Abstract
Extended spectrum β-lactamases (ESBL) are increasing rapidly worldwide. E. coli producing CTX-M type ESBLs are the most common clinically encountered. The majority of E. coli ESBL infections are represented by urinary tract infections, but they can also cause severe infections, for example, in the blood stream and central nervous system. Since E. coli is a common colonizer of the normal gut microbiota, increasing prevalence of ESBL-producing strains is particularly worrisome. Once disseminated in the community, containment of this resistance type will be challenging. The driver of ESBL-producing Enterobacteriaceae (EPE) is debated. Some suggest that the ESBL genes were introduced to particularly successful bacterial clones. Others imply that very successful plasmids drive the rapid dissemination. More research and epidemiological studies of strain types, plasmids and mobile genetic elements are needed for these questions to be answered. In order to combat, or at least slow down, the worrisome trend of increasing numbers of EPE more knowledge is also needed on persistence of EPE in carriers as well as better understanding of how antibiotic treatment and other risk factors affect persistence and further dissemination. This review aims at giving an overview of this global problem from a Nordic perspective.
Keywords: antibiotic resistance, extended spectrum β-lactamase, E. coli, K. pneumoniae, epidemiology
Extended spectrum β-lactamases (ESBLs) are enzymes produced by bacteria that degrade β-lactam antibiotics with extended spectrum (such as third-generation cephalosporins) (1). β-Lactams are extensively used for the treatment of common infections, for example, pneumonia and urinary tract infections (UTI), as well as for severe and life threatening infections such as bloodstream infections (BSI). β-Lactams are also frequently used as prophylaxis treatment before surgery.
Different nomenclatures have been proposed and debated for β-lactamases which includes several hundreds of enzymes (http://www.lahey.org/studies/webt.asp). Also what to include in the ESBL definition is debated. In this review, ESBLA as defined by Giske et al. will be used (2). The Giske et al. definition divides ESBL enzymes in three main groups; ESBLA, ESBLM and ESBLCARBA. ESBLA includes the most frequently found CTX-M enzymes as well as SHV and TEM enzymes that are horizontally transferrable and degraded by clavulanic acid. ESBLM are miscellaneous ESBLs where acquired AmpC represent the most common type. ESBLCARBA are enzymes conferring carbapenemase activity, such as metallo β-lactamases and KPC (2).
The CTX-Ms were first discovered in the 1980s and since 1995 they have spread to all continents. The numbers have since increased tremendously. The bla CTX-M genes are divided into five phylogroups; CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9 and CTX-M-25 (3, 4). The CTX-M-1 phylogroup includes bla CTX-M-15 which is the most dominant is found globally.
Enterobacteriaceae is the main bacterial family associated to ESBL production, of which E. coli and K. pneumoniae are most important.
Enterobacteriaceae are of clinical importance causing infections in the central nervous system, lower respiratory tract, bloodstream, gastrointestinal and urinary tract, but are also common colonizers of the gastrointestinal tract (GIT).
E. coli is the main causative agent of community-acquired UTI (5). Women are more affected than men due to anatomical differences.
K. pneumoniae can cause pneumonia, mostly in immunocompromised humans, and BSI. They are the second most common cause of UTI.
K. pneumoniae are to a larger extent acquired in hospital environments than in the community, for example, in patients with urinary catheters or immunocompromised patients (6).
As the ESBL-carrier rate in the population increases and nosocomial transmission becomes more common, the risk of being infected increases. ESBL-producing Enterobacteriaceae (EPE) often displays multidrug-resistant phenotypes further limiting the therapeutic options. Co-resistance with fluoroquinolones, aminoglycosides and trimethoprim are often found (7) which are common empirical treatment options for severely ill patients resulting in increased morbidity and mortality.
ESBLs also constitute a burden on health care systems conferring prolonged hospital stay. de Kraker and co-workers describe BSIs in Europe caused by E. coli isolates resistant to extended spectrum cephalosporins. They estimate the cost to >2,700 excess deaths and 18.1 million EUR, represented by >120,000 excess days of hospital stay (8).
Patients with severe infections are highly dependent on receiving adequate treatment early in the course of infection for a benign outcome. Many times the diagnostic procedures available are too slow and empirical treatment necessary. Knowledge of the local epidemiology is therefore very important. For a patient with BSI caused by resistant bacteria, such as an ESBL-producer, administration of an ineffective antibiotic can be lethal. Changing to an effective regimen after treatment failure might be too late (9). Peralta and coworkers studied the rate of adequate empirical treatment in BSIs caused by Enterobacteriaceae in 19 Spanish hospitals during 4 years. They found the empirical treatment to be inadequate in 48.8% of the cases. Twenty-four of the 387 patients included in the study died during the first 3 days of inadequate therapy (10).
National and global epidemiology is also important since it provides ways to detect emerging clones or new types of resistant bacteria. A wider perspective can bring valuable information to the practitioners treating patients associated to other parts of the country or the world. Governmental institutions and agencies play an important role gathering data through different surveillance tools.
Epidemiology of CTX-M-genes
Different ESBL genotypes have different substrate specificities for the many β-lactam antibiotics (11), also between CTX-M enzymes. As mentioned bla CTX-Ms are found all over the world and bla CTX-M-15 is the most dominating genotype (12). However, local variations occur. In the Nordic countries, the distribution of genotypes is homogenous. In Sweden, 50–60% of ESBL-producing E. coli harbor bla CTX-M-15 followed by bla CTX-M-14 found in 10–15% (7). In Norway and Denmark, about 50% and 20% of the genotypes are bla CTX-M-15 and bla CTX-M-14 respectively (13, 14). The ESBL epidemiology in Finland has been sparsely reported, but a predominance of the CTX-M-1 group (including bla CTX-M-15) is found (15, 16).
