Abstract
Background:
Nonmydriatic digital imaging (NMDI) is ideal for screening diabetic retinopathy (DR), but its use in Indian eyes has not been evaluated.
Aim:
The aim was to evaluate the sensitivity and specificity of NMDI as a screening tool in detecting DR in Indian eyes.
Design:
A prospective, nonrandomized, noncomparative, noninterventional study.
Materials and Methods:
A total of 500 diabetic patients visiting the endocrinology clinic (September 2008-June 2010) underwent NMDI (Zeiss Procam), followed by routine dilated fundus photography (FP; Zeiss Visupac 450+) of 345° retinal fields (1) optic disc and macula, (2) superotemporal, and (3) nasal to optic disc. Two-masked retina specialists graded the images for quality and severity of DR, and compared between NMDI and dilated FP.
Statistical Analysis:
SPSS Windows 17 for version.
Results:
Mean age was 52.97 ± 13.46 years (306 males: 194 females). The rate of ungradable images was 30.6% and 31% by the two observers. By observer 1, the sensitivity and specificity of detecting any DR was 58.8% and 69.1%, respectively, (κ = 0.608) and sight-threatening DR (STDR) was 63.1% and 68.9%, respectively, (κ = 0.641). By observer 2, the sensitivity and specificity was 57.3% and 68.3%, respectively, for any DR (κ = 0.593) and 62.8% and 68.3%, respectively, for STDR (κ = 0.637). The level of agreement between two observers was high (κ = 0.96).
Conclusion:
A high rate of poor quality photographs and low sensitivity limited the use of NMDI as a perfect screening system, particularly in dark iris population with diabetes as seen in Indian eyes.
Keywords: Diabetic retinopathy, Indian eyes, nonmydriatic imaging, screening
Diabetic retinopathy (DR) poses a major public health burden in India. According to the World Health Organization, the number of cases of type 2 diabetes mellitus (DM) will grow from 19 million in 1995 to nearly 80 million in 2030 and India will emerge one of the major hubs of diabetic population.[1,2] Nearly half of the patients with diabetes would have some degree of DR at any given time.[3,4] After 15 years of duration of DM, DR will develop in nearly all patients with type 1 DM and about 75% of those with type 2 DM.[2,3] In Indian subcontinent, the prevalence rate of DR is reported between 12% and 37% in type 2 DM.[5,6]
Diabetic retinopathy constitutes sixth common cause of blindness in India.[2] The major contributing factor toward the development of blindness is the progression to an advanced stage of the disease. The patients themselves never visit an ophthalmologist in the early stage of DR when it is often asymptomatic. The key to maintenance of good vision in these eyes is the early detection and treatment that can be done by screening all the patients with a diagnosis of DM. Screening is vital not only to prevent visual loss, but it is also a highly cost-effective health intervention program.
The gold standard for evaluating DR is the seven standard stereoscopic 30° field photographs.[7] The screening strategy recommended by the American Diabetes Association and the American Academy of Ophthalmology comprises of a dilated fundus examination with stereoscopic evaluation of the posterior pole on slit lamp biomicroscopy.[8,9,10,11] Alternative methods include direct ophthalmoscopy or nonstereoscopic 45° retinal photography through an undilated pupil, and nonstereoscopic 45° retinal photography through a dilated pupil. Nonmydriatic cameras (NMC) have been used for evaluating DR for the last 25 years.[12,13] In the USA and Europe, regular screening of DR using nonmydriatic fundus camera is being widely recommended, but their sensitivity and specificity is not known in Indian eyes with dark brown irides. The present study evaluates the sensitivity and specificity of nonmydriatic digital imaging (NMDI) in screening DR in Indian eyes.
Materials and Methods
Patients and imaging
Five hundred patients (1000 eyes) with type 1 or type 2 DM attending the endocrinology clinic of our hospital, were imaged between September 2008 and June 2010 and analyzed. Institute ethics committee approval was obtained, and the study was conducted in accordance with Helsinki declaration. Patients with following criteria were excluded:
History of previous laser treatment or vitreous surgery
Patients already following up under a retina specialist
Patients with significant physical or mental disabilities who were unable to sit at the camera or cooperate with photography
Patients with significant cataract and corneal opacity in one or both eyes precluding fundus photography.
