kühn G, Renno JH, Banerjee M, et al. A critical role for collagen II in cartilage matrix degradation: Collagen II induces pro-inflammatory cytokines and MMPs in primary human chondrocytes. Journal of Orthopaedic Research. 2009;27:65–70. doi: 10.1002/jor.20716. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz I, Ariyoshi W, Takahashi N, Knudson CB, Knudson W. Hyaluronan oligosaccharide treatment of chondrocytes stimulates expression of both HAS-2 and MMP-3, but by different signaling pathways. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:447–454. doi: 10.1016/j.joca.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neu CP, Hull ML, Walton JH. Heterogeneous three-dimensional strain fields during unconfined cyclic compression in bovine articular cartilage explants. J Orthop Res. 2005;23:1390–1398. doi: 10.1016/j.orthres.2005.03.022.1100230622. [DOI] [PubMed] [Google Scholar]
- 48.Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, et al. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19172–19177. doi: 10.1073/pnas.1210422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chundru R, Baum T, Nardo L, Nevitt MC, Lynch J, McCulloch CE, et al. Focal knee lesions in knee pairs of asymptomatic and symptomatic subjects with OA risk factors--data from the Osteoarthritis Initiative. European journal of radiology. 2013;82:e367–373. doi: 10.1016/j.ejrad.2013.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trippel S, Cucchiarini M, Madry H, Shi S, Wang C. Gene therapy for articular cartilage repair. Proceedings of the Institution of Mechanical Engineers Part H, Journal of engineering in medicine. 2007;221:451–459. doi: 10.1243/09544119JEIM237. [DOI] [PubMed] [Google Scholar]
- 51.Trippel SB, Ghivizzani SC, Nixon AJ. Gene-based approaches for the repair of articular cartilage. Gene therapy. 2004;11:351–359. doi: 10.1038/sj.gt.3302201. [DOI] [PubMed] [Google Scholar]
- 52.Valonen PK, Moutos FT, Kusanagi A, Moretti MG, Diekman BO, Welter JF, et al. In vitro generation of mechanically functional cartilage grafts based on adult human stem cells and 3D-woven poly(epsilon-caprolactone) scaffolds. Biomaterials. 2010;31:2193–2200. doi: 10.1016/j.biomaterials.2009.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu L, Cai X, Zhang S, Karperien M, Lin Y. Regeneration of articular cartilage by adipose tissue derived mesenchymal stem cells: perspectives from stem cell biology and molecular medicine. Journal of cellular physiology. 2013;228:938–944. doi: 10.1002/jcp.24255. [DOI] [PubMed] [Google Scholar]
- 54.Mow VC, Ratcliffe A, Poole AR. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 55.Lefevre N, Naouri JF, Bohu Y, Klouche S, Herman S. Sensitivity and specificity of bell-hammer tear as an indirect sign of partial anterior cruciate ligament rupture on magnetic resonance imaging. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2013 doi: 10.1007/s00167-013-2511-2. [DOI] [PubMed] [Google Scholar]
- 56.Zilkens C, Miese F, Kim YJ, Hosalkar H, Antoch G, Krauspe R, et al. Three-dimensional delayed gadolinium-enhanced magnetic resonance imaging of hip joint cartilage at 3T: a prospective controlled study. European journal of radiology. 2012;81:3420–3425. doi: 10.1016/j.ejrad.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 57.Creaby MW, Bennell KL, Hunt MA. Gait differs between unilateral and bilateral knee osteoarthritis. Archives of physical medicine and rehabilitation. 2012;93:822–827. doi: 10.1016/j.apmr.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 58.Kon E, Di Martino A, Filardo G, Tetta C, Busacca M, Iacono F, et al. Second-generation autologous chondrocyte transplantation: MRI findings and clinical correlations at a minimum 5-year follow-up. European journal of radiology. 2011;79:382–388. doi: 10.1016/j.ejrad.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Irrechukwu ON, Reiter DA, Lin PC, Roque RA, Fishbein KW, Spencer RG. Characterization of engineered cartilage constructs using multiexponential T(2) relaxation analysis and support vector regression. Tissue engineering Part C, Methods. 2012;18:433–443. doi: 10.1089/ten.tec.2011.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raynauld JP, Goldsmith CH, Bellamy N, Torrance GW, Polisson R, Belovich D, et al. Effectiveness and safety of repeat courses of hylan G-F 20 in patients with knee osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2005;13:111–119. doi: 10.1016/j.joca.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 61.Kerckhofs G, Sainz J, Wevers M, Van de Putte T, Schrooten J. Contrast-enhanced nanofocus computed tomography images the cartilage subtissue architecture in three dimensions. European cells & materials. 2013;25:179–189. doi: 10.22203/ecm.v025a13. [DOI] [PubMed] [Google Scholar]
