Abstract
Spontaneous coronary artery dissection (SCAD) is a rare and often lethal cause of acute coronary syndrome, which typically affects young women and otherwise healthy individuals. SCAD can be diagnosed in patients undergoing coronary angiography and can be underestimated. Special techniques such as optical coherence tomography (OCT) and intravascular ultrasound should be used when there is suspicion of the condition. In the majority of cases, the left anterior descending (LAD) artery is involved; however, a few cases of the right coronary artery (RCA) involvement have been reported. This article describes three cases of SCAD in women of different ages, all presenting with chest pain. Coronary angiography in conjunction with OCT was used for diagnosis in two of the cases. One of the patients had involvement of the proximal RCA and underwent percutaneous coronary intervention, whereas the other two patients had mid-LAD disease and were treated conservatively with medical therapy. Presently, there are no specific guidelines for the treatment of SCAD, and therapy is individualized according to extent and severity of the condition.
Keywords: spontaneous coronary artery dissection, coronary angiography, optical coherence tomography, intravascular ultrasound, myocardial infarction, angina
Spontaneous coronary artery dissection (SCAD) is the separation of the media by hemorrhage with or without an associated intimal tear. It is not associated with aortic root dissection or a consequence of coronary angioplasty/angiography, cardiac surgery, or chest trauma (1). SCAD more commonly occurs in women, usually causing acute coronary syndrome and sudden death (2).
Coronary angiography is frequently used to diagnose SCAD. Optical coherence tomography (OCT) and intravascular ultrasound (IVUS) may also provide additional detailed luminal information (3, 4). In the majority of SCAD cases, the left anterior descending (LAD) coronary artery is involved. The aim of this study is to present three different clinical presentations of SCAD involving the LAD and right coronary artery (RCA), as well as review current literature recommendations for evaluation and treatment.
Case series
Case 1
A 30-year-old Caucasian woman presented to the emergency department with self-limited severe chest heaviness, which started at rest and lasted for three hours. She had associated dyspnea on exertion and diaphoresis. Her past medical history was significant for panic disorder and postpartum depression. Risk factors for coronary artery disease (CAD) included obesity. Medication usage included an oral contraceptive (OCP), alprazolam, citalopram, and lansoprazole. Vital signs demonstrated blood pressure 157/105 mmHg and heart rate 79 beats/min. Physical exam was remarkable for obesity, otherwise normal. Laboratory data were significant for a white blood cell count of 11.10 th/mm3, serum potassium of 3.4 mmol/L, aspartate transaminase of 70 U/L, and human chorionic gonadotropin was negative. Cardiac enzymes were significant for elevated troponin I 27.64 ng/ml (normal value [NV]: 0–0.78), creatine kinase (CK) of 484 U/L (NV: 10–150 U/L), and CK-MB of 56 ng/mL (NV: 0–5 ng/mL). Initial electrocardiogram (EKG) was unremarkable. Computed tomography angiogram showed no evidence of acute pulmonary embolism. Patient was admitted and started on intravenous heparin infusion. The following day the patient underwent transthoracic echocardiogram, which demonstrated mild hypokinesis of the inferior and septal walls with a normal ejection fraction. Patient was transferred to the catheterization lab and a coronary angiogram was performed. There was a 70% stenosis in the proximal RCA and diffuse luminal irregularities of the vessel (Fig. 1). No significant lesions were seen in the left coronary system. Percutaneous coronary angioplasty was performed. A 3.0 x 38 mm drug eluting stent and 4.0 x 38 mm bare metal stent were inserted in the RCA. Patient was started on aspirin, prasugrel, metoprolol, lisinopril, and atorvastatin. She was advised to stop the OCP.