In other parts of Europe, accumulation of different genotypes is found. In Spain, there is a high level of bla CTX-M-14 and bla CTX-M-9, and in Poland as well as several other eastern European countries bla CTX-M-3 has been reported as frequent (17, 18).
In the African and Australian continents, bla CTX-M-15 is dominating (19). In Canada, bla CTX-M-15 is also the most frequent and increasingly so in the United States of America, where bla SHV-phenotypes used to be the most encountered (20). Differences are seen between Asian countries. In Japan, the CTX-M-9 phylogroup is largely dominating (21) and also in China bla CTX-M-14 is more commonly reported (22). In India, bla CTX-M-15 is almost exclusively found (23). South America stands out with a wide distribution of the CTX-M 2 phylogroup. Also the CTX-M-8 phylogroup is found in South America which is rare on other continents (12, 19).
Dissemination of ESBLs
Transmission of genes encoding ESBL enzymes can occur either by emerging bacterial clones or by horizontal gene transfer. In the latter case, plasmids containing resistance genes are spread between bacteria of the same and/or different species.
The gut flora is an ideal reservoir for antibiotic resistance genes, where kilograms of bacteria of different species can interact, most often without causing disease and hence to much interference with the immune system. When antibiotics are used resistant strains gain a selective advantage and are accumulated. This increases the probability for genes important for survival to be further disseminated. Plasmids can contain resistance genes to several classes of antibiotics (24). This can explain the multiresistance often found amongst ESBL-producing bacteria.
Clonal expansion
Clonal expansion refers to a particular bacterial cell line multiplying and disseminating in a community or causing an outbreak, for example, in a hospital. Rapid detection of clonal expansions is important in order to be able to stop or diminish the source of dissemination. Hence, effective molecular epidemiological strain typing tools are crucial.
An example of clonal expansion is the E. coli sequence type 131 (ST131) that is found globally (25). ST131 is highly associated to bla CTX-M-15 (25). In the Nordic countries, ST131 represents 20–40% of the ESBL-producing E. coli (13, 14, 26). However, ST131 is also found in non-ESBL E. coli, suggesting that ST131 is a dominant E. coli type that has acquired ESBLs which may have contributed to the successful dissemination of these resistance genes (25. Successful STs of K. pneumoniae are also described. An example is the STs of clonal complex 11 (CC11) which have been associated to CTX-M dissemination (4, 27).
Outbreaks caused by EPE have occurred in the Nordic countries. The largest ones occurred in Uppsala, Sweden and Stavanger, Norway. The Uppsala outbreak, in 2005, was due to a bla CTX-M-15 producing K. pneumoniae that was spread over several ward departments of the University Hospital. In total, 64 patients were affected (28). Another large outbreak of bla CTX-M-15 producing K. pneumoniae occurred in a neonatal ward department of Stavanger University Hospital in 2008–2009. Fifty-eight infants were infected, probably for receiving contaminated breast milk (29).
Although ST131 represent a large fraction of the ESBL-producing E. coli population, still ~50% of Swedish isolates are genetically unrelated isolates. This indicates that horizontal gene transfer is a major route for disseminating ESBLs and that these resistance genes have a remarkable ability to enter E. coli of many different back-bones (7).
Horizontal dissemination
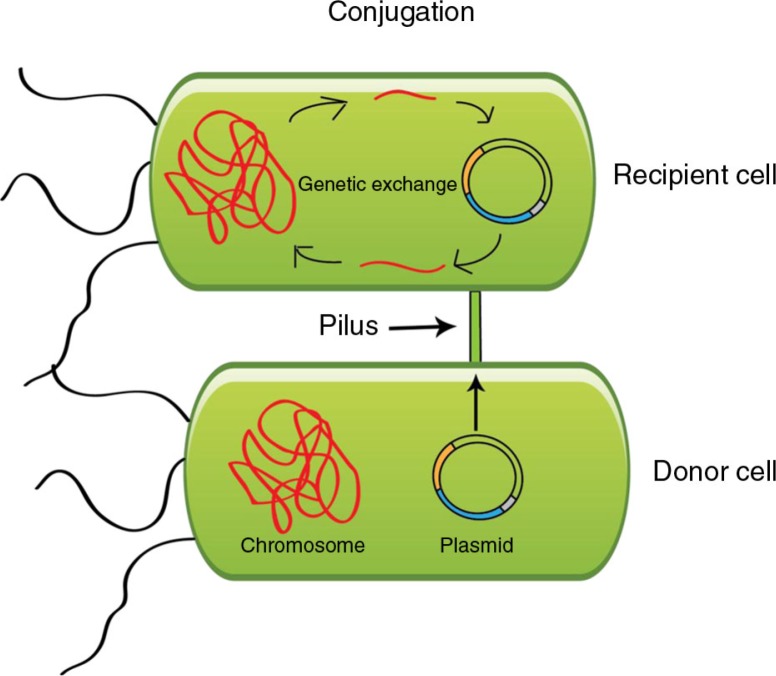
Plasmids are double-stranded extra-chromosomal DNA that replicate independently of the chromosome in the bacterial cell hosting it. Plasmids are highly diverse and the plasmid genome is often scattered with mobile genetic elements that can move genes around within the plasmid as well as between chromosome and plasmid(s). Examples of mobile genetic elements found on plasmids are IS-elements, transposons, integrons and insertion sequence common regions (ISCR), of which the latter is associated to ESBL genes. Plasmids have developed ways to secure their persistence in the bacterial cell. One example is through toxin–antitoxin systems that act by eliminating cells that have lost the plasmid after cell division (24). Figure 1 pictures a schematic illustration of plasmid conjugation which is a common route for plasmid dissemination.
Fig. 1.
Illustration of bacterial conjugation.
Plasmids can be classified according to incompatibility (Inc) groups, which are based on the principle that plasmids with the same replicon cannot be propagated stably in the same cell (30). The explanation for this is that similar plasmids compete over common cellular functions involved in, for example, plasmid replication control (31).
