Abstract
Objective
To compare quantitative magnetization transfer (qMT) parameters of patellar cartilage measured using cross relaxation imaging (CRI) in asymptomatic volunteers and patients with osteoarthritis.
Design
The study was performed with Institutional Review Board approval and with all subjects signing informed consent. CRI of the knee joint was performed at 3.0T on 20 asymptomatic volunteers and 11 patients with osteoarthritis. The fraction of macromolecular bound protons (f), the exchange rate constant between macromolecular bound protons and free water protons (k), and the T2 relaxation time of macromolecular bound protons (T2B) of patellar cartilage were measured. Mann-Whitney-Wilcoxon rank-sum tests were used to compare qMT parameters between asymptomatic volunteers and patients with osteoarthritis.
Results
Average f, k, and T2B of patellar cartilage was 12.46%, 7.22 s−1, and 6.49 μs respectively for asymptomatic volunteers and 12.80%, 6.13 s−1, and 6.80 μs respectively for patients with osteoarthritis. There were statistically significant differences between groups of subjects for k (p<0.01) and T2B (p<0.0001) but not f (p=0.38) of patellar cartilage.
Conclusion
Patients with osteoarthritis had significantly lower k and significantly higher T2B of patellar cartilage than asymptomatic volunteers which suggests that qMT parameters can detect changes in the macromolecular matrix of degenerative cartilage. Key Words: Cartilage; MRI; Osteoarthritis; Magnetization Transfer
Introduction
Osteoarthritis is one of the most prevalent chronic diseases in the United States and worldwide [1]. Characteristic changes in the macromolecular matrix of articular cartilage occur during osteoarthritis including a decrease in proteoglycan content and disruption of the highly organized collagen fiber network [2–4]. Techniques to non-invasively assess the cartilage macromolecular matrix would be beneficial in osteoarthritis research studies to monitor disease-related and treatment-related changes in the composition and ultra-structure of cartilage [5]. Sensitive methods to detect early cartilage degeneration would also be useful in clinical practice to identify the cause of joint pain in symptomatic patients [6] and to allow early initiation of interventions such as weight loss, aerobic activity, and range of motion and strengthening exercises which may alleviate symptoms and potentially slow the rate of joint degeneration [7].
Various quantitative magnetic resonance (MR) imaging techniques have been used to evaluate articular cartilage. Multiple techniques including gadolinium enhanced spin-lattice relaxation time (T1) imaging [8, 9], sodium imaging [10, 11], spin-lattice relaxation time in the rotating frame (T1rho) imaging [12, 13], and chemical exchange-dependent saturation transfer (CEST) imaging [14, 15] have been shown to be sensitive for detecting changes in the proteoglycan content of cartilage. However, only a few MR techniques including spin-spin relaxation time (T2) imaging [16–18] and diffusion tensor imaging [19–22] have been used to identify alterations in cartilage ultra-structure, and all currently used methods have limitations. T2 relaxation time is a nonspecific parameter which is influenced by multiple factors including organization of the collagen fiber network [16–18], water and macromolecular content [23–26], and orientation of cartilage relative to the main magnetic field [27]. While diffusion tensor imaging may provide sensitive and specific information regarding cartilage ultra-structure, it is technically challenging and typically requires the use of high field strength scanners and custom made coils which has limited its use for evaluating human articular cartilage in-vivo [19–22].
Quantitative magnetization transfer (qMT) imaging is MR technique which utilizes a two-pool model of magnetization exchange to acquire information regarding the cartilage macromolecular matrix [28–30]. qMT imaging techniques typically require multiple MT-contrast images with different magnetization preparatory pulses resulting in long scan times which have limited cartilage assessment to ex-vivo specimens [31–33]. Cross-relaxation imaging (CRI) is a qMT method which can create three-dimensional parametric maps of articular cartilage measuring the fraction of macromolecular bound protons (f), the exchange rate constant between macromolecular bound protons and free water protons (k), and the T2 relaxation time of macromolecular bound protons (T2B) with high resolution and relatively short scan time based upon a limited number of MT-contrast images [34–36]. The parameter f provides an indirect measure of macromolecular content, while the parameters k, and T2B reflect the efficiency of magnetization exchange between macromolecular bound protons and free water protons and the spin diffusion between proton sites in macromolecules respectively which may be influenced by macromolecular organization and ultra-structure [30, 37, 38]. We have developed a CRI protocol for evaluating human patellar cartilage in-vivo at 3.0T which can provide robust measurements of f, k, and T2B in a 19 minute scan time. This study was performed to compare qMT parameters of patellar cartilage measured using CRI in asymptomatic volunteers and patients with osteoarthritis to determine whether the MR technique can detect changes in the macromolecular matrix of degenerative cartilage.
Materials and Methods
Study Group
The study was performed in compliance with HIPAA regulations and with approval from our Institutional Review Board. All subjects signed informed consent prior to their participation in the study. The study group consisted of 20 asymptomatic volunteers (15 males and five females between 23 and 45 years of age with an average age of 32.3 years) and 11 patients with osteoarthritis of the knee joint (seven males and four females between 45 and 62 years of age with an average age of 52.6 years). All patients with osteoarthritis of the knee joint complained of chronic knee pain and stiffness for a minimum of six months and showed definitive grade 2 osteophytes with no associated joint space loss on standing anterior-posterior radiographs of the knee [46, 47] . All patients had mild osteoarthritis within the patellofemoral compartment with 6 patients showing small grade 1 osteophytes and 5 patients showing definitive grade 2 osteophytes on the patella and femoral trochlea and no patients showing joint space narrowing on axial radiographs of the knee [46].
MR Examination
An MR examination of the knee joint was performed on all subjects in the study group using a 3.0T scanner (Discovery MR750, GE Healthcare, Waukesha, WI) and an 8-channel phased-array extremity coil (In Vivo, Orlando, FL). Foam padding was used to firmly secure the knee within the coil to minimize subject motion during the MR examination. All MR examinations consisted of the following sequences performed in the axial plane through the patellofemoral compartment of the knee joint: 1) the CRI protocol, 2) a frequency-selective fat-suppressed T2-weighted fast spin-echo sequence acquired using a 4050 ms repetition time, 85 ms echo time, 90° excitation flip angle, 31 kHz bandwidth, 14 cm field of view, 256 x 256 matrix, 4 mm slice thickness, and four signal averages, and 3) an SPGR sequence with iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) fat-water separation [48] acquired using a 12.4 ms repetition time, 3.4 ms, 4.2 ms, and 5.0 ms echo times, 14° excitation flip angle, 31 kHz bandwidth, 14 cm field of view, 256 x 256 matrix, 4 mm slice thickness, and one signal average. A frequency-selective fat-suppressed three-dimensional intermediate-weighted fast spin-echo sequence was also performed in the sagittal plane through the knee joint using a 2217 ms repetition time, 23.6 ms echo time, 90° excitation flip angle, 31 kHz bandwidth , 15 cm field of view, 256 x 256 matrix, 1 mm slice thickness, and one signal average. Coronal and axial reformat images were created from the volumetric fast spin-echo source data.