The nonmydriatic fundus camera (Zeiss Procam) was stationed in the endocrinology clinic, which is located within 300 m of the Ophthalmology Department. After explaining the nature and need for fundus photography, relevant history was taken to rule out exclusion criteria. A trained ophthalmic technician conducted three 45° retinal field imaging in color and red free (1) Optic disc and macula, (2) Superotemporal to optic disc (3) Nasal to optic disc. Three color 45° nonmydriatic fundus fields have been considered as an effective screening tool for quantifying DR.[14]
All patients were made to wait in a dark room prior to taking photographs. A single external image of each eye was acquired to rule out any significant corneal opacity or cataract. Imaging of the posterior segment followed this. After doing few patients, it was found that after exposure to an initial flash, it took several minutes for the pupil to redilate enough for posterior segment to be photographed again. We increased the time interval between successive images in steps of 1 min until a good quality image could be taken. The successive photographs were taken at interval of 3-6 min. The patients were then directed to the Advanced Eye Centre for dilated fundus photography in the same fields (Zeiss Visupac 450 + fundus camera). Based on dilated fundus imaging, each patient was managed for DR accordingly.
Grading of retinal images
The images were displayed on the monitor of the respective cameras from the database. Each observer separately reviewed the external image to look for the size of pupil, status of lens, presence of any corneal opacity, or any lid abnormality. All six fundus images (both in color and red-free mode) from each eye were then reviewed independently by the two experienced retina specialists (VG, RB) and graded for quality of photograph and retinopathy. Any microaneurysms, retinal hemorrhages, venous dilatation or venous caliber abnormalities, retinal exudates (hard or soft), cotton wool spots, retinal thickening, retinal detachment, vitreous hemorrhage, or neovascularization of the disc or retina were looked for. The quality of photographs was assessed as per the following grading scheme used by Higgs et al.[15]
Grade 1: Excellent
Grade 2: Definition of most retinal detail clear
Grade 3: Definition limited, but most detail visible; minor degrees of retinopathy and fine disc or retinal new vessels might be missed
Grade 4: Only gross detail visible; larger hemorrhages and exudates may occasionally be detectable
Grade 5: No detail visible.
The DR was characterized as per Airlie house classification of ETDRS as listed.[9]
No DR: No abnormalities
Mild nonproliferative DR (NPDR): Occasional Microaneurysms or hemorrhages only
Moderate NPDR: Moderate intraretinal hemorrhages, hard or soft exudates
Severe NPDR: Numerous peripheral retinal hemorrhages (each of 4 quadrants) and/or definite venous beading in 2 + quadrants and/or moderate intraretinal microvascular abnormalities (1 + quadrant); and no signs of proliferative DR (PDR)
Proliferative DR: New vessels on disc or elsewhere on retina, vitreous/preretinal hemorrhage.
As ophthalmoscopic examination was done in selected patients only (requiring retinal services care after referral), grading of maculopathy was not done separately in patients detected to have any level of DR. Any grade worse than mild NPDR was labeled as sight-threatening DR (STDR) such as moderate NPDR or worse, or clinically significant maculopathy in one or both eyes needing prompt referral for investigations and management.[16] These clinical levels were graded on dilated fundus photography and nonmydriatic retinal imaging (NMRI) and then compared to evaluate the sensitivity and specificity of NMRI.
Results
A total of 500 patients (1000 eyes) with both type 1 and type 2 diabetes could be photographed both on nonmydriatic and dilated fundus camera. There were 306 males and 194 females. The mean age was 52.97 ± 13.46 years (range: 9-84 years).
Their DR status was assessed by dilated fundus grading (reference) for images captured after pharmacological pupil dilatation. Digital NMRI of these patients was analyzed by two masked observers (number 1 and number 2) and compared. The dilated fundus imaging was considered to be the reference for detecting any form of DR. Images were classified as no DR or presence of any type of DR. DR was further classified as mild NPDR, moderate NPDR, severe NPDR, or PDR.