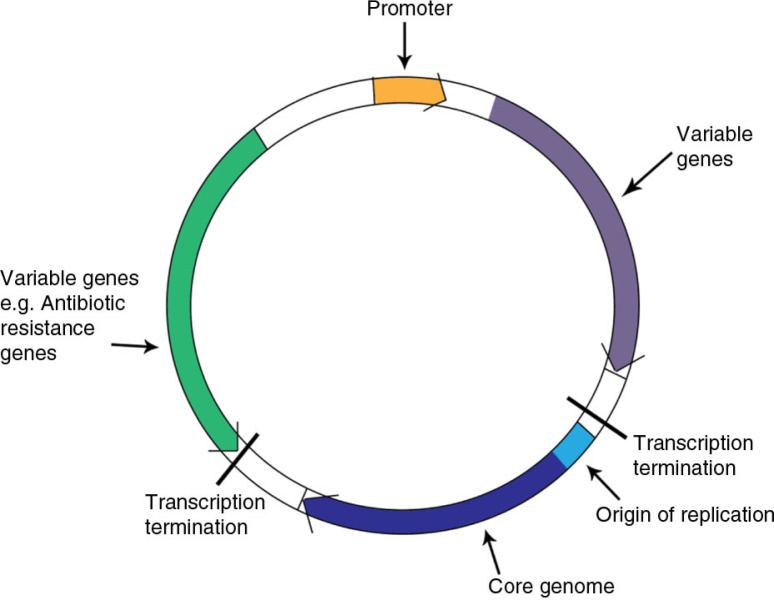
A replicon is a highly conserved part of the plasmid where genes encoding replication initiation, control, copy number, etc. are situated (illustrated as the plasmid core genome in Figure 2). Nowadays incompatibility grouping have been replaced by replicon typing which is simpler experimentally and also allows for a subdivision of some of the Inc groups. One example is the IncF1 plasmids that include repFIA, FIB and FIC of which repFIA and FIB plasmids are commonly found to harbor ESBL genes.
Fig. 2.
A schematic illustration of a plasmid.
The host range of plasmids is largely determined by the Inc type. IncF plasmids are large narrow host range plasmids with low copy number. They are the most common Inc type associated to CTX-M ESBLs. IncA/C plasmids, found associated to ESBLM and ESBLCARBA, are contrarily broad host range plasmids that are found in a wide variety of bacterial species. Some Inc groups are considered as endemic resistance plasmids in Enterobacteriaceae. These are IncF, IncA/C, IncL/M, IncN and IncI (32).
Among fully sequenced E. coli plasmids harboring bla CTX-M-genes in GenBank, the following plasmid families are found: IncF, IncN, IncN2, IncI1, IncHI2, IncL/M, IncA/C, IncK, IncX4, IncU and RCR (33).
Plasmid variability within incompatibility groups is high. In Norwegian and Swedish, ESBL-producing E. coli IncF plasmids are almost exclusively found (13, 34). Subtyping by replicon sequence typing (RST) however shows a great variability within F-plasmids, and it is not possible to link a specific IncF-plasmid to the successful dissemination of bla CTX-Ms (35). IncF plasmids are found also in antibiotic-sensitive Enterobacteriaceae suggesting that the ESBL genes have entered an already well-established plasmid type which has been beneficial for their dissemination (36).
Epidemiology of EPE
The Nordic countries are considered low prevalence countries, but the EPE numbers are increasing fast. In Sweden, national surveillance has been performed since 2007 when ESBLs were included in the communicable disease act. The number of cases have increased from 2099 in 2007 (Feb–Dec) to 8131 in 2013.
Several studies pictures the local frequency in different parts of Sweden; Helldal et al. described the prevalence of ESBL-producing E. coli in South Western Sweden during 2004–2008 with a higher increase in nosocomially acquired isolates in comparison to community-acquired, 0.2–2.5% and 0.2–1.6%, respectively (37). A study from the county of Östergötland indicated an increased prevalence of EPE from 2002 to 2007, yet the numbers were very low (<1%) (38). An investigation of the fecal carriage of ESBL-producing bacteria in both Primary Health Care Units and University Hospital setting in southern Sweden was conducted in 2008–2010. The only species found was E. coli, and the authors described the prevalence as higher than expected in both groups, 2.1–3% and 1.8–6.8%, respectively (39). A recent study from Uppsala investigated the prevalence of EPE in healthy preschool children compared to patients in the same age group. The authors found a carrier rate of 2.9% in healthy children compared to 8.4% in patients of the corresponding age group (40). In Helsinki, Finland, the annual incidence rate of EPE has gone from 0.5/100 000 inhabitants in the year 2000 to 69/100 000 in 2004 (15). In Denmark, the rate of invasive EPE has increased from 2.5% in 2006 to 6.2% in 2009 (41). The annual report of antibiotic resistance isolates in Norway, Norm/Norm-Vet, published in 2012 states that EPE is increasing steadily, and the number of EPE in blood cultures has increased from 3.3% in 2011 to 5.5% in 2012 (42).