The CRI protocol consisted of six MT-prepared SPGR scans and four non-MT-prepared SPGR scans. The MT-prepared SPGR scans were performed with different combinations of MT offset frequencies and flip angles (2.5 kHz/1550°, 5 kHz/1550°, 10 kHz/1550°, 20 kHz/1550°, 2.5 kHz/890°, and 5 kHz/890°) with an 18-ms Fermi MT pulse, 42 ms repetition time, 3.2 ms echo time, and 13° excitation flip angle. The non-MT-prepared SPGR scans were performed over a range of excitation flip angles (4°, 10°, 20°, and 30°) with a 24 ms repetition time and 3.2 ms echo time. Actual flip angle imaging (AFI) was also performed for flip angle mapping using an SPGR scan consisting of two identical radiofrequency pulses followed by repetition times of 30 ms and 150 ms acquired with a 2.2 ms echo time and 55° excitation flip angle [45]. All SPGR scans were performed using a 31 kHz bandwidth, 14 cm field of view, 256 x 256 matrix for the MT-prepared and non-MT-prepared scans and 128 x 128 matrix for the AFI scans, 4 mm slice thickness, 10 slices, and one signal average. Total scan time for the CRI protocol was 19 minutes. In order to measure MTR, one additional MT-prepared SPGR scan was performed using the same imaging parameters except for a 250 kHz offset frequency and 1550° flip angle to create negligible MT effect.
Cartilage qMT Parameter Map Reconstruction
Quantitative MR parameter maps of patellar cartilage were reconstructed using in-house software developed in MATLAB (MATLAB 2011b, MathWorks Inc, Natick, MA). Image registration software (FLIRT, Functional Magnetic Resonance Imaging of the Brain Analysis Group, Oxford University, United Kingdom) was used to correct for any subject motion which may have occurred between the multiple scans. MT-prepared SPGR scans, non-MT-prepared SPGR scans, and AFI scans were co-registered using the IDEAL-SPGR scan as the reference. The MT-prepared and non-MT-prepared SPGR datasets were simultaneously fitted on a pixel-by-pixel basis using a non-linear least squares two-pool model to create cartilage f, k and T2B maps [35]. Both excitation flip angle and MT saturation power were corrected for each pixel using the flip angle maps acquired from the AFI scans. In addition, cartilage MTR maps were created using a pixel-by-pixel measurement of the difference in the signal of the SPGR scan with negligible MT effect (250kHz/1550°) and the SPGR scan with strongest MT effect (2.5kHz/1550°) divided by the signal of the SPGR scan with negligible MT effect [43, 49].
Comparison of Morphologic and Quantitative MR Parameters Between Groups of Subjects
Morphologic joint analysis was performed by a fellowship-trained musculoskeletal radiologist with 12 years of clinical experience who was blinded to whether a subject was an asymptomatic volunteer or patient with osteoarthritis. The radiologist used the axial fat-suppressed T2-weighted fast spin-echo, axial IDEAL-SPGR, and multi-planar fat-suppressed three-dimensional intermediate-weighted fast spin-echo images to grade the severity of degeneration within the knee joint using the Boston-Leeds Osteoarthritis Knee (BLOK) scoring system [50]. A patellar BLOK score and total knee BLOK score was calculated for each subject using the semi-quantitative scoring system.
Quantitative cartilage analysis was performed by a research assistant with four years of cartilage segmentation experience under the supervision of the fellowship-trained musculoskeletal radiologist using software developed in MATLAB (MATLAB 2011b, MathWorks Inc, Natick, MA). Regions of interest were placed around the patellar cartilage on each slice of the IDEAL-SPGR images of each subject to create a three-dimensional contour of patellar cartilage. The three-dimensional contour was then superimposed over the cartilage MTR, f, k, and T2B maps to measure the average quantitative MR parameters of patellar cartilage. Non-parametric two-tailed Mann-Whitney-Wilcoxon rank-sum tests were used to compare MTR, f, k, and T2B of patellar cartilage between asymptomatic volunteers and patients with osteoarthritis. Two-tailed Mann-Whitney-Wilcoxon rank-sum tests were also used to compare MTR, f, k, and T2B of patellar cartilage in patients with osteoarthritis who had small grade 1 osteophytes and definitive grade 2 osteophytes on the patella and femoral trochlea. Spearman’s rank correlation coefficients were used to determine the association between MTR, f, k, and T2B of patellar cartilage and the patellar and total knee BLOK scores in patients with osteoarthritis.
Non-parametric statistical analysis was chosen due to the presence of outliers and non-constant variance of qMT parameters in the subject populations which violated the assumptions of normality and homoscedasticity. The Bonferonni method was used to account for comparison of four quantitative MR parameters between groups of subjects with statistical significance defined as a p-value less than 0.01 due to
Assessment of Repeatability of Cartilage Quantitative MR Measurements
The CRI protocol was performed twice on both knee joints of five asymptomatic volunteers (five males between 28 and 32 years of age with an average age of 29.2 years) with the subjects taken out of the scanner and allowed to rest in a sitting position for 10 minutes between the scans for a total of four scans per subject. Average MTR, f, k, and T2B of patellar cartilage were measured using the previously described methods. Repeatability of cartilage qMT measurements was assessed using conventional [51–53] and standardized [54] coefficients of variance. Confidence intervals for coefficient of variance were calculated using the approximate pivotal method [55]. Bland-Altman analysis was also performed to assess the estimated bias or mean of differences and 95% limits of agreement for the repeat cartilage qMT measurements obtained using the two CRI scans [56]. Student t-tests were used to determine the significance of the estimated bias of the repeat measurements by testing whether the mean of differences was equal to 0. Significance of the estimated bias was also determined by observing whether the 95% confidence intervals of the mean of differences on the Bland-Altman plots included 0.