Statistical analysis was performed using SPSS Windows 17 for version (SPSS Inc., Chicago, IL). Chi-square test was applied to find the association between the grading done by the observers and their measure of agreement (Kappa coefficient). The gradation by each observer was also compared with the grades of DR by dilated fundus imaging which was considered gold standard for their association and agreement. We also calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of NMRI for detecting any form of DR by both the observers separately. Wilcoxon signed ranks test was applied to compare the distributions of grading by the observers and the grading by gold standard.
Observer number 1
Of 1000 eyes, 256 (25.6%) eyes could not be graded as they were either partially or completely unassessable. Of 500 patients, 153 (30.6%) patients had ungradable images in at least 1 eye. In 347 (69.4%) patients, both eyes could be graded from undilated images. The mean age of patients with ungradable images was 55.14 ± 12.65 years (median 56 years, range: 15-81 years) and with gradable images was 50.71 ± 13.60 (median 53 years, range: 9-84 years). The difference was statistically significant (P < 0.001).
When analyzed in this subset of 347 patients with gradable images by digital nonmydriatic photography (after exclusion of patients with ungradable images), the sensitivity of detecting any type of DR was 84.7%. The specificity was 99.6%. The PPV was 98.9% and NPV 93.3%. The weighted kappa (κ) denoting the level of agreement between the undilated and the dilated images of these 347 patients was 0.876.
However, since only 69.4% patients could be graded from undilated images, the overall sensitivity and specificity in the screened population were reduced to 58.8% and 69.1%, respectively. The Overall PPV and NPV were 68.6% and 64.7%, respectively. The level of agreement was also reduced (κ = 0.608).
Similarly, the sensitivity of detecting STDR in 347 patients was 91.1% and the specificity was 99.3% (κ =0.924). The PPV was 96.2% and NPV 98.3%. However, the overall sensitivity, specificity, PPV, and NPV were reduced to 63.1%, 68.9%, 66.8%, and 68.2%, respectively, in the 500 patients screened (κ = 0.641).
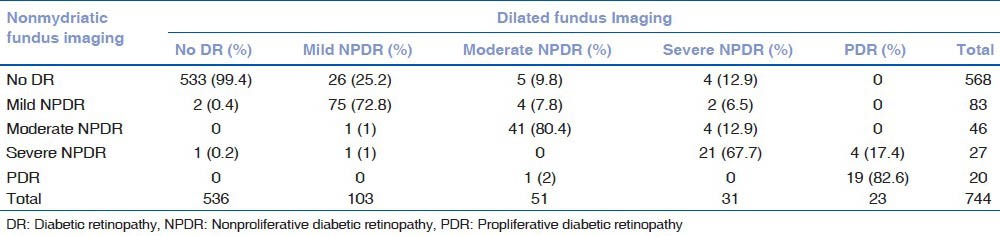
Of 744 eyes that could be graded from nonmydriatic imaging, there was concurrence in 689 (92.6%) eyes between the dilated and undilated photographic grading of the absence and presence of (including severity of) DR (κ = 0.827). While 6 eyes were falsely assigned more severe grades, 49 eyes were falsely assigned lower grades of DR and were underestimated by the observer from undilated images when compared with dilated fundus photographs in terms of severity of DR as shown in Table 1 (P < 0.001).
Table 1.
Distribution of absence or presence of diabetic retinopathy and its severity as assessed by the observer number 1 from nonmydriatic imaging compared to conventional dilated fundus imaging
Observer number 2
A total of 254 eyes (25.4%) could not be graded for absence or presence of any type of DR due to partially or totally unassessable images by the NMC. The results by observer number 2 are summarized in Table 2. The level of agreement between the two observers was high as denoted by κ = 0.96. Table 3 shows the inter observer variability between the absence, presence, and severity of DR.
Table 2.