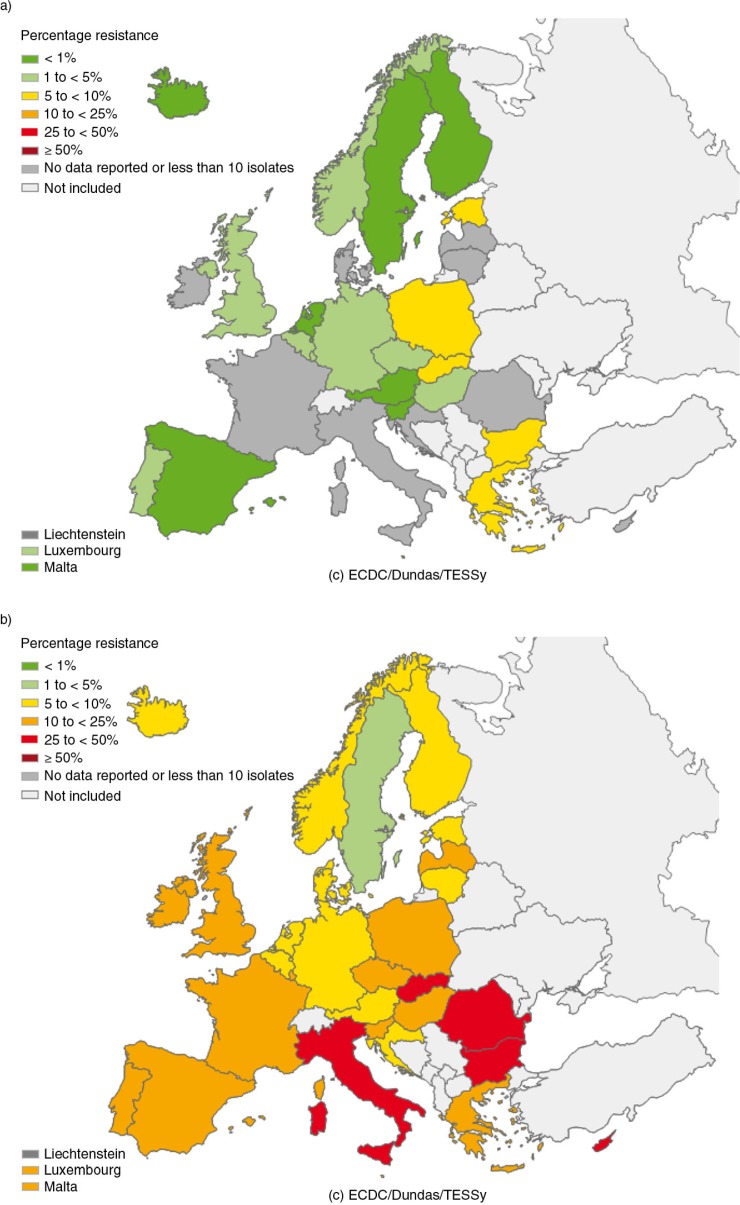
In Europe, the prevalence of bacteria resistant to extended spectrum cephalosporins differs a lot between countries. They are less frequent in the north and more common in the southern and eastern parts of Europe. The European network of national surveillance systems of antimicrobial resistance summarizes the levels of invasive infections caused by resistant bacteria in the European countries in a yearly report available on the ECDC homepage: http://ecdc.europa.eu/. The results are also illustrated on color-coded maps. In Fig. 3, levels of invasive E. coli infections resistant to third-generation cephalosporins are illustrated over time. However, one must keep in mind that the results are biased by differences in reporting, national monitoring and sampling.
Fig. 3.
Maps generated by EARS-Net illustrating the proportion of invasive E. coli isolates resistant or intermediately resistant to third-generation cephalosporins from reporting countries in a) 2001 and b) 2012.
Figure 3 is reprinted with permission from ECDC. Source: ECDC/EARS-Net 2001 and 2012.
Looking at countries outside Europe the ESBL prevalence also varies a lot. A recent study from the USA presents results from the SENTRY surveillance program including 26 hospitals from 20 states. The resistance levels to cephalosporins and/or aztreonam in invasive Enterobacteriaceae was 6.4% (43).
A Canadian study reported ESBL-production in almost 5% of the E. coli population – numbers similar to northern Europe (44).
The level of resistant bacteria is truly troublesome in other parts of the world. In Thailand, carriage of ESBL-producing bacteria, in as high numbers as 52% of healthy volunteers, has been reported (45). In India, the reports are also alarming with high numbers seen among patients. A study from two hospitals in Ujjain, India, collected consecutive isolates from incoming patients with abscesses, UTI, BSI, etc. and found ESBL production in 69% of E. coli (46).
One factor contributing to the low EPE numbers in the Nordic countries is the restricted antibiotic treatment policies. Antibiotics can only be purchased through prescriptions. But despite the strict antibiotic policy and decreasing antibiotic consumption, EPE is still increasing (47). It has been shown that one entry of EPE to low prevalence countries is through traveling to countries with higher prevalence. In 2010, Tängden et al. studied the acquisition of resistance genes as a result of travel outside northern Europe. Healthy travelers voluntarily participated in taking fecal samples before and after travel. The study resulted in 24% returning as new carriers of EPE after travel (48). Patients admitted to an Infectious Disease department in Aarhus, Denmark, who had been abroad within 3 months were identified and subjected to screening for multidrug resistant bacteria. EPE colonization was found in 12.5% of which >80% had been abroad for longer than 2 weeks (49).
Many countries outside Scandinavia (e.g. the member states of the European Union) also have antibiotic prescribing policies where prescription is required for antibiotics to be purchased. Other countries lack such regulations and the implementation of the policies differs between countries. Acquisition of antibiotics ‘over the counter’ in pharmacies or equivalents still occurs in many places. There are also differences in prescribing tradition and culture between countries. The climate affects the frequency of infections and also the rate of dissemination. Economical status, household conditions and population density also clearly play a role.
Infection control routines and programs have a large impact on dissemination of bacteria (50–52). Differences in infection control routines are substantial between different countries, also between European countries. Furthermore, the microbiological diagnostic traditions and setup of surveillance programs differ. If awareness of the regional situation is low, preventive measures will also be. More and more studies show that there is dissemination of bacteria between food, animal (both food-production animals and pets) and humans (53–56). Consequently antibiotic usage and inadequate infection control in veterinary medicine, fish farming, irrigation, etc. also contribute to the increased spread of antibiotic resistance.