- 62.Henderson JT, Shannon G, Veress AI, Neu CP. Direct measurement of intranuclear strain distributions and RNA synthesis in single cells embedded within native tissue. Biophysical journal. 2013;105:2252–2261. doi: 10.1016/j.bpj.2013.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Millington SA, Grabner M, Wozelka R, Anderson DD, Hurwitz SR, Crandall JR. Quantification of ankle articular cartilage topography and thickness using a high resolution stereophotography system. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;15:205–211. doi: 10.1016/j.joca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Xiao J, He J. Multispectral quantitative photoacoustic imaging of osteoarthritis in finger joints. Applied optics. 2010;49:5721–5727. doi: 10.1364/AO.49.005721. [DOI] [PubMed] [Google Scholar]
- 65.Halilaj E, Rainbow MJ, Got C, Schwartz JB, Moore DC, Weiss AP, et al. In Vivo Kinematics of the Thumb Carpometacarpal Joint During Three Isometric Functional Tasks. Clinical orthopaedics and related research. 2013 doi: 10.1007/s11999-013-3063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Griebel AJ, Trippel SB, Neu CP. Noninvasive dualMRI-based strains vary by depth and region in human osteoarthritic articular cartilage. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:394–400. doi: 10.1016/j.joca.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee JH, Dyke JP, Ballon D, Ciombor DM, Rosenwasser MP, Aaron RK. Subchondral fluid dynamics in a model of osteoarthritis: use of dynamic contrast-enhanced magnetic resonance imaging. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009;17:1350–1355. doi: 10.1016/j.joca.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McKinley TO, Bay BK. Trabecular bone strain changes associated with cartilage defects in the proximal and distal tibia. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2001;19:906–913. doi: 10.1016/S0736-0266(01)00011-0. [DOI] [PubMed] [Google Scholar]
- 69.Shakoor N, Dua A, Thorp LE, Mikolaitis RA, Wimmer MA, Foucher KC, et al. Asymmetric loading and bone mineral density at the asymptomatic knees of patients with unilateral hip osteoarthritis. Arthritis and rheumatism. 2011;63:3853–3858. doi: 10.1002/art.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang M, Radjenovic A, Stapleton TW, Venkatesh R, Williams S, Ingham E, et al. A novel and non-destructive method to examine meniscus architecture using 9. 4 Tesla. MRI Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:1417–1420. doi: 10.1016/j.joca.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan SM, Neu CP, Duraine G, Komvopoulos K, Reddi AH. Atomic force microscope investigation of the boundary-lubricant layer in articular cartilage. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:956–963. doi: 10.1016/j.joca.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 72.Ko SU, Ling SM, Schreiber C, Nesbitt M, Ferrucci L. Gait patterns during different walking conditions in older adults with and without knee osteoarthritis--results from the Baltimore Longitudinal Study of Aging. Gait & posture. 2011;33:205–210. doi: 10.1016/j.gaitpost.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thorp LE, Sumner DR, Block JA, Moisio KC, Shott S, Wimmer MA. Knee joint loading differs in individuals with mild compared with moderate medial knee osteoarthritis. Arthritis and rheumatism. 2006;54:3842–3849. doi: 10.1002/art.22247. [DOI] [PubMed] [Google Scholar]
- 74.Favre J, Erhart-Hledik JC, Andriacchi TP. Age-related differences in sagittal-plane knee function at heel-strike of walking are increased in osteoarthritic patients. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2014 doi: 10.1016/j.joca.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thorp LE, Sumner DR, Wimmer MA, Block JA. Relationship between pain and medial knee joint loading in mild radiographic knee osteoarthritis. Arthritis and rheumatism. 2007;57:1254–1260. doi: 10.1002/art.22991. [DOI] [PubMed] [Google Scholar]