Assessment of Signal-to-Noise Ratio (SNR) of the CRI Protocol
Monte Carlo simulations were used to compare the signal-to-noise ratio (SNR) to the variations due to error in MTR, f, k, and T2B [57]. One hundred digital phantoms, each containing 2000 pixels, were created using the average MTR, f, k, and T2B of all subjects in the study. Different levels of normally distributed noise were added to the digital signals to simulate SNR levels from 0 to 160 in increments of 20. For each SNR level, the digital phantom data was used to obtain estimates of MTR, f, k, and T2B [35]. The average MTR, f, k, and T2B were calculated over each digital phantom at each SNR level. Standard deviations of the averages were taken at each SNR level. The standard deviations were then normalized as a percentage of the average MTR, f, k, and T2B values. The results were compared to the observed differences in MTR, f, k, and T2B between asymptomatic volunteers and patients with osteoarthritis at the reference SNR for the CRI protocol. The reference SNR for the CRI protocol was determined using the addition/subtraction method in which two identical SPGR scans with negligible MT effect were performed on an asymptomatic volunteer one immediately following the other. Signal was defined as the signal of patellar cartilage on the addition images divided by 2, while noise was defined as the standard deviation of patellar cartilage on the subtraction images divided by the square root of 2 [58].
Results
All asymptomatic volunteers had a patellar BLOK score and total knee BLOK score of 0 indicating no degeneration within the knee joint. Patients with osteoarthritis had patellar BLOK scores ranging between 6 and 13 with an average value of 8.0 and total knee BLOK scores ranging between 27 and 58 with an average value of 36.1. All patients with osteoarthritis had osteophytes and focal areas of partial-thickness cartilage loss on the patella with two patients also having subchondral bone marrow edema.
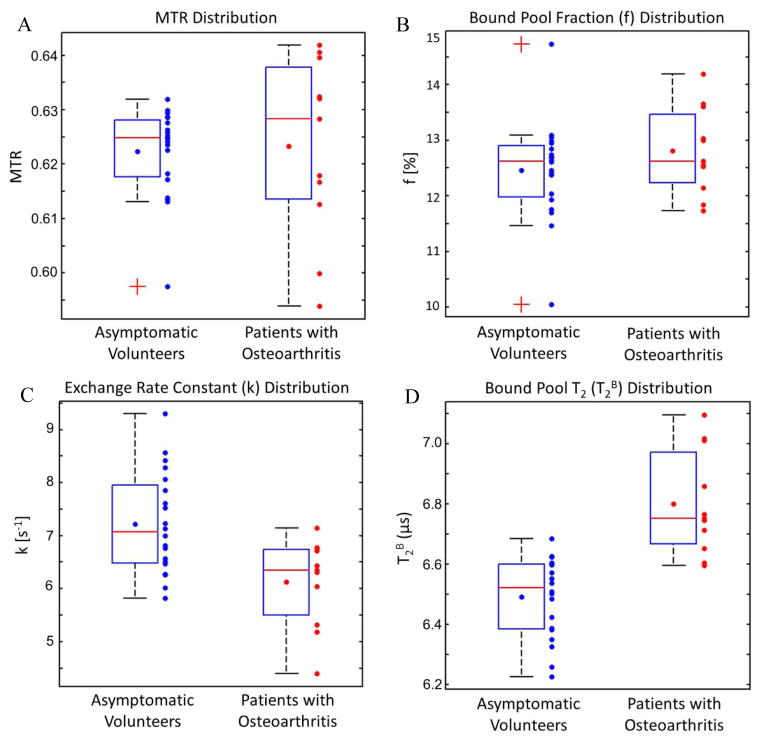
Figure 1 shows box plots illustrating the distribution of MTR, f, k, and T2B values of patellar cartilage for asymptomatic volunteers and patients with osteoarthritis. Table 1 shows average MTR, f, k, and T2B values of patellar cartilage with standard deviations and 95% confidence intervals for asymptomatic volunteers and patients with osteoarthritis. There was significantly lower k (p<0.01) and significantly higher T2B (p<0.0001) of patellar cartilage in patients with osteoarthritis than asymptomatic volunteers. There was no significant difference in MTR (p=0.52) and f (p=0.38) of patellar cartilage between groups of subjects. There was no significantly difference in MTR (p=0.100), f (p=0.13), k (p=1.00), and T2B (p=1.00) of patellar cartilage between patients with osteoarthritis who had small grade 1 osteophytes and definitive grade 2 osteophytes on the patella and femoral trochlea. There was no significant correlation in patients with osteoarthritis between patellar BLOK scores and total knee BLOK scores and MTR (p=0.56 and p=0.77 respectively), f (p=0.45 and p=0.77 respectively), k (p=0.51 and p=0.53 respectively), and T2B (p=0.83 and p=0.82 respectively) of patellar cartilage. Figures 2, 3, and 4 illustrate examples of differences in the qMT parameters f, k, and T2B of patellar cartilage between asymptomatic volunteers and patients with osteoarthritis.
Figure 1.
Box plots illustrating the distribution of (A) MTR, (B) f, (C) k, and (D) T2B values of patellar cartilage in asymptomatic volunteers and patients with osteoarthritis. The boxes indicate interquartile ranges, the blue dots within the boxes indicate average values, the red lines within the boxes indicate median values, the whiskers extend to the most extreme data points not considered outliers (1.5 of the interquartile range between the first and third quartiles), and the crosses indicate outliers.
Table 1.
Average MTR, f, k, and T2B values of patellar cartilage with standard deviations (SD) and 95% confidence intervals (CI) in asymptomatic volunteers and patients with osteoarthritis
| CRI Parameter | Asymptomatic Volunteers | Patients with Osteoarthritis | ||
|---|---|---|---|---|
| Average Value (SD) | 95% CI | Average Value (SD) | 95% CI | |
| MTR | 0.62 (0.01) | 0.62–0.63 | 0.62 (0.02) | 0.62–0.64 |
| f (%) | 12.46 (0.89) | 12.32–12.93 | 12.80 (0.78) | 12.16–13.09 |
| k (s–1) | 7.22 (0.95) | 6.57–7.56 | 6.13 (0.83) | 5.86–6.85 |
| T2B (μs) | 6.49 (0.13) | 6.45–6.60 | 6.80 (0.14) | 6.63–6.87 |
Figure 2.