Persistence of ESBLs
Little is known about the persistence of ESBL-producing bacteria in patients. This is mainly due to difficulties in studying persistence. Fecal cultures represent a snapshot of the flora at the time of study, and low numbers of bacteria can be difficult to detect. It has been shown that a person can present several negative samples and then later on still come up with a positive one (57). Another difficulty is that negative samples prior to colonization or confirmed infection seldom exist.
There are however some studies on the persistence of EPE. Most are conducted after detecting nosocomial outbreaks. One example is Löhr et al. who have followed children and their family members for 3 years after an outbreak in the neonatal intensive care unit in Stavanger, Norway. The authors observed carrier length of up to 2 years in some of the children (58). Persistence in neonates is proposed to be longer than in older children and adults since they have not yet established a naturally protective microbial flora. They also have an undeveloped immune system and are more often treated with antibiotics (58).
Alsterlund et al. studied EPE fecal colonization after a hospital outbreak in southern Sweden and found some individuals to still be colonized after 5 years (57). Another Swedish study by Tham et al. describes persistence of ESBL in 10% of patients after 3 years. The authors detected two new ESBL-producing strain types in one of the patients indicating transfer of the resistance phenotype (59). An alternative interpretation would be that this patient was infected with additional strains, although given the low frequency of EPE in Sweden the probability seems low. Low risk of re-colonization is beneficial when studying colonization rates, since the probability of finding the true colonization rate, duration of carriage and migration of plasmids between species increases. Titelman et al. studied persistence of EPE in 61 patients over the course of 1 year. By the end of the study period, 43% of the patients were still colonized (60). This study resulted in different scenarios in different patients. Some of the patients presented EPE of different species and molecular strain types carrying the same resistance gene over time picturing the interaction between the bacteria in the GIT. Colonization in other patients in the same study, however, presented one identical strain type with the same resistance gene over time (60). The factors behind these differences would be highly interesting to identify.
Dissemination and persistence of ESBL-producing bacteria have also been studied within households. A Spanish study reported that 16.7% of people living together with persons with known community-acquired infections also were colonized (61). Another Spanish study found fecal carriage in 27.4% of household members of known carriers. Relatives outside the household had a 15.6% carrier rate. These numbers can be compared with a control group of unrelated persons where the carrier rate was 7.4% (62). A Chinese study investigated ESBL carriage in children 0–5 years of age admitted to a pediatric clinic in Hong Kong and found CTX-M producing bacteria in 43.5%. In 83% of these children, at least one of the household members was also a carrier (63). A French study found intra-family transmission from adoptive children to their new family members at a rate of 23% (64).
A different way of studying persistence is through plasmid dissemination. Cottell et al. investigated a plasmid, pCT, harboring bla CTX-M-14 (the only resistance gene on that plasmid). They transformed the plasmid into an E. coli strain and found that it provided little or no additional fitness to the bacteria, but still the plasmid persisted successfully in the absence of selective antibiotic pressure. This suggests that persistence of the plasmid is not always dependent on the resistance gene (65).
The studies mentioned show that ESBLs can persist in carriers for several years. They are shared within households and disseminated in the community. More research is however needed to understand how persistence and dissemination can be minimized. Dissemination through plasmids adds a second dimension to this problem, and most probably prolongs the ability for resistance phenotypes to persist.
Conclusions
The impact of antibiotic treatment on the dissemination and persistence of resistant bacteria cannot be underestimated. In order to use these extremely important drugs as rational as possible, we need to gain full understanding of how resistance arises and all the factors that influence dissemination. Optimized antibiotic treatment is in turn dependent on the development of faster diagnostic tools in order to minimize empirical treatment.
Much research is still needed in this field. Strain and plasmid interaction and dissemination needs further attention in order to be fully understood. The persistence of EPE in the healthy population is also important to learn more about. Such knowledge would contribute to important information regarding how to handle patients colonized with EPE in hospitals and for how long persistence is to be expected under different conditions. It could also give us insight in how to optimize antibiotic treatment to be as effective as possible for patients and yet minimize further antibiotic resistance from arising and disseminating.
For antibiotics to be potent also in the future, promoting rational antibiotic use in all sectors minimizes the selective pressure for resistant bacteria. New antibiotic agents are also needed as well as optimizing the usage of the already available antibiotics. New treatment options can buy us time to address this important issue that constitutes a major public health problem.
Acknowledgements
This review is based on the doctoral thesis ‘Plasmid mediated antibiotic resistance – with focus on extended spectrum β-lactamases (ESBL),’ by Alma Brolund, 2013, published by Karolinska Institutet, ISBN 978–91–7549–210–0.
I gratefully acknowledge Karin Tegmark Wisell, Christian G Giske and Öjar Meleford for supervision during my research education. A special thanks also to countries providing data for EARS-Net at ECDC.
Conflict of interest and funding
The author has no conflict of interest.