- 76.Okamoto N, Breslauer L, Hedley AK, Mizuta H, Banks SA. In vivo knee kinematics in patients with bilateral total knee arthroplasty of 2 designs. The Journal of arthroplasty. 2011;26:914–918. doi: 10.1016/j.arth.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 77.Deneweth JM, Bey MJ, McLean SG, Lock TR, Kolowich PA, Tashman S. Tibiofemoral joint kinematics of the anterior cruciate ligament-reconstructed knee during a single-legged hop landing. The American journal of sports medicine. 2010;38:1820–1828. doi: 10.1177/0363546510365531. [DOI] [PubMed] [Google Scholar]
- 78.Leng S, Zhao K, Qu M, An KN, Berger R, McCollough CH. Dynamic CT technique for assessment of wrist joint instabilities. Medical physics. 2011;38 (Suppl 1):S50. doi: 10.1118/1.3577759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolfe SW, Neu C, Crisco JJ. In vivo scaphoid, lunate, and capitate kinematics in flexion and in extension. The Journal of hand surgery. 2000;25:860–869. doi: 10.1053/jhsu.2000.9423. [DOI] [PubMed] [Google Scholar]
- 80.Borotikar BS, Sheehan FT. In vivo patellofemoral contact mechanics during active extension using a novel dynamic MRI-based methodology. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:1886–1894. doi: 10.1016/j.joca.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borotikar BS, Sipprell WH, 3rd, Wible EE, Sheehan FT. A methodology to accurately quantify patellofemoral cartilage contact kinematics by combining 3D image shape registration and cine-PC MRI velocity data. Journal of biomechanics. 2012;45:1117–1122. doi: 10.1016/j.jbiomech.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalichman L, Zhu Y, Zhang Y, Niu J, Gale D, Felson DT, et al. The association between patella alignment and knee pain and function: an MRI study in persons with symptomatic knee osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;15:1235–1240. doi: 10.1016/j.joca.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 83.Hunter DJ, Wilson DR. Role of alignment and biomechanics in osteoarthritis and implications for imaging. Radiologic clinics of North America. 2009;47:553–566. doi: 10.1016/j.rcl.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 84.Abebe ES, Utturkar GM, Taylor DC, Spritzer CE, Kim JP, Moorman CT, 3rd, et al. The effects of femoral graft placement on in vivo knee kinematics after anterior cruciate ligament reconstruction. J Biomech. 2011;44:924–929. doi: 10.1016/j.jbiomech.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wainright WB, Spritzer CE, Lee JY, Easley ME, DeOrio JK, Nunley JA, et al. The effect of modified Brostrom-Gould repair for lateral ankle instability on in vivo tibiotalar kinematics. Am J Sports Med. 2012;40:2099–2104. doi: 10.1177/0363546512454840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wan L, de Asla RJ, Rubash HE, Li G. In vivo cartilage contact deformation of human ankle joints under full body weight. J Orthop Res. 2008;26:1081–1089. doi: 10.1002/jor.20593. [DOI] [PubMed] [Google Scholar]
- 87.Myers CA, Torry MR, Shelburne KB, Giphart JE, LaPrade RF, Woo SL, et al. In vivo tibiofemoral kinematics during 4 functional tasks of increasing demand using biplane fluoroscopy. Am J Sports Med. 2012;40:170–178. doi: 10.1177/0363546511423746. [DOI] [PubMed] [Google Scholar]
- 88.Hamerman D. Osteoarthritis. Orthopaedic review. 1988;17:353–360. [PubMed] [Google Scholar]
- 89.Neu CP, Reddi AH, Komvopoulos K, Schmid TM, Di Cesare PE. Increased friction coefficient and superficial zone protein expression in patients with advanced osteoarthritis. Arthritis and rheumatism. 2010;62:2680–2687. doi: 10.1002/art.27577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Picha BM, Konstantakos EK, Gordon DA. Incidence of bilateral scapholunate dissociation in symptomatic and asymptomatic wrists. The Journal of hand surgery. 2012;37:1130–1135. doi: 10.1016/j.jhsa.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 91.Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol. 2004;183:17–23. doi: 10.2214/ajr.183.1.1830017. [DOI] [PubMed] [Google Scholar]