Distribution of absence or presence of diabetic retinopathy and its severity as assessed by the observer number 2 from nonmydriatic imaging compared to conventional dilated fundusss imaging
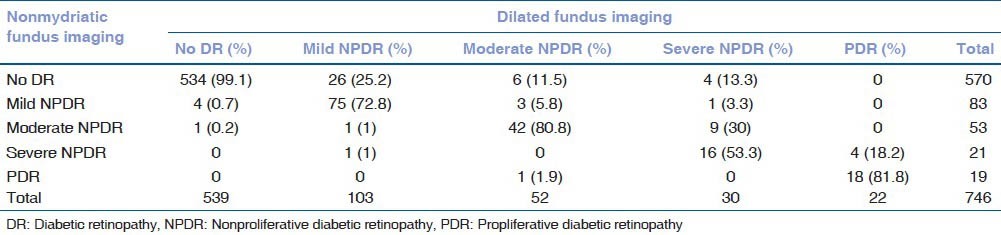
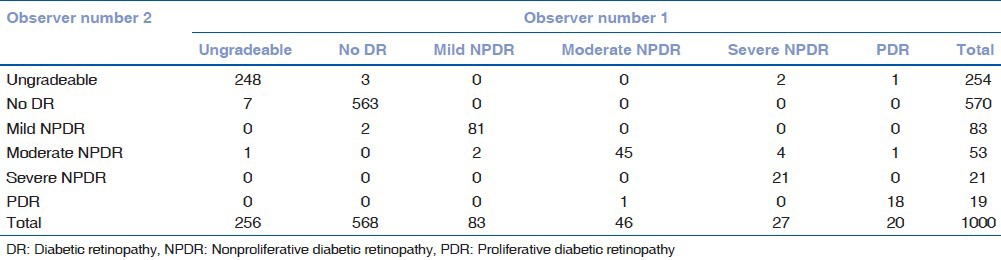
Table 3.
Distribution of ungradable images, absence and presence of diabetic retinopathy (including its severity) as assessed by the two observers from nonmydriatic imaging (κ=0.96)
The rate of ungradable images was 30.6% and 31% by the two observers, respectively [Figs. 1 and 2]. As per the exclusion criteria, uncooperative patients and those with significant media opacities on gross external eye examination (such as corneal opacities or dense white cataract) were not recruited in the study. However, 19 eyes (7.5% of ungradable images by nonmydriatic imaging) of 12 patients remained ungradable even on dilated fundus imaging due to significant cataract in 10 eyes (that could not be excluded by gross examination), posterior capsular opacification (5 eyes), and asteroid hyalosis (4 eyes).
Figure 1.

Anterior segment photographs of the right (a) and left (b) eyes of a 57-year-old male showing undilated pupils just before obtaining nonmydriatic retinal imaging (NMRI). The NMRI showed grade 4 quality photographs in both right (c) and left (d) eyes. Both eyes were ungradable for detection of diabetic retinopathy (DR). Dilated fundus photographs of right (e) and left (f) eyes of same patient as in a-d, showing grade 1 quality images and absence of any DR
Figure 2.
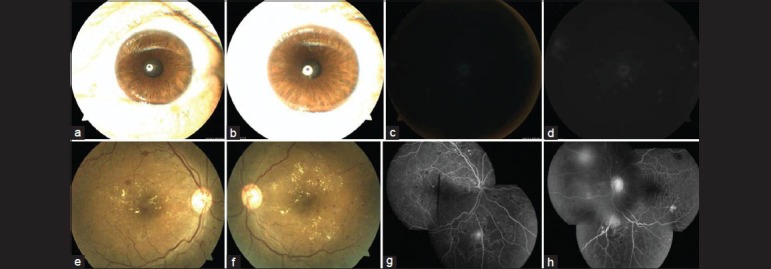
Anterior segment photographs of the right (a) and left (b) eyes of a 41-year-old male showing undilated pupils just before obtaining NMRI. The NMRI showed grade 5 quality photographs in both right (c) and left (d) eyes. Both eyes were ungradable for detection of diabetic retinopathy. Dilated fundus photographs (e and f) and fluorescein angiography photographs (g and h) of same patient as in a-d, showing grade 1 quality images. There is presence of clinically significant macular edema and neovascularization elsewhere on the retina in both eyes, suggestive of proliferative diabetic retinopathy
Discussion
The main aim of screening DR is to detect referable cases that need full ophthalmic evaluation and treatment. Any delay in this would lead to irreversible visual loss. Our results show a sensitivity of 84.7% and 83% by two independent observers among patients with gradable fundus images for detecting any form of DR by the NMRI system. The accepted target for an effective screening program is 80%.[17] The sensitivity in our study decreases further if all patients with gradable and ungradable images are included.