References
- 1.Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18:657–86. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giske CG, Sundsfjord AS, Kahlmeter G, Woodford N, Nordmann P, Paterson DL, et al. Redefining extended-spectrum beta-lactamases: balancing science and clinical need. J Antimicrob Chemother. 2009;63:1–4. doi: 10.1093/jac/dkn444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birkett CI, Ludlam HA, Woodford N, Brown DF, Brown NM, Roberts MT, et al. Real-time TaqMan PCR for rapid detection and typing of genes encoding CTX-M extended-spectrum beta-lactamases. J Med Microbiol. 2007;56:52–5. doi: 10.1099/jmm.0.46909-0. [DOI] [PubMed] [Google Scholar]
- 4.D'Andrea MM, Arena F, Pallecchi L, Rossolini GM. CTX-M-type beta-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol. 2013;303:305–17. doi: 10.1016/j.ijmm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Abraham S, Chapman TA, Zhang R, Chin J, Mabbett AN, Totsika M, et al. Molecular characterization of Escherichia coli strains that cause symptomatic and asymptomatic urinary tract infections. J Clin Microbiol. 2012;50:1027–30. doi: 10.1128/JCM.06671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madigan MT, Martinko JM, Parker J. 10th ed. NJ, USA: Pearson Education, Inc., Upper Saddle iver; 2003. Brock biology of microorganisms. [Google Scholar]
- 7.Brolund A, Edquist PJ, Makitalo B, Olsson-Liljequist B, Soderblom T, Wisell KT, et al. Epidemiology of extended-spectrum beta-lactamase-producing Escherichia coli in Sweden 2007–2011. Clin Microbiol Infect. 2014;20:O344–52. doi: 10.1111/1469-0691.12413. [DOI] [PubMed] [Google Scholar]
- 8.de Kraker ME, Davey PG, Grundmann H, BURDEN study group Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med. 2011;8:e1001104. doi: 10.1371/journal.pmed.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giske CG, Monnet DL, Cars O, Carmeli Y, ReAct-Action on Antibiotic Resistance Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother. 2008;52:813–21. doi: 10.1128/AAC.01169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peralta G, Lamelo M, Alvarez-Garcia P, Velasco M, Delgado A, Horcajada JP, et al. Impact of empirical treatment in extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp. bacteremia. A multicentric cohort study. BMC Infect Dis. 2012;12:245. doi: 10.1186/1471-2334-12-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnet R. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother. 2004;48:1–14. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canton R, Coque TM. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol. 2006;9:466–75. doi: 10.1016/j.mib.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Naseer U, Haldorsen B, Tofteland S, Hegstad K, Scheutz F, Simonsen GS, et al. Molecular characterization of CTX-M-15-producing clinical isolates of Escherichia coli reveals the spread of multidrug-resistant ST131 (O25:H4) and ST964 (O102:H6) strains in Norway. APMIS. 2009;117:526–36. doi: 10.1111/j.1600-0463.2009.02465.x. [DOI] [PubMed] [Google Scholar]
- 14.Olesen B, Hansen DS, Nilsson F, Frimodt-Moller J, Leihof RF, Struve C, et al. Prevalence and characteristics of the epidemic multiresistant Escherichia coli ST131 clonal group among extended-spectrum beta-lactamase-producing E. coli isolates in Copenhagen, Denmark. J Clin Microbiol. 2013;51:1779–85. doi: 10.1128/JCM.00346-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forssten SD, Kolho E, Lauhio A, Lehtola L, Mero S, Oksaharju A, et al. Emergence of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae during the years 2000 and 2004 in Helsinki, Finland. Clin Microbiol Infect. 2010;16:1158–61. doi: 10.1111/j.1469-0691.2010.03068.x. [DOI] [PubMed] [Google Scholar]
- 16.Nyberg SD, Osterblad M, Hakanen AJ, Huovinen P, Jalava J, Resistance TF. Detection and molecular genetics of extended-spectrum beta-lactamases among cefuroxime-resistant Escherichia coli and Klebsiella spp. isolates from Finland, 2002–2004. Scand J Infect Dis. 2007;39:417–24. doi: 10.1080/00365540601105731. [DOI] [PubMed] [Google Scholar]
- 17.Baraniak A, Fiett J, Sulikowska A, Hryniewicz W, Gniadkowski M. Countrywide spread of CTX-M-3 extended-spectrum beta-lactamase-producing microorganisms of the family Enterobacteriaceae in Poland. Antimicrob Agents Chemother. 2002;46:151–9. doi: 10.1128/AAC.46.1.151-159.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livermore DM, Canton R, Gniadkowski M, Nordmann P, Rossolini GM, Arlet G, et al. CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother. 2007;59:165–74. doi: 10.1093/jac/dkl483. [DOI] [PubMed] [Google Scholar]
- 19.Naseer U, Sundsfjord A. The CTX-M conundrum: dissemination of plasmids and Escherichia coli clones. Microb Drug Resist. 2011;17:83–97. doi: 10.1089/mdr.2010.0132. [DOI] [PubMed] [Google Scholar]
- 20.Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, et al. Community-associated extended-spectrum beta-lactamase-producing Escherichia coli infection in the United States. Clin Infect Dis. 2013;56:641–8. doi: 10.1093/cid/cis942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong Y, Shimoda S, Yakushiji H, Ito Y, Miyamoto T, Kamimura T, et al. Community spread of extended-spectrum beta-lactamase-producing Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis: a long-term study in Japan. J Med Microbiol. 2013;62:1038–43. doi: 10.1099/jmm.0.059279-0. [DOI] [PubMed] [Google Scholar]