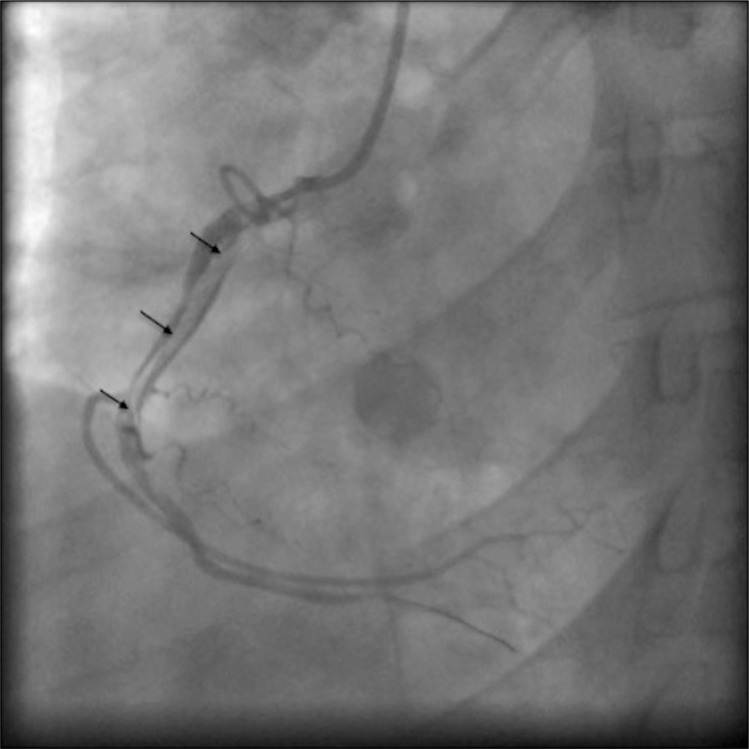
Fig. 1.
Angiographic image of right coronary artery demonstrating diffuse luminal irregularities and double lumen (indicated by black arrows) diagnostic of spontaneous coronary artery dissection.
Case 2
A 38-year-old Caucasian woman presented to an outpatient cardiology clinic with sudden, severe, midsternal chest pressure that began after a rigorous exercise session. She had been training at a vigorous level in preparation for a local triathlon. Training sessions had lasted approximately 2 h daily. She denied previous history of collagen vascular disease and pregnancy. Risk factor for CAD included a family history of premature cardiovascular disease. Past medical history was significant for fatty liver. Patient medication usage included an antihistamine; she denied OCP use and drug abuse. Vital signs demonstrated blood pressure 126/80 mmHg and heart rate 61 beats/min. Physical examination was unremarkable and included a normal cardiovascular exam. Initial EKG and chest x-rays were unrevealing. Laboratory data was negative for hematologic, renal, and hepatic disease. Cardiac enzymes were unremarkable. Transthoracic echocardiogram demonstrated normal ventricular size and function with no wall motion abnormalities. She was admitted and coronary angiogram was performed with suspicion of acute coronary syndrome, coronary spasm, and/or SCAD. Patient was found to have a 20% stenosis in mid-LAD (Fig. 2). Within the involved area, contrast was seen to swirl and stay longer than usual, consistent with self-limiting SCAD. Additionally, OCT was performed and did not show any large dissection (Fig. 3). RCA and left circumflex artery were normal. No interventions were performed and patient was discharged on life-long aspirin and clopidogrel for 1 year. Beta-blocker was not indicated because of sinus bradycardia. Avoidance of strenuous exercise was recommended.
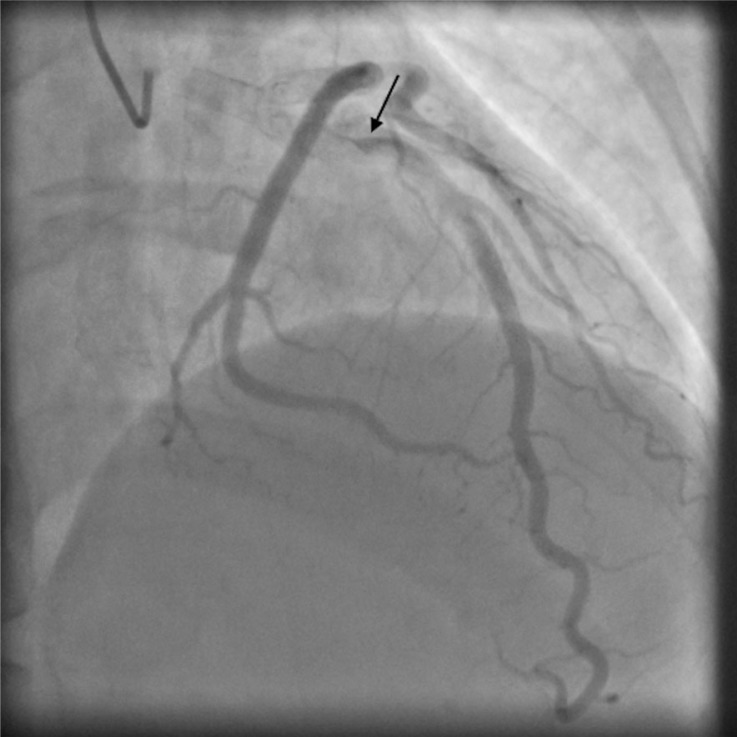
Fig. 2.