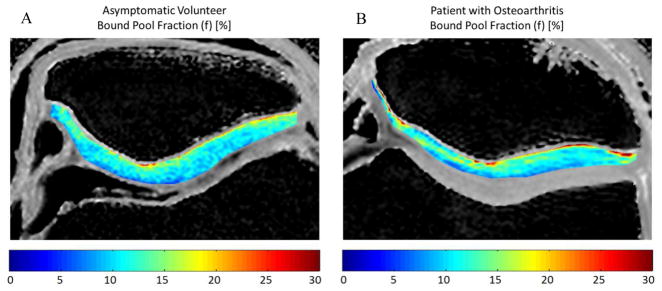
Comparison of f of patellar cartilage in (A) a 25 year old male asymmetric volunteer and (B) a 52 year old male patient with osteoarthritis. Note that there are no visible differences in f between the asymptomatic volunteer and the patient with osteoarthritis.
Figure 3.
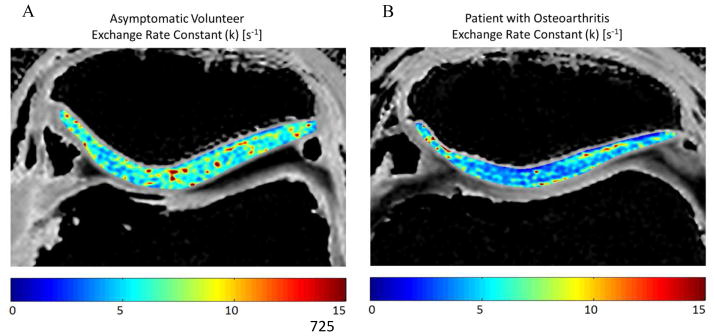
Comparison of k of patellar cartilage in (A) a 27 year old male asymmetric volunteer and (B) a 45 year old male patient with osteoarthritis. Note that the patient with osteoarthritis has lower k of patellar cartilage than the asymptomatic volunteer.
Figure 4.
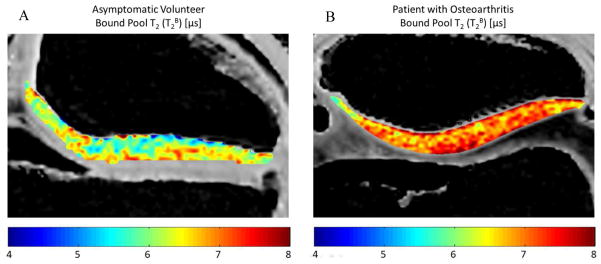
Comparison of T2B of patellar cartilage in (A) a 27 year old male asymmetric volunteer and (B) a 45 year old male patient with osteoarthritis. Note that the patient with osteoarthritis has higher T2B of patellar cartilage than the asymptomatic volunteer.
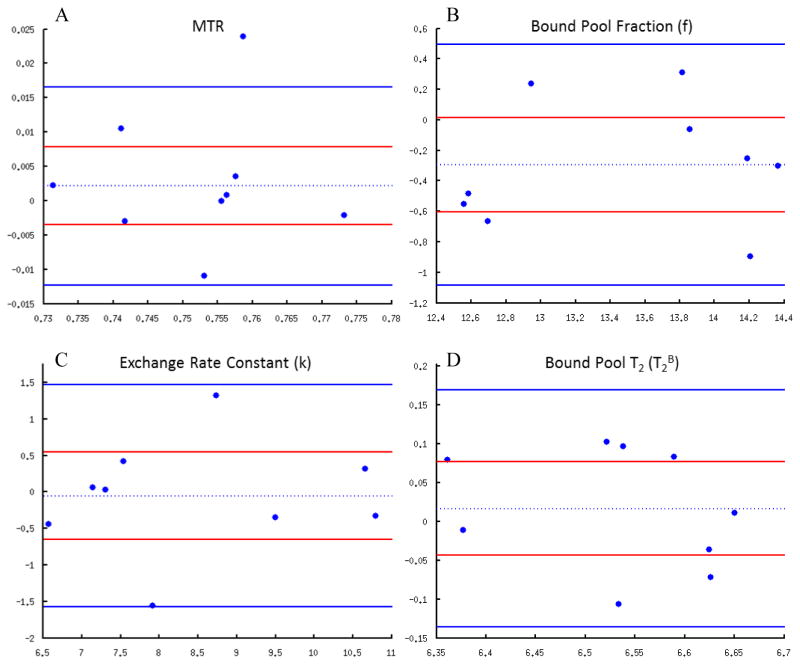
Conventional coefficients of variance for repeat qMT measurements obtained using the two CRI scans performed on the same knee of the same subject were 0.45% for MTR (95% confidence intervals 0.44% to 0.48%), 2.21% for f (95% confidence intervals 2.15% to 2.35%), 4.56% for k (95% confidence intervals 4.44% to 4.87%), and 0.72% for T2B (95% confidence intervals 0.70% to 0.77%). Standardized coefficients of variance for repeat qMT measurements were 10.28% for MTR (95% confidence intervals 10.02% to 10.96%), 9.55% for f (95% confidence intervals 9.30% to 10.18%), 6.22% for k (95% confidence intervals 6.06% to 6.63%), and 10.81% for T2B (95% confidence intervals 10.53% to 11.53. Figure 5 shows the Bland-Altman plots with estimated bias or mean of differences with 95% confidence intervals and 95% limits of agreement for repeat qMT measurements. The estimated biases for repeat measurements on Bland-Altman analysis were 0.01 for MTR, −0.24 for f, −0.05 for k, and 0.02 for T2B. The estimated biases for the repeat measurements were not statistically significant with the p-values for the student t-tests ranging between 0.06 and 0.84 and all 95% confidence intervals for the mean of differences including 0.
Figure 5.
Bland-Altman plots for (A) MTR, (B) f, (C) k, and (D) T2B. The two solid blue lines indicate 95% limits of agreement, and the dotted blue line indicates the estimated bias or mean of differences. The two solid red lines indicate 95% confidence intervals (CI) of the mean of differences which can be used to determine the significance of the estimated bias. Note that for all qMT parameters, the 95% CI of the mean of differences includes 0 indicating no statistically significant estimated bias between the repeat measurements.