- 92.Sharma L, Chmiel JS, Almagor O, Dunlop D, Guermazi A, Bathon J, et al. Significance of pre-radiographic MRI lesions in persons at higher risk for knee osteoarthritis. Arthritis Rheumatol. 2014 [Google Scholar]
- 93.Choo HJ, Lee SJ, Kim DW, Jeong HW, Gwak H. Multibanded anterior talofibular ligaments in normal ankles and sprained ankles using 3D isotropic proton density-weighted fast spin-echo MRI sequence. AJR Am J Roentgenol. 2014;202:W87–94. doi: 10.2214/AJR.13.10727. [DOI] [PubMed] [Google Scholar]
- 94.Bae WC, Dwek JR, Znamirowski R, Statum SM, Hermida JC, D’Lima DD, et al. Ultrashort echo time MR imaging of osteochondral junction of the knee at 3 T: identification of anatomic structures contributing to signal intensity. Radiology. 2010;254:837–845. doi: 10.1148/radiol.09081743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kazakia GJ, Kuo D, Schooler J, Siddiqui S, Shanbhag S, Bernstein G, et al. Bone and cartilage demonstrate changes localized to bone marrow edema-like lesions within osteoarthritic knees. Osteoarthritis Cartilage. 2013;21:94–101. doi: 10.1016/j.joca.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Henak CR, Anderson AE, Weiss JA. Subject-specific analysis of joint contact mechanics: application to the study of osteoarthritis and surgical planning. Journal of biomechanical engineering. 2013;135:021003. doi: 10.1115/1.4023386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kerr R, Resnick D, Pineda C, Haghighi P. Osteoarthritis of the glenohumeral joint: a radiologic-pathologic study. AJR American journal of roentgenology. 1985;144:967–972. doi: 10.2214/ajr.144.5.967. [DOI] [PubMed] [Google Scholar]
- 98.Buckland W. Radiographic assessment of osteoarthritis: comparison between existing methodologies. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 1999;7:430–433. doi: 10.1053/joca.1998.0234. [DOI] [PubMed] [Google Scholar]
- 99.Le Graverand MP, Buck RJ, Wyman BT, Vignon E, Mazzuca SA, Brandt KD, et al. Change in regional cartilage morphology and joint space width in osteoarthritis participants versus healthy controls: a multicentre study using 3. 0 Tesla MRI and Lyon-Schuss radiography. Annals of the rheumatic diseases. 2010;69:155–162. doi: 10.1136/ard.2008.099762. [DOI] [PubMed] [Google Scholar]
- 100.Xie L, Lin AS, Guldberg RE, Levenston ME. Nondestructive assessment of sGAG content and distribution in normal and degraded rat articular cartilage via EPIC-microCT. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:65–72. doi: 10.1016/j.joca.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burnett WD, Kontulainen SA, McLennan CE, Hunter DJ, Wilson DR, Johnston JD. Regional depth-specific subchondral bone density measures in osteoarthritic and normal patellae: in vivo precision and preliminary comparisons. Osteoporos Int. 2014;25:1107–1114. doi: 10.1007/s00198-013-2568-2. [DOI] [PubMed] [Google Scholar]
- 102.Shin CS, Souza RB, Kumar D, Link TM, Wyman BT, Majumdar S. In vivo tibiofemoral cartilage-to-cartilage contact area of females with medial osteoarthritis under acute loading using MRI. Journal of magnetic resonance imaging : JMRI. 2011;34:1405–1413. doi: 10.1002/jmri.22796. [DOI] [PubMed] [Google Scholar]
- 103.Wan L, de Asla RJ, Rubash HE, Li G. Determination of in-vivo articular cartilage contact areas of human talocrural joint under weightbearing conditions. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14:1294–1301. doi: 10.1016/j.joca.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 104.Hinterwimmer S, Gotthardt M, von Eisenhart-Rothe R, Sauerland S, Siebert M, Vogl T, et al. In vivo contact areas of the knee in patients with patellar subluxation. Journal of biomechanics. 2005;38:2095–2101. doi: 10.1016/j.jbiomech.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 105.Cotofana S, Ring-Dimitriou S, Hudelmaier M, Himmer M, Wirth W, Sanger AM, et al. Effects of exercise intervention on knee morphology in middle-aged women: a longitudinal analysis using magnetic resonance imaging. Cells, tissues, organs. 2010;192:64–72. doi: 10.1159/000289816. [DOI] [PubMed] [Google Scholar]