Angiographic image demonstrating left anterior descending artery with 20% stenosis in the mid-section. Black arrow indicates area where the contrast was seen to swirl and stay longer than usual, consistent with self-limiting spontaneous coronary artery dissection.
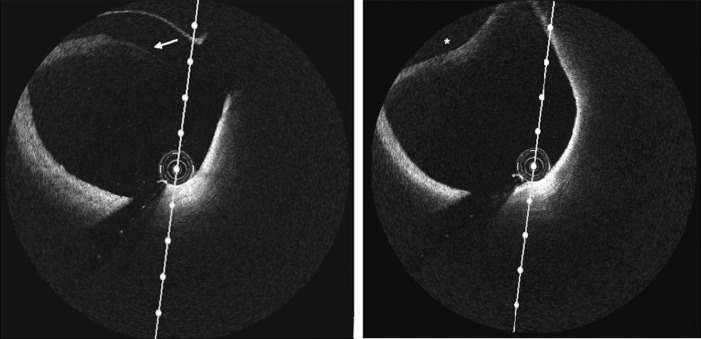
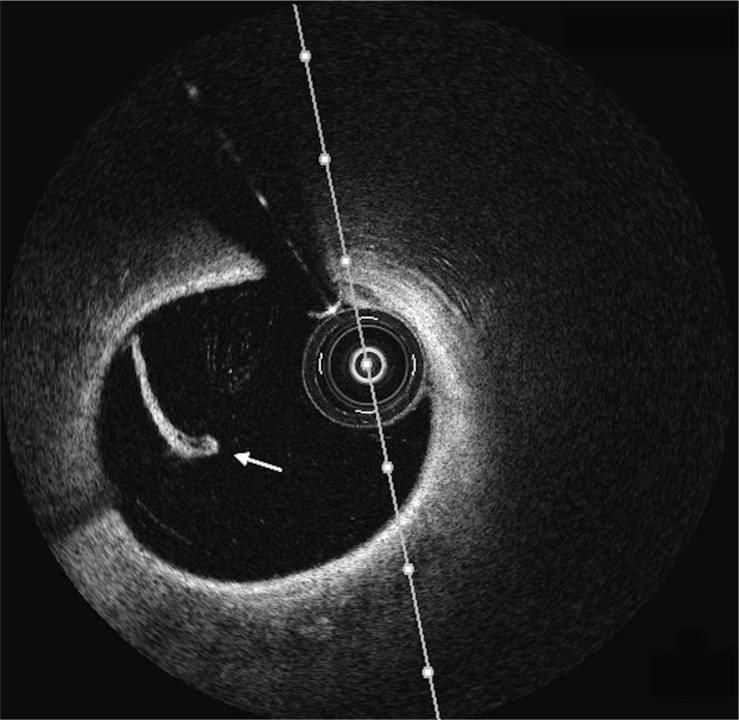
Fig. 3.
(Left-hand panel) Optical coherence tomography image of the left anterior descending artery demonstrating intimal tear (indicated by white arrow). (Right-hand panel) Optical coherence tomography image of the left anterior descending artery demonstrating intramural hematoma (asterisk) compromising the true lumen.