Nearly 80% of people with diabetes are in developing countries, where its health burden is alarming in China and India.[18,19] Every fifth diabetic in the world is an Indian. The aim of screening DR is to detect retinopathy, determine its severity, and to decide which patients require referral for further investigation and possible treatment. This requires a good screening tool with good sensitivity and specificity.
Several studies have demonstrated its high sensitivities and specificities for detecting Mohan et al., in their study on two ethnic groups, namely, Indian and European diabetic patients, found only 6% of photographs totally unassessable and an additional 12% only partially assessable.[20] However, there was 72% agreement between ophthalmoscopy by an ophthalmologist and the photographic assessment, and 100% agreement on clinically important lesions requiring treatment in their study. Baeza et al. found that screening for STDR with NMC can be performed effectively with one nonstereoscopic retinal photograph per eye with mydriasis.[21] However, when screening without mydriasis was performed, the percentage of referred patients to ophthalmologists increased from 5% to 15% because of ungradable photographs. Ahmed et al. found 35% of the images were not gradable.[22] Of the gradable images, they found 86% agreement in the grading between nonmydriatic digital stereoscopic retinal imaging (NMDSRI) and dilated fundus examination. Of the gradable NMDSRI results, the overall sensitivity of NMDSRI was 98% and specificity was 100% for retinopathy within one grade of the dilated fundus examination. For macular edema and clinically significant macular edema, there was 100% agreement in the stereoscopic retinal imaging of gradable eyes. A low rate (7.2%) of poor quality images (that required mydriasis) has been reported by Lopez-Bastida et al. who reported sensitivity of 92% and specificity of 96% for detecting any DR, and sensitivity of 100% for detecting STDR.[23] In a recent study in south Israel, the sensitivity of NMDI, when compared to mydriatic imaging was 99.3%, and specificity 88.3%.[24]
A very important aspect of designing and implementing a screening program with a nonmydriatic digital photographic camera is the rate of ungradable images. In our study, the rate of ungradable photos by nonmydriatic imaging modality is higher than that reported (12-25%) in many of the previous studies.[12,13,19,23,25,26,27,28,29,30] Light irises have been shown to transmit more light than eyes with dark irises. The high pigment content blocks light from passing through the iris to the retina, restricting it to the pupil. The iris controls the amount of light that enters the eye. Bergamin et al. studied the effect of iri color on pupillary light reflex in normal healthy volunteers and found that it significantly influenced the amplitude and velocity of pupillary contraction, but not the pupil size and latency time.[31] A stronger pupillary light reflex in terms of increased, faster, and prolonged pupillary contraction (as assessed by the amplitude, contraction velocity, and redilation velocity, respectively) was a significant finding in the brown iris group as compared to blue iris group in this study. Klein et al. found that patients with brown irises were more discomforted by the flash than patients with blue irises.[25]
A higher rate of ungradable images (35%) has been reported by Choremis and Chow,[32] and Ahmed et al.[22] Choremis and Chow found that images obtained from patients aged 65 years or more significantly poorer consistently than those obtained from patients < 65 years.[32] Ahmed et al. too found that the patients with ungradable eyes were significantly older (65 ± 10.6 years) than those with gradable eyes (57 ± 10.6 years). Higgs et al. have reported 34% of patients in whom one or both eyes could not be assessed due to poor quality photos.[14] They attributed this higher number of ungradable photos as compared to other series to higher proportion of elderly diabetic patients in their community with a mean age of 60 years (range: 6-89 years). Lens opacities and small pupils in older patients reduce the quality of photographs with a NMC. We also observed a higher mean age of patients with ungradable images as compared to those with gradable images. But the median age of patients with ungradable and gradable images did not show a large difference (56 and 53 years, respectively). Our patients were, however, much younger with a mean age of 52.07 ± 13.46 years (range: 9-84 years). This also concurs with the fact that diabetes in Asian countries is disproportionately high in young to middle-aged adults when compared to west, where older persons are most affected.[33] This could have long-lasting adverse effects on a nation's health and economy, especially for developing countries. Lack of symptoms and insidious onset of diabetes in adults may result in development of DR at an early stage. To overcome these challenges, we need to device strategies to diagnose DR at the earliest, so that timely detection and management can prevent vision-threatening complications of DR.