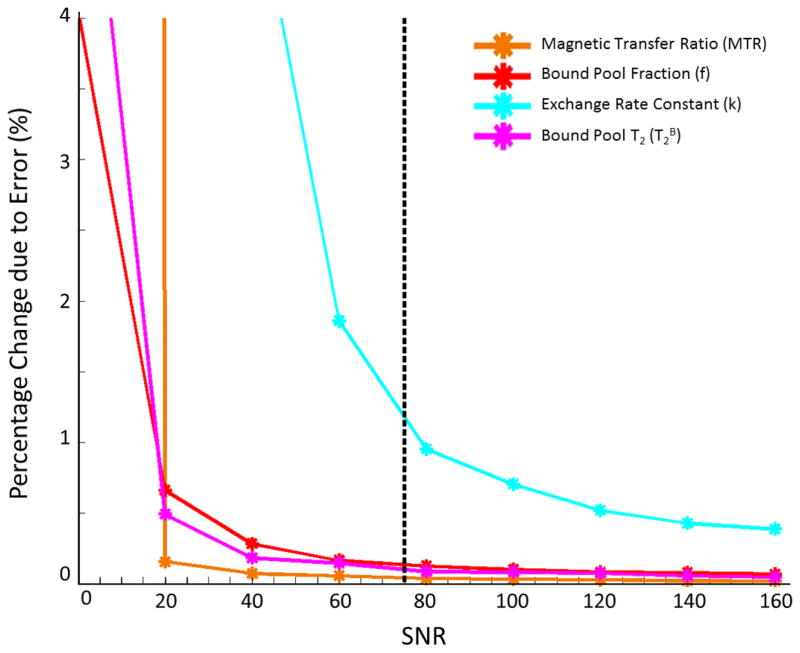
The relationships between signal-to-noise ratio (SNR) and variations due to error in MTR, f, k, and T2B are illustrated in Figure 6. The normalized standard deviation for a given parameter may be interpreted as the percent change expected due to noise and data fit instability at a certain SNR level. At the reference SNR of 75 for the CRI protocol, the percent change expected due to noise and data fit instability was approximately 0.1% in MTR, 0.1% in f, 1% in k, and 0.1% in T2B. In comparison, the percent change of the differences in qMT values of patellar cartilage between asymptomatic volunteers and patients with osteoarthritis was 0.1% for MTR, 2.8% in f, −15.9% in k, and 4.7% in T2B.
Figure 6.
Relationship between normalized standard deviation due to error and SNR for MTR, f, k, and T2B of patellar cartilage. The reference SNR of 75 for the CRI protocol (dotted black vertical line) provides low percent change due to error in all qMT parameters.
Discussion
Our study has demonstrated the feasibility of using CRI to measure the qMT parameters f, k, and T2B of the articular cartilage of the knee joint in human subjects which has never been previously performed to the best of our knowledge. Our study found significant differences in f and T2B of patellar cartilage between asymptomatic volunteers and patients with osteoarthritis which suggests that qMT parameters can detect changes in the macromolecular matrix of degenerative cartilage. MTR could not distinguish between groups of subjects which is similar to the findings of a previous study which found no significant difference in MTR of patellar cartilage between asymptomatic volunteers and patients with osteoarthritis [59]. These results suggest that a more detailed analysis of the magnetization exchange between macromolecular bound protons and free water protons provided by CRI is needed to detect changes in the cartilage macromolecular matrix.
Our study found significantly lower k and significantly higher T2B of patellar cartilage in patients with osteoarthritis when compared to asymptomatic volunteers. Few previous studies have investigated k and T2B of cartilage, and thus the exact mechanisms of the observed changes in the qMT parameters remain unknown. However, previous ex-vivo studies have shown that k decreases [60, 61] and T2B increases [60] with proteoglycan loss due to trypsin degradation of bovine cartilage specimens. Furthermore, thermal denaturation of collagen solution has been shown to cause an increase in T2B although the change was accompanied by a decrease in f [60]. Thus, the decrease in k and increase in T2B with cartilage degeneration in our study may be due to the combined effects of proteoglycan loss and collagen denaturation which both occur during the early stages of osteoarthritis [2–4]. Fragmentation of collagen decreases proton binding sites on the macromolecule and may thereby reduce the exchange rate between macromolecular bound and free water protons. Fragmentation of collagen may also increase the mobility of the macromolecule within cartilage and thereby increase T2B. Decreased organization of the cartilage matrix due to collagen denaturation and proteoglycan loss may increase spin diffusion of macromolecular-bound protons, the primary mechanism defining T2 relaxation time in the semisolid fraction, and thereby also increase T2B [38].
Our study found no significant difference in f of patellar cartilage in patients with osteoarthritis when compared to asymptomatic volunteers. The factors responsible for the measured f values of cartilage are incompletely understood. A previous study using CRI to investigate ex-vivo human cadaveric cartilage specimens found a moderate correlation between f and the proteoglycan content of cartilage [36]. However, the two-pool model used in the study fixed T2B which has been shown in our study to change with cartilage degeneration. Another study using proteoglycan and collagen phantoms and a similar CRI protocol as our study has documented large increases in f with increasing collagen concentration but only negligible increases in f with increasing proteoglycan concentration. The same study found no change in f with proteoglycan loss due to trypsin degradation of ex-vivo bovine cartilage specimens suggesting that proteoglycan content has a minimal effect on f [60]. Proteoglycan has an abundance of macromolecular bound protons, but its concentration within cartilage is lower [2] and its protons are more mobile [62] when compared to collagen which may limit its contribution to f. The absence of changes in f with cartilage degeneration in our study may be due to the fact that f is primarily a measure of the collagen content of cartilage which decreases by only 5% during the late stages of osteoarthritis [63]. However, additional studies are needed to investigate the influence of collagen and proteoglycan on the measured f values of both normal and degenerative cartilage.
The CRI protocol used in our study had adequate SNR to detect differences in qMT parameters between groups of subjects and high repeatability with conventional coefficients of variance which compared favorably with other quantitative cartilage imaging techniques [51–53]. However, k was more sensitive to measurement errors when compared to f and T2B with higher percent change expected due to noise and data fit instability. These results are consistent with the findings of a previous study investigating qMT parameters within neural tissue which showed that small fluctuations in MT signals due to measurement error had a more significant effect on k than f and T2B [64]. One method to improve measurements of k of articular cartilage would be to acquire SPGR scans at higher MT flip angles than those used in our study. However, using higher MT flip angles would increase specific absorption rate (SAR) so SAR would need to be reduced by using lower field strength scanners at the expense of decreased SNR or longer repetition times at the expense of increased scan time.