- 22.Xia S, Fan X, Huang Z, Xia L, Xiao M, Chen R, et al. Dominance of CTX-M-type extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli isolated from patients with community-onset and hospital-onset infection in China. PLoS One. 2014;9:e100707. doi: 10.1371/journal.pone.0100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muzaheed , Doi Y, Adams-Haduch JM, Endimiani A, Sidjabat HE, Gaddad SM, et al. High prevalence of CTX-M-15-producing Klebsiella pneumoniae among inpatients and outpatients with urinary tract infection in Southern India. J Antimicrob Chemother. 2008;61:1393–4. doi: 10.1093/jac/dkn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett PM. Plasmid encoded antibiotic resistance: acquisition and transfer of antibiotic resistance genes in bacteria. Br J Pharmacol. 2008;153:S347–57. doi: 10.1038/sj.bjp.0707607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolas-Chanoine MH, Bertrand X, Madec JY. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev. 2014;27:543–74. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brolund A, Edquist PJ, Makitalo B, Olsson-Liljequist B, Soderblom T, Tegmark Wisell K. Epidemiology of ESBL-producing Escherichia coli in Sweden 2007–2011. Clin Microbiol Infect. 2013;20(6):O344–52. doi: 10.1111/1469-0691.12413. [DOI] [PubMed] [Google Scholar]
- 27.Andrade LN, Vitali L, Gaspar GG, Bellissimo-Rodrigues F, Martinez R, Darini AL. Expansion and evolution of a virulent, extensively drug-resistant (polymyxin B-resistant), QnrS1-, CTX-M-2-, and KPC-2-producing Klebsiella pneumoniae ST11 international high-risk clone. J Clin Microbiol. 2014;52:2530–5. doi: 10.1128/JCM.00088-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lytsy B, Sandegren L, Tano E, Torell E, Andersson DI, Melhus A. The first major extended-spectrum beta-lactamase outbreak in Scandinavia was caused by clonal spread of a multiresistant Klebsiella pneumoniae producing CTX-M-15. APMIS. 2008;116:302–8. doi: 10.1111/j.1600-0463.2008.00922.x. [DOI] [PubMed] [Google Scholar]
- 29.Rettedal S, Lohr IH, Natas O, Giske CG, Sundsfjord A, Oymar K. First outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a Norwegian neonatal intensive care unit; associated with contaminated breast milk and resolved by strict cohorting. APMIS. 2012;120:612–21. doi: 10.1111/j.1600-0463.2012.02879.x. [DOI] [PubMed] [Google Scholar]
- 30.Novick RP. Plasmid incompatibility. Microbiol Rev. 1987;51:381–95. doi: 10.1128/mr.51.4.381-395.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Couturier M, Bex F, Bergquist PL, Maas WK. Identification and classification of bacterial plasmids. Microbiol Rev. 1988;52:375–95. doi: 10.1128/mr.52.3.375-395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carattoli A. Plasmids in Gram negatives: molecular typing of resistance plasmids. Int J Med Microbiol. 2011;301:654–8. doi: 10.1016/j.ijmm.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Carattoli A. Plasmids and the spread of resistance. Int J Med Microbiol. 2013;303:298–304. doi: 10.1016/j.ijmm.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Brolund A, Franzen O, Melefors O, Tegmark-Wisell K, Sandegren L. Plasmidome-analysis of ESBL-producing Escherichia coli using conventional typing and high-throughput sequencing. PLoS One. 2013;8:e65793. doi: 10.1371/journal.pone.0065793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villa L, Garcia-Fernandez A, Fortini D, Carattoli A. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J Antimicrob Chemother. 2010;65:2518–29. doi: 10.1093/jac/dkq347. [DOI] [PubMed] [Google Scholar]
- 36.Bengtsson S, Naseer U, Sundsfjord A, Kahlmeter G, Sundqvist M. Sequence types and plasmid carriage of uropathogenic Escherichia coli devoid of phenotypically detectable resistance. J Antimicrob Chemother. 2012;67:69–73. doi: 10.1093/jac/dkr421. [DOI] [PubMed] [Google Scholar]
- 37.Helldal L, Karami N, Floren K, Welinder-Olsson C, Moore ER, Ahren C. Shift of CTX-M genotypes has determined the increased prevalence of extended-spectrum beta-lactamase-producing Escherichia coli in south-western Sweden. Clin Microbiol Infect. 2013;19:E87–90. doi: 10.1111/1469-0691.12086. [DOI] [PubMed] [Google Scholar]
- 38.Ostholm-Balkhed A, Tarnberg M, Nilsson M, Johansson AV, Hanberger H, Monstein HJ, et al. Prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae and trends in antibiotic consumption in a county of Sweden. Scand J Infect Dis. 2010;42:831–8. doi: 10.3109/00365548.2010.498017. [DOI] [PubMed] [Google Scholar]
- 39.Stromdahl H, Tham J, Melander E, Walder M, Edquist PJ, Odenholt I. Prevalence of faecal ESBL carriage in the community and in a hospital setting in a county of Southern Sweden. Eur J Clin Microbiol Infect Dis. 2011;30:1159–62. doi: 10.1007/s10096-011-1202-5. [DOI] [PubMed] [Google Scholar]
- 40.Kaarme J, Molin Y, Olsen B, Melhus A. Prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in healthy Swedish preschool children. Acta Paediatr. 2013;102:655–60. doi: 10.1111/apa.12206. [DOI] [PubMed] [Google Scholar]
- 41.Hansen F, Olsen SS, Heltberg O, Justesen US, Fuglsang-Damgaard D, Knudsen JD, et al. Characterization of third-generation cephalosporin-resistant Escherichia coli from bloodstream infections in Denmark. Microb Drug Resist. 2014;20:316–24. doi: 10.1089/mdr.2013.0157. [DOI] [PubMed] [Google Scholar]
- 42.NORM/NORM-VET 2012. Tromsø/Oslo: 2013. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. [Google Scholar]
- 43.Castanheira M, Farrell SE, Deshpande LM, Mendes RE, Jones RN. Prevalence of beta-lactamase-encoding genes among Enterobacteriaceae bacteremia isolates collected in 26 U.S. hospitals: report from the SENTRY Antimicrobial Surveillance Program (2010) Antimicrob Agents Chemother. 2013;57:3012–20. doi: 10.1128/AAC.02252-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simner PJ, Zhanel GG, Pitout J, Tailor F, McCracken M, Mulvey MR, et al. Prevalence and characterization of extended-spectrum beta-lactamase- and AmpC beta-lactamase-producing Escherichia coli: results of the CANWARD 2007–2009 study. Diagn Microbiol Infect Dis. 2011;69:326–34. doi: 10.1016/j.diagmicrobio.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 45.Oteo J, Perez-Vazquez M, Campos J. Extended-spectrum [beta]-lactamase producing Escherichia coli: changing epidemiology and clinical impact. Curr Opin Infect Dis. 2010;23:320–6. doi: 10.1097/qco.0b013e3283398dc1. [DOI] [PubMed] [Google Scholar]