Screening is the most vital step for early detection as it is cost-effective. Sheer numbers, lack of awareness, and inadequate control of diabetes are the major patient barriers in providing adequate diabetes care in India.[34] Lack of knowledge among the physicians managing diabetes patients, lack of transportation or clinical facilities, and shortage of trained ophthalmologists to tackle retinal diseases constitute some of the physician barriers. The annual screening by an expert ophthalmologist remains a far reached target with increasing number of diabetic patients and very few ophthalmologists with required expertise. Nonmydriatic fundus camera overcomes some of these barriers by allowing retinal examination in the same visit at the diabetologist's office with a facility for remote reading by the ophthalmologist. Nonmedical personnel with a minimum of training can operate it. NMCs have been cost-effectively used for telemedicine screening of DR.[35,36]
It is not only convenient but also provides the opportunity to involve patients effectively in their care and educate them about their disease. We observed that although majority of eyes do not need dilatation for adequate quality retinal photograph to detect DR, a significant proportion of patients that may not have any DR do need ophthalmic referral solely due to ungradable photos.
Our study has a limitation of not evaluating the studied eyes with ophthalmoscopy. However, several studies have reported a lower sensitivity in detecting STDR by direct ophthalmoscopy as compared to by mydriatic photography (color slide film).[29,37,38]
The acquisition of image quality without the use of dilators needs to be validated in Indian eyes before this technology can be used as a widespread tool for implementation in national screening programs to curb the problem of DR in our population. Older age is a well-known factor that consistently produces poor quality undilated fundus images. But the brown iris may be a crucial factor in Indian population that further compromises the image quality by the NMRI. It has proved to be a useful tool when installed in an endocrinologist's clinic for recognizing any form of DR in diabetic patients, particularly those who never otherwise visit an ophthalmologist, because it improves patient compliance due to a greater comfort and convenience. It also limits the ophthalmic referrals by allowing us to screen a large number of patients attending an endocrinologist's outpatient clinic and identifying those with DR who require management. However, due to a high number of ungradable photos, it creates unnecessary ophthalmic referrals among patients who do not have any DR or have only mild NPDR because all patients with ungradable photos need to be referred for comprehensive eye evaluation. A relatively lower sensitivity and a high rate of poor quality photographs pose major limitations in its usefulness as a perfect screening system, particularly in dark irides population with diabetes involving younger age groups as seen in Indian eyes.
Acknowledgment
We thank Mr. R.C. Goyal for the statistical analysis of this study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Vashist P, Singh S, Gupta N, Saxena R. Role of early screening for diabetic retinopathy in patients with diabetes mellitus: An overview. Indian J Community Med. 2011;36:247–52. doi: 10.4103/0970-0218.91324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, et al. Diabetic retinopathy. Diabetes Care. 1998;21:143–56. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- 4.International diabetes foundation. Diabetes atlas. [Last accessed on 2013 Apr 12]. Available from: http://www.idf.org/diabetesatlas .