- 106.Mosher TJ, Liu Y, Torok CM. Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:358–364. doi: 10.1016/j.joca.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Braunstein EM, Brandt KD, Albrecht M. MRI demonstration of hypertrophic articular cartilage repair in osteoarthritis. Skeletal radiology. 1990;19:335–339. doi: 10.1007/BF00193086. [DOI] [PubMed] [Google Scholar]
- 108.Graichen H, Jakob J, von Eisenhart-Rothe R, Englmeier KH, Reiser M, Eckstein F. Validation of cartilage volume and thickness measurements in the human shoulder with quantitative magnetic resonance imaging. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2003;11:475–482. doi: 10.1016/s1063-4584(03)00077-3. [DOI] [PubMed] [Google Scholar]
- 109.Favre J, Scanlan SF, Erhart-Hledik JC, Blazek K, Andriacchi TP. Patterns of femoral cartilage thickness are different in asymptomatic and osteoarthritic knees and can be used to detect disease-related differences between samples. Journal of biomechanical engineering. 2013;135:101002–101010. doi: 10.1115/1.4024629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Visser SK, Crawford RW, Pope JM. Structural adaptations in compressed articular cartilage measured by diffusion tensor imaging. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16:83–89. doi: 10.1016/j.joca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 111.Geiger D, Bae WC, Statum S, Du J, Chung CB. Quantitative 3D ultrashort time-to-echo (UTE) MRI and micro-CT (muCT) evaluation of the temporomandibular joint (TMJ) condylar morphology. Skeletal Radiol. 2014;43:19–25. doi: 10.1007/s00256-013-1738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Du J, Carl M, Bae WC, Statum S, Chang EY, Bydder GM, et al. Dual inversion recovery ultrashort echo time (DIR-UTE) imaging and quantification of the zone of calcified cartilage (ZCC) Osteoarthritis Cartilage. 2013;21:77–85. doi: 10.1016/j.joca.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Burr DB. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Cartilage. 2004;12 (Suppl A):S20–30. doi: 10.1016/j.joca.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 114.Esen S, Akarirmak U, Aydin FY, Unalan H. Clinical evaluation during the acute exacerbation of knee osteoarthritis: the impact of diagnostic ultrasonography. Rheumatology international. 2013;33:711–717. doi: 10.1007/s00296-012-2441-1. [DOI] [PubMed] [Google Scholar]
- 115.Acebes C, Romero FI, Contreras MA, Mahillo I, Herrero-Beaumont G. Dynamic ultrasound assessment of medial meniscal subluxation in knee osteoarthritis. Rheumatology. 2013;52:1443–1447. doi: 10.1093/rheumatology/ket110. [DOI] [PubMed] [Google Scholar]
- 116.Herberhold C, Stammberger T, Faber S, Putz R, Englmeier KH, Reiser M, et al. An MR-based technique for quantifying the deformation of articular cartilage during mechanical loading in an intact cadaver joint. Magn Reson Med. 1998;39:843–850. doi: 10.1002/mrm.1910390522. [DOI] [PubMed] [Google Scholar]
- 117.Kaufman JH, Regatte RR, Bolinger L, Kneeland JB, Reddy R, Leigh JS. A novel approach to observing articular cartilage deformation in vitro via magnetic resonance imaging. J Magn Reson Imaging. 1999;9:653–662. doi: 10.1002/(sici)1522-2586(199905)9:5<653::aid-jmri6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 118.Li H, Hosseini A, Li JS, Gill TJt, Li G. Quantitative magnetic resonance imaging (MRI) morphological analysis of knee cartilage in healthy and anterior cruciate ligament-injured knees. Knee Surg Sports Traumatol Arthrosc. 2012;20:1496–1502. doi: 10.1007/s00167-011-1723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Widmyer MR, Utturkar GM, Leddy HA, Coleman JL, Spritzer CE, Moorman CT, 3rd, et al. High body mass index is associated with increased diurnal strains in the articular cartilage of the knee. Arthritis Rheum. 2013;65:2615–2622. doi: 10.1002/art.38062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chan SM, Neu CP, DuRaine G, Komvopoulos K, Reddi AH. Tribological altruism: A sacrificial layer mechanism of synovial joint lubrication in articular cartilage. Journal of biomechanics. 2012;45:2426–2431. doi: 10.1016/j.jbiomech.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 121.Kim HR, So Y, Moon SG, Lee IS, Lee SH. Clinical value of (99m)Tc-methylene diphosphonate (MDP) bone single photon emission computed tomography (SPECT) in patients with knee osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16:212–218. doi: 10.1016/j.joca.