Case 3
A 65-year-old Caucasian woman presented to the emergency department with worsening intermittent substernal chest pain radiating to her left arm for 2 days. She had significant past medical history of hypertension, unruptured brain aneurysm, and a previous cardiac arrest due to asystole. Risk factors for CAD included advanced age, family history of premature cardiovascular disease, and tobacco exposure. Patient medications included high dose aspirin, metoprolol, nabumetone, and methocarbamol. Vital signs demonstrated blood pressure 188/100 mmHg and heart rate 74 beats/min. Physical examination was unremarkable with regular heart sounds without murmurs, rubs, or gallops. Initial laboratory data were within normal limits without evidence of hematologic, hepatic, or renal disease. EKG showed sinus rhythm with ST depression in leads V1–V3. Initial chest x-ray was normal without cardiomegaly or effusions. Cardiac biomarkers were normal. Patient was transferred to the catheterization lab and a coronary angiogram was obtained with the suspicion of non-ST elevation myocardial infarction. The RCA was noted to be dominant and the left circumflex artery did not have significant disease. The LAD artery did have a 10% stenotic lesion at the midpoint (Fig. 4). An OCT revealed an isolated limited dissection composed of only the intimal layer (Fig. 5). Atherosclerotic change was not detected in the vessel. No intervention was performed. Patient had a favorable clinical course and was discharged on low dose aspirin, clopidogrel, atorvastatin, low dose lisinopril, and metoprolol.
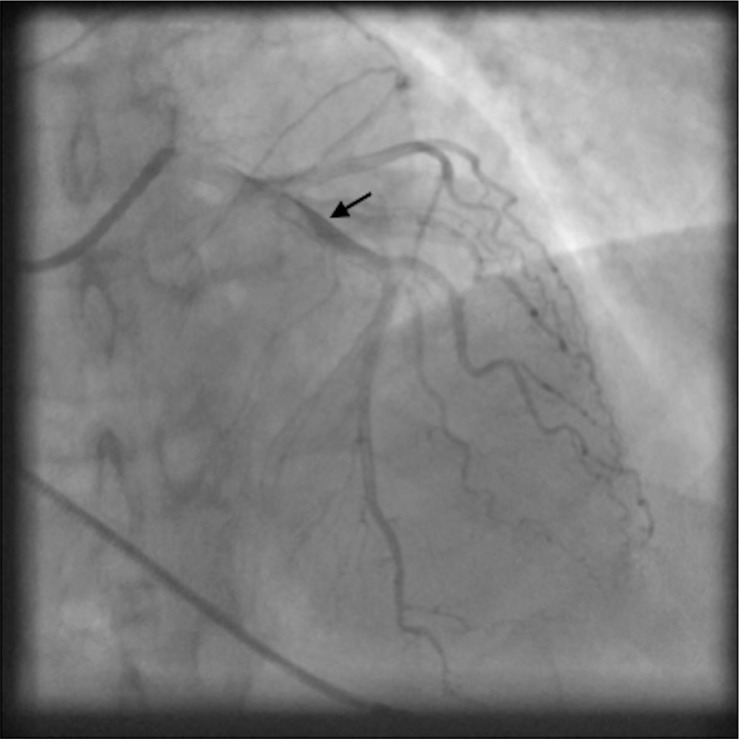
Fig. 4.
Angiographic view of left anterior descending artery with delayed clearing of the contrast suggesting spontaneous coronary artery dissection (indicated by black arrow).
Fig. 5.
Optical coherence tomography image of the left anterior descending artery demonstrating intimal flap (indicated by white arrow).
Discussion
The presented case series described three women of differing ages, who presented with chest pain. Coronary angiography, in conjunction with OCT, was used for SCAD diagnosis in two of the cases. One of the patients had involvement of the proximal RCA and underwent percutaneous coronary intervention (PCI), whereas the other two patients had mid-LAD disease and were treated conservatively with medical therapy. Treatments were in accordance with the degree of stenosis in the involved vessel.
From 1931 to 2013, more than 490 cases of SCAD have been reported in the medical literature (5). Incidence of SCAD is approximately 0.1–0.28% in patients who have had a coronary angiogram. Recently, however, with the availability of advanced intracoronary imaging, more subtle dissections are being detected and the overall incidence of SCAD is as high as 1–4% in patients with acute coronary syndrome.
The average age for the incidence of SCAD is 42 years, though there have been cases reported from age 14 to about the seventh decade of life. Around 80% of the cases are females and of those, 20–25% occur in the peripartum period. SCAD has also been found to account for up to 40% of heart attacks in women under the age of 50. Almost 50% present as ST elevation myocardial infarction and about 25% present with multivessel involvement (6).