Our study has several limitations. One limitation was the relatively small number of subjects which prevented detailed analysis of inter-group variability in qMT parameters and identification of thresholds for k and T2B which could be considered diagnostic for cartilage degeneration. Furthermore, the small number of patients with osteoarthritis provided low statistical power for comparison of qMT parameters with radiographic and MR parameters of cartilage degeneration. Another limitation was that not all patients with osteoarthritis in our study had definitive grade 2 osteophytes within the patellofemoral compartment which is considered to be the radiographic hallmark of the disease [47]. However, all patients did have osteoarthritis of the whole knee joint diagnosed using standardized clinical and radiographic criteria [47, 65]. Furthermore, a group of patients with mild osteoarthritis within the patellofemoral compartment was desired to determine whether CRI could detect macromolecular changes associated with early patellar cartilage degeneration. Another limitation of our study was that it could not identify the mechanisms responsible for changes in k and T2B between asymptomatic volunteers and patients with osteoarthritis. Additional studies correlating qMT parameters in ex-vivo cartilage specimens with proteoglycan and collagen content measured using biochemical assays and tissue ultra-structure measured using polarized light microscopy are needed to investigate the factors responsible for changes in qMT parameters at various stages of cartilage degeneration. A final limitation was that the CRI protocol used in our study had a relatively long scan time which limited qMT assessment to 10 slices through patellar cartilage. We are currently investigating various methods to reduce the scan time of the CRI protocol including use of a smaller number of optimized magnetization preparatory pulses, more rapid methods for flip angle mapping, and compressed sensing with parallel imaging to provide complete anatomic coverage of the knee joint in the sagittal plane.
In conclusion, our study has shown that patients with osteoarthritis have significantly lower k and significantly higher T2B of patellar cartilage than asymptomatic volunteers which suggests that qMT parameters can detect changes in the macromolecular matrix of degenerative cartilage. CRI may provide a new quantitative MR technique to identify patients with early cartilage degeneration and to monitor disease-related and treatment-related changes in the macromolecular matrix of articular cartilage in osteoarthritis research studies. However, further studies are needed to better understand the fundamental mechanisms responsible for changes in qMT parameters in patients with osteoarthritis and to identify thresholds of k and T2B which could be considered diagnostic for cartilage degeneration.
Acknowledgments
We kindly acknowledge Dr. Alejandro Munoz del Rio for his statistical expertise.
Role of Funding Source
We kindly declare NIH NIAMS U01 AR059514-01 as the sole source of funding for this work.
Contributions
A declaration of contributions for each of the authors is provided below:
Nade Sritanyaratana, M.S. (nade@cae.wisc.edu)
Conception and design
Collection and assembly of data
Analysis and interpretation of the data
Drafting of the article
Critical revision of the article for important intellectual content
Final approval of the article
Alexey Samsonov, Ph.D. (samsonov@wisc.edu)
Conception and design
Interpretation of the data
Critical revision of the article for important intellectual content
Final approval of the article
Pouria Mossahebi, Ph.D. (mossahebi@wisc.edu)
Conception and design
Interpretation of the data
Critical revision of the article for important intellectual content
Final approval of the article
John J. Wilson, M.D. (john.wilson@fammed.wisc.edu)
Collection and assembly of data
Interpretation of the data
Critical revision of the article for important intellectual content
Final approval of the article
Walter F. Block, Ph.D. (wfblock@wisc.edu)
Analysis and interpretation of the data
Critical revision of the article for important intellectual content
Final approval of the article
Statistical expertise
Obtaining of funding
Richard Kijowski, M.D. (rkijowski@uwhealth.org)
Conception and design
Analysis and interpretation of the data
Drafting of the article
Critical revision of the article for important intellectual content
Final approval of the article
Statistical expertise
Obtaining of funding
Administrative, technical, or logistic support
Drs. Alexey Samsonov, Walter Block, and Richard Kijowski take responsibility for the integrity of the work as a whole, from inception to finished article.
Footnotes
Competing Interests
None of the authors have any conflicts of interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.From the Centers for Disease Control and Prevention. Arthritis prevalence and activity limitations--United States, 1990. Jama. 1994;272:346–347. [PubMed] [Google Scholar]
- 2.Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Ann Rheum Dis. 1977;36:121–129. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Squires GR, Okouneff S, Ionescu M, Poole AR. The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis. Arthritis Rheum. 2003;48:1261–1270. doi: 10.1002/art.10976. [DOI] [PubMed] [Google Scholar]
- 4.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold GE, Burstein D, Dardzinski B, Lang P, Boada F, Mosher T. MRI of articular cartilage in OA: novel pulse sequences and compositional/functional markers. Osteoarthritis Cartilage. 2006;14 (Suppl A):A76–86. doi: 10.1016/j.joca.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Disler DG, McCauley TR, Kelman CG, Fuchs MD, Ratner LM, Wirth CR, et al. Fat-suppressed three-dimensional spoiled gradient-echo MR imaging of hyaline cartilage defects in the knee: comparison with standard MR imaging and arthroscopy. AJR Am J Roentgenol. 1996;167:127–132. doi: 10.2214/ajr.167.1.8659356. [DOI] [PubMed] [Google Scholar]
- 7.Altman RD. Early management of osteoarthritis. Am J Manag Care. 2010;16(Suppl Management):S41–47. [PubMed] [Google Scholar]