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 122.Butz KD, Chan DD, Nauman EA, Neu CP. Stress distributions and material properties determined in articular cartilage from MRI-based finite strains. Journal of biomechanics. 2011;44:2667–2672. doi: 10.1016/j.jbiomech.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 123.Hardy PA, Ridler AC, Chiarot CB, Plewes DB, Henkelman RM. Imaging articular cartilage under compression--cartilage elastography. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2005;53:1065–1073. doi: 10.1002/mrm.20439. [DOI] [PubMed] [Google Scholar]
- 124.Lopez O, Amrami KK, Manduca A, Ehman RL. Characterization of the dynamic shear properties of hyaline cartilage using high-frequency dynamic MR elastography. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2008;59:356–364. doi: 10.1002/mrm.21474. [DOI] [PubMed] [Google Scholar]
- 125.Lopez O, Amrami KK, Manduca A, Rossman PJ, Ehman RL. Developments in dynamic MR elastography for in vitro biomechanical assessment of hyaline cartilage under high-frequency cyclical shear. Journal of magnetic resonance imaging : JMRI. 2007;25:310–320. doi: 10.1002/jmri.20857. [DOI] [PubMed] [Google Scholar]
- 126.Tervonen O, Dietz MJ, Carmichael SW, Ehman RL. MR imaging of knee hyaline cartilage: evaluation of two- and three-dimensional sequences. Journal of magnetic resonance imaging : JMRI. 1993;3:663–668. doi: 10.1002/jmri.1880030417. [DOI] [PubMed] [Google Scholar]
- 127.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 128.Neu CP, Hull ML, Walton JH. Error optimization of a three-dimensional magnetic resonance imaging tagging-based cartilage deformation technique. Magn Reson Med. 2005;54:1290–1294. doi: 10.1002/mrm.20669. [DOI] [PubMed] [Google Scholar]
- 129.Neu CP, Hull ML, Walton JH, Buonocore MH. MRI-based technique for determining nonuniform deformations throughout the volume of articular cartilage explants. Magn Reson Med. 2005;53:321–328. doi: 10.1002/mrm.20343. [DOI] [PubMed] [Google Scholar]
- 130.Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171:841–845. doi: 10.1148/radiology.171.3.2717762. [DOI] [PubMed] [Google Scholar]
- 131.Mosher TJ, Smith MB. A DANTE tagging sequence for the evaluation of translational sample motion. Magn Reson Med. 1990;15:334–339. doi: 10.1002/mrm.1910150215. [DOI] [PubMed] [Google Scholar]
- 132.Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology. 1988;169:59–63. doi: 10.1148/radiology.169.1.3420283. [DOI] [PubMed] [Google Scholar]
- 133.Neu CP, Walton JH. Displacement encoding for the measurement of cartilage deformation. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2008;59:149–155. doi: 10.1002/mrm.21464. [DOI] [PubMed] [Google Scholar]
- 134.Chan DD, Neu CP. Intervertebral disc internal deformation measured by displacements under applied loading with MRI at 3T. Magn Reson Med. 2013 doi: 10.1002/mrm.24757. [DOI] [PubMed] [Google Scholar]
- 135.Neu CP, Arastu HF, Curtiss S, Reddi AH. Characterization of engineered tissue construct mechanical function by magnetic resonance imaging. Journal of tissue engineering and regenerative medicine. 2009;3:477–485. doi: 10.1002/term.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chan DD, Neu CP, Hull ML. In situ deformation of cartilage in cyclically loaded tibiofemoral joints by displacement-encoded MRI. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009;17:1461–1468. doi: 10.1016/j.joca.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chan DD, Butz KD, Trippel S, Nauman EA, Neu CP. In vivo displacements and strains measured in human knee articular cartilage using dualMRI. Transactions of the Annual Meeting of the Orthopaedic Research Society; New Orleans, LA. 2014. [Google Scholar]