Three different pathophysiological mechanisms have been associated with SCAD. Patients are separated according to underlying condition: 1) non-atherosclerotic/peripartum period group; 2) atherosclerotic group; and 3) idiopathic or heterogeneous group in which one of the following conditions are present: connective tissue disorders (Marfan syndrome, Ehlers–Danlos syndrome, polyarteritis nodosa, systemic lupus erythematous and fibromuscular dysplasia,); medication (OCP, fluorouracil, fenfluramine, cyclosporine); cocaine use; hypertension; cystic medial necrosis; variant angina; and physical exercise (7). The most common conditions related to SCAD are coronary atherosclerosis and those associated with the peripartum period. In the atherosclerotic group, atherosclerotic plaque inflammation and rupture may induce disruption of the intimal-medial junction, leading to an intimal flap and subsequent intramural hematoma formation (7, 8). In contrast, peripartum dissection has not been related to atherosclerotic plaque. Eosinophils infiltrate the uterus and serum collagenase levels increase during labor and the peripartum period. Eosinophilic infiltration in the coronary artery adventitia breaks down the medial-adventitial layer, leading to SCAD (9, 10). In this case series, the first two patients were most likely in the heterogeneous group. The first case only had the risk factor of obesity for atherosclerotic disease and was not in the peripartum period. However, OCP therapy may have played a role in the condition. In the second case, the patient had minimal atherosclerotic risk factors and was not in the peripartum period, but had strenuous exercise at the time of chest pain onset. In contrast, the last patient had significant risk for CAD and fell into the atherosclerotic group.
The clinical presentation of SCAD depends on the extent and flow limiting severity of the coronary dissection. It can range from asymptomatic, to unstable angina, acute myocardial infarction, ventricular arrhythmias, or sudden cardiac death. In the above described cases, one patient presented with the classic features of non-ST elevation myocardial infarction and two patients presented with unstable angina. All three patients underwent invasive imaging studies, which contributed to the diagnosis of SCAD.
SCAD is typically diagnosed with coronary angiography, however it can be underestimated using angiography alone (11). Coronary angiography can indicate a radiolucent intimal flap or a delayed clearance of contrast from the false lumen. If there is not an intimal tear, the medial hematoma may appear as a narrowed vessel (3). Therefore, in some instances it can be difficult to distinguish atherosclerotic stenosis or spasm from intramural hematoma or dissection. The use of OCT is seen as a complementary technique to identify the exact location of the dissection as well as the extent and thickness of the hematoma. Histological and clinical studies have shown that OCT can identify the microstructure of coronary wall and atherosclerotic plaques (12). In addition IVUS has been used to evaluate the relation between the false and the true lumen and dissection. In the first case, the diagnosis was made using coronary angiography alone given the degree of stenosis and the evidence of false lumen seen and corroborated by the inability to advance a guide wire through the RCA. In the second case, SCAD was diagnosed by angiogram, though OCT was performed to evaluate the extent of dissection. In contrast, in the third case the lesion was first suspected to be due to spasm because there was no intimal flap or false lumen visible when coronary angiography was performed. The final diagnosis, coronary artery dissection, was made performing OCT.
Optimal treatments for SCAD have not been clearly determined, with no consensus as to recommendations. Therapeutic strategies depend upon the clinical presentation, location, and extent of dissection as well as the ischemic myocardium area at risk (13). Options include conservative medical therapy, PCI, and coronary artery bypass graft (CABG) (14). Conservative medical therapy can be a reasonable initial option in mid- or distal-single vessel dissection with a lumen diameter limitation <50% (5, 9, 15). Some studies have demonstrated that early intervention with either PCI or CABG following the diagnosis of SCAD lead to better outcomes (5). The role of thrombolytics is controversial, and may be associated with the extension of dissection due to increased blood in the vessel wall or hematoma causing compression of the true lumen (9). PCI can be the therapy of choice in single vessel disease, especially proximal dissection, in which there are ongoing symptoms and persistent restriction of coronary blood flow. However, PCI can be associated with several complications relating to the passage of the coronary wire into the false lumen of the dissected vessel or the loss of coronary flow through the propagation of dissection and displacement of stents, resulting in the propagation of hematoma (15). Adjunctive-imaging technologies such as IVUS and OCT should be considered to determine extent of dissection and to provide real-time guidance for intervention. Finally, CABG is the treatment of choice in multiple vessel disease, especially where there is left main stem involvement with ongoing ischemia refractory to medical or interventional therapy (9). In the first case, PCI was performed given 70% stenosis of the lumen involving the proximal RCA. In the subsequent cases, medical management was pursued due to mild involvement of mid-LAD.