- 5.Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: A worldwide perspective. Surv Ophthalmol. 2012;57:347–70. doi: 10.1016/j.survophthal.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Murthy GV, Gupta SK, Bachani D, Jose R, John N. Current estimates of blindness in India. Br J Ophthalmol. 2005;89:257–60. doi: 10.1136/bjo.2004.056937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grading diabetic retinopathy from stereoscopic color fundus photographs-An extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 8.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102:520–6. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102:527–32. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 10.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Retinopathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S84–7. doi: 10.2337/diacare.27.2007.s84. [DOI] [PubMed] [Google Scholar]
- 11.Preferred Practice Pattern: Diabetic Retinopathy. 2003. [Last accessed on 2013]. Available from: http://www.aao.org .
- 12.Ryder RE, Vora JP, Atiea JA, Owens DR, Hayes TM, Young S. Possible new method to improve detection of diabetic retinopathy: Polaroid non-mydriatic retinal photography. Br Med J (Clin Res Ed) 1985;291:1256–7. doi: 10.1136/bmj.291.6504.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams R, Nussey S, Humphry R, Thompson G. Assessment of non-mydriatic fundus photography in detection of diabetic retinopathy. Br Med J (Clin Res Ed) 1986;293:1140–2. doi: 10.1136/bmj.293.6555.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vujosevic S, Benetti E, Massignan F, Pilotto E, Varano M, Cavarzeran F, et al. Screening for diabetic retinopathy: 1 and 3 nonmydriatic 45-degree digital fundus photographs vs 7 standard early treatment diabetic retinopathy study fields. Am J Ophthalmol. 2009;148:111–8. doi: 10.1016/j.ajo.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Higgs ER, Harney BA, Kelleher A, Reckless JP. Detection of diabetic retinopathy in the community using a non-mydriatic camera. Diabet Med. 1991;8:551–5. doi: 10.1111/j.1464-5491.1991.tb01650.x. [DOI] [PubMed] [Google Scholar]
- 16.Younis N, Broadbent DM, Vora JP, Harding SP Liverpool Diabetic Eye Study. Incidence of sight-threatening retinopathy in patients with type 2 diabetes in the Liverpool Diabetic Eye Study: A cohort study. Lancet. 2003;361:195–200. doi: 10.1016/s0140-6736(03)12267-2. [DOI] [PubMed] [Google Scholar]
- 17.British Diabetic Association. London, England: British Diabetic Association; 1997. Retinal Photographic Screening for Diabetic Eye Disease. A British Diabetic Association Report. [Google Scholar]
- 18.Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375:408–18. doi: 10.1016/S0140-6736(09)60937-5. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran A, Snehalatha C, Shetty AS, Nanditha A. Trends in prevalence of diabetes in Asian countries. World J Diabetes. 2012;3:110–7. doi: 10.4239/wjd.v3.i6.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan R, Kohner EM, Aldington SJ, Nijhar I, Mohan V, Mather HM. Evaluation of a non-mydriatic camera in Indian and European diabetic patients. Br J Ophthalmol. 1988;72:841–5. doi: 10.1136/bjo.72.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baeza M, Orozco-Beltrán D, Gil-Guillen VF, Pedrera V, Ribera MC, Pertusa S, et al. Screening for sight threatening diabetic retinopathy using non-mydriatic retinal camera in a primary care setting: To dilate or not to dilate? Int J Clin Pract. 2009;63:433–8. doi: 10.1111/j.1742-1241.2008.01921.x. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed J, Ward TP, Bursell SE, Aiello LM, Cavallerano JD, Vigersky RA. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205–9. doi: 10.2337/dc06-0295. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Bastida J, Cabrera-Lopez F, Serrano-Aguilar P. Sensitivity and specificity of digital retinal imaging for screening diabetic retinopathy. Diabet Med. 2007;24:403–7. doi: 10.1111/j.1464-5491.2007.02074.x. [DOI] [PubMed] [Google Scholar]