- 8.Bashir A, Gray ML, Boutin RD, Burstein D. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology. 1997;205:551–558. doi: 10.1148/radiology.205.2.9356644. [DOI] [PubMed] [Google Scholar]
- 9.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36:665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 10.Borthakur A, Shapiro EM, Beers J, Kudchodkar S, Kneeland JB, Reddy R. Sensitivity of MRI to proteoglycan depletion in cartilage: comparison of sodium and proton MRI. Osteoarthritis Cartilage. 2000;8:288–293. doi: 10.1053/joca.1999.0303. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro EM, Borthakur A, Gougoutas A, Reddy R. 23Na MRI accurately measures fixed charge density in articular cartilage. Magn Reson Med. 2002;47:284–291. doi: 10.1002/mrm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duvvuri U, Kudchodkar S, Reddy R, Leigh JS. T(1rho) relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthritis Cartilage. 2002;10:838–844. doi: 10.1053/joca.2002.0826. [DOI] [PubMed] [Google Scholar]
- 13.Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38:863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt B, Zbyn S, Stelzeneder D, Jellus V, Paul D, Lauer L, et al. Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and (23)Na MR imaging at 7 T. Radiology. 2011;260:257–264. doi: 10.1148/radiol.11101841. [DOI] [PubMed] [Google Scholar]
- 15.Singh A, Haris M, Cai K, Kassey VB, Kogan F, Reddy D, et al. Chemical exchange saturation transfer magnetic resonance imaging of human knee cartilage at 3 T and 7 T. Magn Reson Med. 2012;68:588–594. doi: 10.1002/mrm.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwin DW, Wadghiri YZ, Zhu H, Vinton CJ, Smith ED, Dunn JF. Macroscopic structure of articular cartilage of the tibial plateau: influence of a characteristic matrix architecture on MRI appearance. AJR Am J Roentgenol. 2004;182:311–318. doi: 10.2214/ajr.182.2.1820311. [DOI] [PubMed] [Google Scholar]
- 17.Nieminen MT, Rieppo J, Toyras J, Hakumaki JM, Silvennoinen J, Hyttinen MM, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46:487–493. doi: 10.1002/mrm.1218. [DOI] [PubMed] [Google Scholar]
- 18.Xia Y, Moody JB, Burton-Wurster N, Lust G. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage. 2001;9:393–406. doi: 10.1053/joca.2000.0405. [DOI] [PubMed] [Google Scholar]
- 19.Meder R, de Visser SK, Bowden JC, Bostrom T, Pope JM. Diffusion tensor imaging of articular cartilage as a measure of tissue microstructure. Osteoarthritis Cartilage. 2006;14:875–881. doi: 10.1016/j.joca.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 20.de Visser SK, Bowden JC, Wentrup-Byrne E, Rintoul L, Bostrom T, Pope JM, et al. Anisotropy of collagen fibre alignment in bovine cartilage: comparison of polarised light microscopy and spatially resolved diffusion-tensor measurements. Osteoarthritis Cartilage. 2008;16:689–697. doi: 10.1016/j.joca.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Raya JG, Arnoldi AP, Weber DL, Filidoro L, Dietrich O, Adam-Neumair S, et al. Ultra-high field diffusion tensor imaging of articular cartilage correlated with histology and scanning electron microscopy. MAGMA. 2011;24:247–258. doi: 10.1007/s10334-011-0259-6. [DOI] [PubMed] [Google Scholar]
- 22.Raya JG, Melkus G, Adam-Neumair S, Dietrich O, Mutzel E, Reiser MF, et al. Diffusion-tensor imaging of human articular cartilage specimens with early signs of cartilage damage. Radiology. 2013;266:831–841. doi: 10.1148/radiol.12120954. [DOI] [PubMed] [Google Scholar]
- 23.Liess C, Lusse S, Karger N, Heller M, Gluer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10:907–913. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- 24.Nishioka H, Hirose J, Nakamura E, Oniki Y, Takada K, Yamashita Y, et al. T1rho and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J Magn Reson Imaging. 2012;35:147–155. doi: 10.1002/jmri.22811. [DOI] [PubMed] [Google Scholar]
- 25.Watrin–Pinzano A, Ruaud JP, Olivier P, Grossin L, Gonord P, Blum A, et al. Effect of proteoglycan depletion on T2 mapping in rat patellar cartilage. Radiology. 2005;234:162–170. doi: 10.1148/radiol.2341030394. [DOI] [PubMed] [Google Scholar]
- 26.Wong CS, Yan CH, Gong NJ, Li T, Chan Q, Chu YC. Imaging biomarker with T1rho and T2 mappings in osteoarthritis - in vivo human articular cartilage study. Eur J Radiol. 2013;82:647–650. doi: 10.1016/j.ejrad.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 27.Xia Y. Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol. 2000;35:602–621. doi: 10.1097/00004424-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Henkelman RM, Huang X, Xiang QS, Stanisz GJ, Swanson SD, Bronskill MJ. Quantitative interpretation of magnetization transfer. Magn Reson Med. 1993;29:759–766. doi: 10.1002/mrm.1910290607. [DOI] [PubMed] [Google Scholar]
- 29.Sled JG, Levesque I, Santos AC, Francis SJ, Narayanan S, Brass SD, et al. Regional variations in normal brain shown by quantitative magnetization transfer imaging. Magn Reson Med. 2004;51:299–303. doi: 10.1002/mrm.10701. [DOI] [PubMed] [Google Scholar]
- 30.Yarnykh VL. Pulsed Z-spectroscopic imaging of cross-relaxation parameters in tissues for human MRI: theory and clinical applications. Magn Reson Med. 2002;47:929–939. doi: 10.1002/mrm.10120. [DOI] [PubMed] [Google Scholar]
- 31.Henkelman RM, Stanisz GJ, Menezes N, Burstein D. Can MTR be used to assess cartilage in the presence of Gd-DTPA2-? Magn Reson Med. 2002;48:1081–1084. doi: 10.1002/mrm.10322. [DOI] [PubMed] [Google Scholar]
- 32.Li W, Hong L, Hu L, Magin RL. Magnetization transfer imaging provides a quantitative measure of chondrogenic differentiation and tissue development. Tissue Eng Part C Methods. 2010;16:1407–1415. doi: 10.1089/ten.tec.2009.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med. 2005;54:507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- 34.Yarnykh VL, Yuan C. Cross-relaxation imaging reveals detailed anatomy of white matter fiber tracts in the human brain. Neuroimage. 2004;23:409–424. doi: 10.1016/j.neuroimage.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 35.Mossahebi P, Yarnykh VL, Samsonov A. Analysis and correction of biases in cross-relaxation MRI due to biexponential longitudinal relaxation. Magn Reson Med. 2013 doi: 10.1002/mrm.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stikov N, Keenan KE, Pauly JM, Smith RL, Dougherty RF, Gold GE. Cross-relaxation imaging of human articular cartilage. Magn Reson Med. 2011;66:725–734. doi: 10.1002/mrm.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sled JG, Pike GB. Quantitative imaging of magnetization transfer exchange and relaxation properties in vivo using MRI. Magn Reson Med. 2001;46:923–931. doi: 10.1002/mrm.1278. [DOI] [PubMed] [Google Scholar]
