Abstract
The choice of small immediate rewards as opposed to larger delayed rewards, or delay discounting, is an important dimension of impulsive decision making. The inability to delay gratification is related to obesity, as well as other maladaptive behaviors such as substance abuse, problem drinking, smoking, pathological gambling, and risky HIV behaviors. One way to reduce delay discounting (DD) may be to use prospective imagery in the form of episodic future thinking (EFT) during inter-temporal decision making. We have recently shown that EFT reduces DD and ad libitum energy intake in obese individuals. However, no studies have examined whether the magnitude of the EFT effect differs between lean and overweight/obese individuals. We conducted a within-subject design experiment to compare the efficacy of EFT versus a control task in reducing DD between lean (N = 24) and overweight/obese (N = 24) women. Participants attended two sessions in which they engaged in either EFT or control episodic thinking during a DD task. We also examined whether individual differences such as trait time perspective, behavioral inhibition or behavioral activation moderated the EFT effect on DD. Results showed EFT reduced DD similarly for lean and overweight/obese individuals. The EFT effect was moderated by behavioral activation. This suggests EFT is just as effective in reducing impulsive decision making in obese individuals as it is in lean individuals and may be useful in reducing other impulsive obesity related behaviors.
Keywords: reducing impulsivity, obesity, episodic future thinking
Obesity is due to energy intake in excess of energy expenditure (Hill, Melanson, & Wyatt, 2000), and is associated with chronic conditions such as cardiovascular disease, stroke and type-2 diabetes (Björntorp, 1990, 2009). Despite these well-publicized adverse outcomes (Winter & Wuppermann, 2013), many individuals often choose to overindulge in high energy-dense and unhealthy foods that result in a positive energy balance. One reason for the excess intake may be a preference for the immediate gratification of high energy-dense foods, as opposed to not consuming these foods to be healthy later. This discounting of larger future rewards in favor of smaller immediate rewards is known as delay discounting (DD) and it is greater as the temporal distance between the immediate and delayed rewards increases (Bickel & Marsch, 2001). Higher DD rates have been associated with substance abuse, problem drinking, smoking, pathological gambling, and risky HIV behaviors (Bickel & Marsch, 2001; Bickel, Odum, & Madden, 1999; Kollins, 2003; Madden, Francisco, Brewer, & Stein, 2011; Vuchinich & Simpson, 1998).
The inability to delay gratification or greater DD is also cross-sectionally and prospectively related to obesity (Davis, Patte, Curtis, & Reid, 2010; Francis & Susman, 2009; Weller, Cook, Avsar, & Cox, 2008). Higher DD predicts greater consumption of high energy-dense ready-to-eat and away-from-home foods in obese women (Appelhans et al., 2012). DD also interacts with food reinforcement to predict energy intake such that individuals with a high motivation to eat and high levels of DD consume more calories in ad libitum eating sessions (Appelhans et al., 2011; Rollins, Dearing, & Epstein, 2010). Furthermore, the ability to delay gratification predicts success in weight loss treatment which suggests that modifying DD may improve behaviors related to weight loss (Best et al., 2012).
Individuals making impulsive decisions typically focus on the present and de-value future rewards signifying a shortened time perspective during inter-temporal decision making (Petry, Bickel, & Arnett, 1998). One approach to reducing the bias towards immediate gratification or DD is to vividly imagine the future during decision making using episodic future thinking (Benoit, Gilbert, & Burgess, 2011; Peters & Büchel, 2010). Episodic future thinking (EFT) is a type of prospective imagery that involves self-projection to pre-experience future events (Atance & O'Neill, 2001). EFT engages the episodic memory network; the memory system for autobiographical details, in mentally simulating the future (Atance & O'Neill, 2001; Schacter, Addis, & Buckner, 2007). It has been suggested that episodic simulation of the future steers decision-making towards long term benefits (Boyer, 2008) and EFT has been shown to reduce DD (Peters & Büchel, 2010).
We have shown that EFT reduces both DD (ES$10=1.44, ES$100=1.51, p=0.017) and ad libitum energy intake (ES =1.09, p=.011) in obese individuals in a tempting food situation (Daniel, Stanton, & Epstein, in press). However, research suggests that obese individuals often show suboptimal functioning in certain brain regions that could lead to a blunted EFT effect in obese individuals (Volkow et al., 2008). Higher BMI is associated with decreased regional cerebral blood flow in the dorsolateral prefrontal cortex, anterior prefrontal cortex, orbitofrontal area and the anterior cingulate cortex (Willeumier, Taylor, & Amen, 2011). Deficits in these regions may limit EFT’s effectiveness as the dorsolateral prefrontal cortex and anterior cingulate cortex have been implicated in the EFT effect (Peters & Büchel, 2010).