- 24.Mizrachi Y, Knyazer B, Guigui S, Rosen S, Lifshitz T, Belfair N, et al. Evaluation of diabetic retinopathy screening using a non-mydriatic retinal digital camera in primary care settings in south Israel. Int Ophthalmol. 2014;34:831–7. doi: 10.1007/s10792-013-9887-3. [DOI] [PubMed] [Google Scholar]
- 25.Klein R, Klein BE, Neider MW, Hubbard LD, Meuer SM, Brothers RJ. Diabetic retinopathy as detected using ophthalmoscopy, a nonmydriatic camera and a standard fundus camera. Ophthalmology. 1985;92:485–91. doi: 10.1016/s0161-6420(85)34003-4. [DOI] [PubMed] [Google Scholar]
- 26.Taylor R, Lovelock L, Tunbridge WM, Alberti KG, Brackenridge RG, Stephenson P, et al. Comparison of non-mydriatic retinal photography with ophthalmoscopy in 2159 patients: Mobile retinal camera study. BMJ. 1990;301:1243–7. doi: 10.1136/bmj.301.6763.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paton RC. Non-mydriatic Polaroid photography in screening for diabetic retinopathy. Br Med J (Clin Res Ed) 1988;296:1399. doi: 10.1136/bmj.296.6633.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murgatroyd H, Ellingford A, Cox A, Binnie M, Ellis JD, MacEwen CJ, et al. Effect of mydriasis and different field strategies on digital image screening of diabetic eye disease. Br J Ophthalmol. 2004;88:920–4. doi: 10.1136/bjo.2003.026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scanlon PH, Malhotra R, Greenwood RH, Aldington SJ, Foy C, Flatman M, et al. Comparison of two reference standards in validating two field mydriatic digital photography as a method of screening for diabetic retinopathy. Br J Ophthalmol. 2003;87:1258–63. doi: 10.1136/bjo.87.10.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leese GP, Morris AD, Swaminathan K, Petrie JR, Sinharay R, Ellingford A, et al. Implementation of national diabetes retinal screening programme is associated with a lower proportion of patients referred to ophthalmology. Diabet Med. 2005;22:1112–5. doi: 10.1111/j.1464-5491.2005.01603.x. [DOI] [PubMed] [Google Scholar]
- 31.Bergamin O, Schoetzau A, Sugimoto K, Zulauf M. The influence of iris color on the pupillary light reflex. Graefes Arch Clin Exp Ophthalmol. 1998;236:567–70. doi: 10.1007/s004170050122. [DOI] [PubMed] [Google Scholar]
- 32.Choremis J, Chow DR. Use of telemedicine in screening for diabetic retinopathy. Can J Ophthalmol. 2003;38:575–9. doi: 10.1016/s0008-4182(03)80111-4. [DOI] [PubMed] [Google Scholar]
- 33.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: Epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–40. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 34.Joshi SR, Das AK, Vijay VJ, Mohan V. Challenges in diabetes care in India: Sheer numbers, lack of awareness and inadequate control. J Assoc Physicians India. 2008;56:443–50. [PubMed] [Google Scholar]
- 35.Li Z, Wu C, Olayiwola JN, Hilaire DS, Huang JJ. Telemedicine-based digital retinal imaging vs standard ophthalmologic evaluation for the assessment of diabetic retinopathy. Conn Med. 2012;76:85–90. [PubMed] [Google Scholar]
- 36.Mansberger SL, Gleitsmann K, Gardiner S, Sheppler C, Demirel S, Wooten K, et al. Comparing the effectiveness of telemedicine and traditional surveillance in providing diabetic retinopathy screening examinations: A randomized controlled trial. Telemed J E Health. 2013;19:942–8. doi: 10.1089/tmj.2012.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin DY, Blumenkranz MS, Brothers RJ, Grosvenor DM. The sensitivity and specificity of single-field nonmydriatic monochromatic digital fundus photography with remote image interpretation for diabetic retinopathy screening: A comparison with ophthalmoscopy and standardized mydriatic color photography. Am J Ophthalmol. 2002;134:204–13. doi: 10.1016/s0002-9394(02)01522-2. [DOI] [PubMed] [Google Scholar]
- 38.Harding SP, Broadbent DM, Neoh C, White MC, Vora J. Sensitivity and specificity of photography and direct ophthalmoscopy in screening for sight threatening eye disease: The liverpool diabetic eye study. BMJ. 1995;311:1131–5. doi: 10.1136/bmj.311.7013.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]