- 38.Edzes HT, Samulski ET. Cross relaxation and spin diffusion in the proton NMR or hydrated collagen. Nature. 1977;265:521–523. doi: 10.1038/265521a0. [DOI] [PubMed] [Google Scholar]
- 39.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 40.Dardzinski BJ, Mosher TJ, Li S, Van Slyke MA, Smith MB. Spatial variation of T2 in human articular cartilage. Radiology. 1997;205:546–550. doi: 10.1148/radiology.205.2.9356643. [DOI] [PubMed] [Google Scholar]
- 41.Burstein D, Gray ML, Hartman AL, Gipe R, Foy BD. Diffusion of small solutes in cartilage as measured by nuclear magnetic resonance (NMR) spectroscopy and imaging. J Orthop Res. 1993;11:465–478. doi: 10.1002/jor.1100110402. [DOI] [PubMed] [Google Scholar]
- 42.Mlynarik V, Sulzbacher I, Bittsansky M, Fuiko R, Trattnig S. Investigation of apparent diffusion constant as an indicator of early degenerative disease in articular cartilage. J Magn Reson Imaging. 2003;17:440–444. doi: 10.1002/jmri.10276. [DOI] [PubMed] [Google Scholar]
- 43.Gray ML, Burstein D, Lesperance LM, Gehrke L. Magnetization transfer in cartilage and its constituent macromolecules. Magn Reson Med. 1995;34:319–325. doi: 10.1002/mrm.1910340307. [DOI] [PubMed] [Google Scholar]
- 44.Kim DK, Ceckler TL, Hascall VC, Calabro A, Balaban RS. Analysis of water-macromolecule proton magnetization transfer in articular cartilage. Magn Reson Med. 1993;29:211–215. doi: 10.1002/mrm.1910290209. [DOI] [PubMed] [Google Scholar]
- 45.Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med. 2007;57:192–200. doi: 10.1002/mrm.21120. [DOI] [PubMed] [Google Scholar]
- 46.Altman RD, Hochberg M, Murphy WA, Jr, Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3 (Suppl A):3–70. [PubMed] [Google Scholar]
- 47.Felson DT, McAlindon TE, Anderson JJ, Naimark A, Weissman BW, Aliabadi P, et al. Defining radiographic osteoarthritis for the whole knee. Osteoarthritis Cartilage. 1997;5:241–250. doi: 10.1016/s1063-4584(97)80020-9. [DOI] [PubMed] [Google Scholar]
- 48.Reeder SB, McKenzie CA, Pineda AR, Yu H, Shimakawa A, Brau AC, et al. Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging. 2007;25:644–652. doi: 10.1002/jmri.20831. [DOI] [PubMed] [Google Scholar]
- 49.Laurent D, Wasvary J, Yin J, Rudin M, Pellas TC, O'Byrne E. Quantitative and qualitative assessment of articular cartilage in the goat knee with magnetization transfer imaging. Magn Reson Imaging. 2001;19:1279–1286. doi: 10.1016/s0730-725x(01)00433-7. [DOI] [PubMed] [Google Scholar]
- 50.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67:206–211. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 51.Multanen J, Rauvala E, Lammentausta E, Ojala R, Kiviranta I, Hakkinen A, et al. Reproducibility of imaging human knee cartilage by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) at 1. 5 Tesla. Osteoarthritis Cartilage. 2009;17:559–564. doi: 10.1016/j.joca.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 52.Glaser C, Mendlik T, Dinges J, Weber J, Stahl R, Trumm C, et al. Global and regional reproducibility of T2 relaxation time measurements in human patellar cartilage. Magn Reson Med. 2006;56:527–534. doi: 10.1002/mrm.21005. [DOI] [PubMed] [Google Scholar]
- 53.Mosher TJ, Zhang Z, Reddy R, Boudhar S, Milestone BN, Morrison WB, et al. Knee articular cartilage damage in osteoarthritis: analysis of MR image biomarker reproducibility in ACRIN-PA 4001 multicenter trial. Radiology. 2011;258:832–842. doi: 10.1148/radiol.10101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Njeh CF, Hans D, Li J, Fan B, Fuerst T, He YQ, et al. Comparison of six calcaneal quantitative ultrasound devices: precision and hip fracture discrimination. Osteoporos Int. 2000;11:1051–1062. doi: 10.1007/s001980070027. [DOI] [PubMed] [Google Scholar]
- 55.Riley PO, DellaCroce U, Kerrigan DC. Effect of age on lower extremity joint moment contributions to gait speed. Gait Posture. 2001;14:264–270. doi: 10.1016/s0966-6362(01)00133-3. [DOI] [PubMed] [Google Scholar]
- 56.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 57.Velikina J, Alexander AL, Samsonov A. Accelerating Multi-Component Relaxometry in Steady State with an Application of Constrained Reconstruction in Parametric Dimension. Proc. of ISMRM; Montreal, Canada. 2011. p. 2740. [Google Scholar]
- 58.Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging. 2007;26:375–385. doi: 10.1002/jmri.20969. [DOI] [PubMed] [Google Scholar]
- 59.Yao W, Qu N, Lu Z, Yang S. The application of T1 and T2 relaxation time and magnetization transfer ratios to the early diagnosis of patellar cartilage osteoarthritis. Skeletal Radiol. 2009;38:1055–1062. doi: 10.1007/s00256-009-0769-8. [DOI] [PubMed] [Google Scholar]
- 60.Mossehebi P, Chaudhary R, Sritanyaratana N, Block W, Samsonov A, Kijowski R. Factors influencing cross relaxation imaging parameters of articular cartilage. Proceedings of the 21st Annual Meeting of ISMRM; Salt Lake City. 2013. p. abstract 431. [Google Scholar]
- 61.Lin PC, Reiter DA, Spencer RG. Sensitivity and specificity of univariate MRI analysis of experimentally degraded cartilage. Magn Reson Med. 2009;62:1311–1318. doi: 10.1002/mrm.22110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torchia DA, Hasson MA, Hascall VC. Investigation of molecular motion of proteoglycans in cartilage by 13C magnetic resonance. J Biol Chem. 1977;252:3617–3625. [PubMed] [Google Scholar]
- 63.Lippiello L, Hall D, Mankin HJ. Collagen-Synthesis in Normal and Osteoarthritic Human Cartilage. Journal of Clinical Investigation. 1977;59:593–600. doi: 10.1172/JCI108676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yarnykh VL. Fast macromolecular proton fraction mapping from a single off-resonance magnetization transfer measurement. Magn Reson Med. 2012;68:166–178. doi: 10.1002/mrm.23224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]