- 46.Pathak A, Marothi Y, Kekre V, Mahadik K, Macaden R, Lundborg CS. High prevalence of extended-spectrum beta-lactamase-producing pathogens: results of a surveillance study in two hospitals in Ujjain, India. Infect Drug Resist. 2012;5:65–73. doi: 10.2147/IDR.S30043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.SWEDRES-SVARM 2012. 2012. Use of antimicrobials and occurrence of antimicrobial resistance in Sweden. [Google Scholar]
- 48.Tangden T, Cars O, Melhus A, Lowdin E. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum beta-lactamases: a prospective study with Swedish volunteers. Antimicrob Agents Chemother. 2010;54:3564–8. doi: 10.1128/AAC.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lausch KR, Fuursted K, Larsen CS, Storgaard M. Colonisation with multi-resistant Enterobacteriaceae in hospitalised Danish patients with a history of recent travel: a cross-sectional study. Travel Med Infect Dis. 2013;11:320–3. doi: 10.1016/j.tmaid.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 50.March A, Aschbacher R, Pagani E, Sleghel F, Soelva G, Hopkins KL, et al. Changes in colonization of residents and staff of a long-term care facility and an adjacent acute-care hospital geriatric unit by multidrug-resistant bacteria over a four-year period. Scand J Infect Dis. 2014;46:114–22. doi: 10.3109/00365548.2013.859392. [DOI] [PubMed] [Google Scholar]
- 51.Jadhav S, Sahasrabudhe T, Kalley V, Gandham N. The microbial colonization profile of respiratory devices and the significance of the role of disinfection: a blinded study. J Clin Diagn Res. 2013;7:1021–6. doi: 10.7860/JCDR/2013/5681.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deege MP, Paterson DL. Reducing the development of antibiotic resistance in critical care units. Curr Pharm Biotechnol. 2011;12:2062–9. doi: 10.2174/138920111798808301. [DOI] [PubMed] [Google Scholar]
- 53.Trott D. β-Lactam resistance in gram-negative pathogens isolated from animals. Curr Pharm Des. 2013;19:239–49. [PubMed] [Google Scholar]
- 54.Reuland EA, Al Naiemi N, Raadsen SA, Savelkoul PH, Kluytmans JA, Vandenbroucke-Grauls CM. Prevalence of ESBL-producing Enterobacteriaceae in raw vegetables. Eur J Clin Microbiol Infect Dis. 2014 doi: 10.1007/s10096-014-2142-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Egervarn M, Borjesson S, Byfors S, Finn M, Kaipe C, Englund S, et al. Escherichia coli with extended-spectrum beta-lactamases or transferable AmpC beta-lactamases and Salmonella on meat imported into Sweden. Int J Food Microbiol. 2014;171:8–14. doi: 10.1016/j.ijfoodmicro.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Tschudin-Sutter S, Frei R, Stephan R, Hachler H, Nogarth D, Widmer AF. Extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae: a threat from the kitchen. Infect Control Hosp Epidemiol. 2014;35:581–4. doi: 10.1086/675831. [DOI] [PubMed] [Google Scholar]
- 57.Alsterlund R, Axelsson C, Olsson-Liljequist B. Long-term carriage of extended-spectrum beta-lactamase-producing Escherichia coli . Scand J Infect Dis. 2012;44:51–4. doi: 10.3109/00365548.2011.592987. [DOI] [PubMed] [Google Scholar]
- 58.Lohr IH, Rettedal S, Natas OB, Naseer U, Oymar K, Sundsfjord A. Long-term faecal carriage in infants and intra-household transmission of CTX-M-15-producing Klebsiella pneumoniae following a nosocomial outbreak. J Antimicrob Chemother. 2013;68:1043–8. doi: 10.1093/jac/dks502. [DOI] [PubMed] [Google Scholar]
- 59.Tham J, Walder M, Melander E, Odenholt I. Duration of colonization with extended-spectrum beta-lactamase-producing Escherichia coli in patients with travellers’ diarrhoea. Scand J Infect Dis. 2012;44:573–7. doi: 10.3109/00365548.2011.653582. [DOI] [PubMed] [Google Scholar]
- 60.Titelman E, Hasan CM, Iversen A, Naucler P, Kais M, Kalin M. Faecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae is common 12 months after infection and is related to strain factors. Clin Microbiol Infect. 2014 doi: 10.1111/1469-0691.12559. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 61.Valverde A, Grill F, Coque TM, Pintado V, Baquero F, Canton R, et al. High rate of intestinal colonization with extended-spectrum-beta-lactamase-producing organisms in household contacts of infected community patients. J Clin Microbiol. 2008;46:2796–9. doi: 10.1128/JCM.01008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez-Bano J, Lopez-Cerero L, Navarro MD, Diaz de Alba P, Pascual A. Faecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli: prevalence, risk factors and molecular epidemiology. J Antimicrob Chemother. 2008;62:1142–9. doi: 10.1093/jac/dkn293. [DOI] [PubMed] [Google Scholar]
- 63.Lo WU, Ho PL, Chow KH, Lai EL, Yeung F, Chiu SS. Fecal carriage of CTXM type extended-spectrum beta-lactamase-producing organisms by children and their household contacts. J Infect. 2010;60:286–92. doi: 10.1016/j.jinf.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Tande D, Boisrame-Gastrin S, Munck MR, Hery-Arnaud G, Gouriou S, Jallot N, et al. Intrafamilial transmission of extended-spectrum-beta-lactamase-producing Escherichia coli and Salmonella enterica Babelsberg among the families of internationally adopted children. J Antimicrob Chemother. 2010;65:859–65. doi: 10.1093/jac/dkq068. [DOI] [PubMed] [Google Scholar]
- 65.Cottell JL, Webber MA, Piddock LJ. Persistence of transferable extended-spectrum-beta-lactamase resistance in the absence of antibiotic pressure. Antimicrob Agents Chemother. 2012;56:4703–6. doi: 10.1128/AAC.00848-12. [DOI] [PMC free article] [PubMed] [Google Scholar]