Close and long-term follow up is indicated in SCAD patients given that 10-year recurrence rates up to 20% have been reported (6). Our three patients are being followed up on a 6-month basis in an outpatient cardiology clinic.
Conclusion
SCAD is an uncommon condition and serious cause of acute coronary syndrome. Consideration of SCAD can be very important in young healthy females, especially when it is difficult to explain the pathogenesis of the stenotic lesion and no atherosclerotic risk factors. Early coronary angiography remains essential in the diagnosis of SCAD. OCT and IVUS can be important tools for obtaining additional information on extent and location of the dissection. LAD is the most common site of dissection; however, as seen within this case series, patients can have RCA involvement.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Waller BF. Nonatherosclerotic coronary heart disease. In: Fuster V, Walsh RA, Harrington RA, editors. Hurst's the heart. 13th ed. New York: McGraw-Hill; 2011. pp. 1257–86. [Google Scholar]
- 2.Xin-He Y, Cheng-Jian Y, Yan J, Xin X, Jia-Ning C, Zhen-Jie Y, et al. A successful emergency management of spontaneous coronary artery dissection and review of literature. Am J Emerg Med. 2013;31:1156.e1–3. doi: 10.1016/j.ajem.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Arnold JR, West NE, Van Gaal WJ, Karamitos TD, Banning AP. The role of intravascular ultrasound in the management of spontaneous coronary artery dissection. Cardiovasc Ultrasound. 2008;6:24. doi: 10.1186/1476-7120-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfonso F, Canales E, Aleong G. Spontaneous coronary artery dissection diagnosis: diagnosis by optical coherence tomography. Eur Heart J. 2009;30:385. doi: 10.1093/eurheartj/ehn441. [DOI] [PubMed] [Google Scholar]
- 5.Shamloo BK, Chintala RS, Nasur A, Ghazvini M, Shariat P, Diggs JA, et al. Spontaneous coronary artery dissection: aggressive vs. conservative therapy. J Invasive Cardiol. 2010;22:222–8. [PubMed] [Google Scholar]
- 6.Hayes SN. Spontaneous coronary artery dissection (SCAD): new insights into this not-so-rare condition. Tex Heart Inst J. 2014;41:295–98. doi: 10.14503/THIJ-14-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almafragi A, Convens C, Heuvel PV. Spontaneous healing of spontaneous coronary artery dissection. Cardiol J. 2010;17:92–5. [PubMed] [Google Scholar]
- 8.Borczuk AC, van Hoeven KH, Factor SM. Review and hypothesis: the eosinophil and peripartum heart disease (myocarditis and coronary artery dissection) – coincidence or pathogenetic significance? Cardiovasc Res. 1997;33:527–32. doi: 10.1016/s0008-6363(96)00257-x. [DOI] [PubMed] [Google Scholar]
- 9.Paraskevaidis S, Theofilogiannakos EK, Chatzizisis YS, Mantziari L, Economou F, Ziakas A, et al. Spontaneous dissection of right coronary artery manifested with acute myocardial infarction. Open Cardiovasc Med J. 2010;4:178–80. doi: 10.2174/1874192401004010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinowitz M, Virmani R, McAllister HA., Jr. Spontaneous coronary artery dissection and eosinophilic inflammation: a cause and effect relationship? Am J Med. 1982;72:923–28. doi: 10.1016/0002-9343(82)90853-1. [DOI] [PubMed] [Google Scholar]
- 11.Jorgensen MB, Aharonian V, Mansukhani P, Mahrer PR. Spontaneous coronary dissection: a cluster of cases with this rare finding. Am Heart J. 1994;127:1382–7. doi: 10.1016/0002-8703(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 12.Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–9. doi: 10.1016/j.jacc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 13.Almeda FQ, Barkatullah S, Kavinsky CJ. Spontaneous coronary artery dissection. Clin Cardiol. 2004;27:377–80. doi: 10.1002/clc.4960270702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanzetto G, Berger-Coz E, Barone-Rochette G, Chavanon O, Bouvaist H, Hacini R, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg. 2009;35:250–4. doi: 10.1016/j.ejcts.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579–88. doi: 10.1161/CIRCULATIONAHA.112.105718. [DOI] [PubMed] [Google Scholar]