- 138.Bruns K, Svensson F, Turkiewicz A, Wirth W, Guermazi A, Eckstein F, et al. Meniscus body position and its change over four years in asymptomatic adults: a cohort study using data from the Osteoarthritis Initiative (OAI) BMC Musculoskelet Disord. 2014;15:32. doi: 10.1186/1471-2474-15-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lin W, Alizai H, Joseph GB, Srikhum W, Nevitt MC, Lynch JA, et al. Physical activity in relation to knee cartilage T2 progression measured with 3 T MRI over a period of 4 years: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2013;21:1558–1566. doi: 10.1016/j.joca.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gold GE, Burstein D, Dardzinski B, Lang P, Boada F, Mosher T. MRI of articular cartilage in OA: novel pulse sequences and compositional/functional markers. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14 (Suppl A):A76–86. doi: 10.1016/j.joca.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 141.Eckstein F, Glaser C. Measuring cartilage morphology with quantitative magnetic resonance imaging. Seminars in musculoskeletal radiology. 2004;8:329–353. doi: 10.1055/s-2004-861579. [DOI] [PubMed] [Google Scholar]
- 142.Eckstein F, Reiser M, Englmeier KH, Putz R. In vivo morphometry and functional analysis of human articular cartilage with quantitative magnetic resonance imaging--from image to data, from data to theory. Anatomy and embryology. 2001;203:147–173. doi: 10.1007/s004290000154. [DOI] [PubMed] [Google Scholar]
- 143.Chan DD, Neu CP. Probing articular cartilage damage and disease by quantitative magnetic resonance imaging. Journal of the Royal Society, Interface / the Royal Society. 2013;10:20120608. doi: 10.1098/rsif.2012.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36:665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 145.Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38:863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 146.Lammentausta E, Kiviranta P, Toyras J, Hyttinen MM, Kiviranta I, Nieminen MT, et al. Quantitative MRI of parallel changes of articular cartilage and underlying trabecular bone in degeneration. Osteoarthritis Cartilage. 2007;15:1149–1157. doi: 10.1016/j.joca.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 147.Samosky JT, Burstein D, Eric Grimson W, Howe R, Martin S, Gray ML. Spatially-localized correlation of dGEMRIC-measured GAG distribution and mechanical stiffness in the human tibial plateau. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2005;23:93–101. doi: 10.1016/j.orthres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 148.Regatte RR, Akella SV, Wheaton AJ, Lech G, Borthakur A, Kneeland JB, et al. 3D-T1rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Academic radiology. 2004;11:741–749. doi: 10.1016/j.acra.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 149.Wheaton AJ, Dodge GR, Elliott DM, Nicoll SB, Reddy R. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2005;54:1087–1093. doi: 10.1002/mrm.20678. [DOI] [PubMed] [Google Scholar]
- 150.Koff MF, Amrami KK, Kaufman KR. Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;15:198–204. doi: 10.1016/j.joca.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 151.Griebel AJ, Trippel SB, Emery NC, Neu CP. Noninvasive assessment of osteoarthritis severity in human explants by multicontrast MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2013 doi: 10.1002/mrm.24725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin PC, Reiter DA, Spencer RG. Classification of degraded cartilage through multiparametric MRI analysis. Journal of magnetic resonance. 2009;201:61–71. doi: 10.1016/j.jmr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rudin M, Beckmann N, Porszasz R, Reese T, Bochelen D, Sauter A. In vivo magnetic resonance methods in pharmaceutical research: current status and perspectives. NMR in biomedicine. 1999;12:69–97. doi: 10.1002/(sici)1099-1492(199904)12:2<69::aid-nbm548>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 154.Ginat DT, Hung G, Gardner TR, Konofagou EE. High-resolution ultrasound elastography of articular cartilage in vitro. Conf Proc IEEE Eng Med Biol Soc. 2006;(Suppl):6644–6647. doi: 10.1109/IEMBS.2006.260910. [DOI] [PubMed] [Google Scholar]
- 155.McCredie AJ, Stride E, Saffari N. Ultrasound elastography to determine the layered mechanical properties of articular cartilage and the importance of such structural characteristics under load. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4262–4265. doi: 10.1109/IEMBS.2009.5334589. [DOI] [PubMed] [Google Scholar]
- 156.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]