Furthermore, the EFT effect is associated with increased neural interaction between the anterior cingulate cortex and the hippocampus (Peters & Büchel, 2010). Animal studies show that high-fat diets similar to those associated with obesity in humans, creates deficits in the prefrontal cortex and the ventral and dorsal hippocampus in rats (Kanoski, Meisel, Mullins, & Davidson, 2007). Research in humans indicates that some hormonal deficits associated with decreased hippocampal volume in pre-diabetics and type 2 diabetics (Bruehl, Wolf, & Convit, 2009) may be mediated by higher BMI (Ursache, Wedin, Tirsi, & Convit, 2012). In addition, elderly obese individuals show atrophy in the frontal lobes, anterior cingulate gyrus and hippocampus compared to elders with a normal BMI (Raji et al., 2010). This converging evidence suggests that the neural deficits related to obesity may lead to a reduced EFT effect in obese compared to lean individuals. Thus, the main aim of the present study was to compare the effect of EFT versus a control imagery task on DD in non-overweight and overweight/obese women. We used a within subject design as opposed to the between design used in our previous study (Daniel et al., in press). We examined differences in the magnitude of change in DD due to EFT between lean and obese individuals.
There are individual differences in the magnitude of change in DD as a function of EFT. Identification of traits that moderate the effect of DD may provide insight into who may benefit the most from EFT. As EFT targets a temporal bias toward immediate rewards, trait level temporal orientation (Henson, Carey, Carey, & Maisto, 2006; Zimbardo & Boyd, 1999) may be related to responsiveness to EFT. Behavioral inhibition, the sensitivity to punishment and novelty; and behavioral activation, the sensitivity to reward and motivation for goal-directed activity (Carver & White, 1994), have been associated with delay of gratification (Corvi, Juergensen, Weaver, & Demaree, 2012; Davis et al., 2007) and overeating (Davis et al., 2007). Sensitivity to reward and individual’s motivation for goal directed activity may influence responsiveness to EFT.
Methods
Participants
Lean (Body Mass Index (BMI = kg/m2) <25, N = 24) and overweight/obese (BMI≥ 25, N = 24) women between the ages of 18 and 40 were recruited for the study. Thirty-three percent of the participants were minority, 44% were high school or vocational graduates, with 56% of participants with at least some college. Women were recruited through a laboratory database, flyers posted around the University at Buffalo campus, community settings and web-based advertisements. Participants were screened by telephone to guarantee they met inclusion criteria; no current depression or psychopathology and no previous experience with study procedures or measures. This study was approved by the Social and Behavioral Sciences IRB at the University at Buffalo.
Experimental Design and Procedures
Participants attended two 90 minute sessions, 3 to 7 days apart, in which they engaged in EFT or control episodic thinking (CET) during a DD task, with the order of the conditions counterbalanced. Prior to attending the session, subjects completed demographic forms, the Zimbardo Time Perspective Scale (Zimbardo & Boyd, 1999) and the Behavioral Activation and Behavioral Inhibition Scales (Carver & White, 1994) online. At the end of the second session, participants had their height and weight measured, after which they were debriefed and mailed a $30 compensation check.
Episodic Future Thinking Task
The EFT task was a modification of a personal future thinking task used to assess the anticipation of future experiences in depressed individuals (MacLeod, Pankhania, Lee, & Mitchell, 1997; MacLeod & Salaminiou, 2001). The EFT task required participants to generate positive future events that they were looking forward to for 7 time periods corresponding to the time periods in the DD task (1 day, 2 days, 1 week, 2 weeks, 1 month, 6 months, and 2 years). Participants rated the valence, salience and arousal of each event on scales of 1 to 6 (1 for very low and 6 for very high), which requires thinking about autobiographical details of the events. Participants also rated the frequency and vividness of the episodic associations evoked by each event on a scale of 1 to 6 (1 for not at all to 6 for very high) (Peters & Büchel, 2010). Since individuals pre-experience positive future events more vividly (D’Argembeau & Van der Linden, 2004), and vividness of episodic imagery predicts the amount of reduction in DD caused by EFT (Peters & Büchel, 2010), an imagery score was calculated for each event by averaging the ratings of frequency and vividness of the episodic association, and only events with the highest imagery scores were used during the DD task.
Control Episodic Thinking (CET) Task
The CET task was similar to the EFT task except that instead of generating future events, participants read the first two chapters of Pinocchio (Collodi, 1995) which was divided into seven pages. This story was selected because it contained vivid imagery. After reading the story, participants listed as many events they remembered and enjoyed from each of the seven pages. Participants also rated the valence, salience and arousal of each story event as in the EFT task, and ratings of the frequency and vividness of episodic associations evoked by each story event were used to calculate an imagery score. Only story events with the highest imagery scores were used during the DD task. In this way, participants were engaged in imagery of a recently experienced event, rather than pre-experiencing a future event.
Delay Discounting Task
Discounting of hypothetical monetary rewards ($10 and $100) were assessed at increasing time delays (Rollins et al., 2010). Two stacks of index cards were placed on a table in front of participants, with the left stack of cards displaying a monetary amount that was available immediately (e.g., “Would you like $100 now”) and the right stack of cards showing a monetary amount available at a later time (e.g., “ Would you like $100 in 1 day”). The value of the money available at a delayed time remained constant while the money available immediately decreased with each trial going for example from $100 to $99, $97.5, $95, $92.5, $90.0, $85, $80, $75, $70, $65, $60, $55, $50, $45, $40, $35, $30, $25, $20, $15, $10, $7.5, $5, $2.5 and $1.00. Participants were asked to choose the monetary amount they would prefer at each trial. The immediate rewards cards were titrated in ascending then descending order for half of the participants, and vice versa for the other participants. Participants were asked to make choices regarding delayed rewards of both $10 and $100 at the following time delays: 1 day, 2 days, 1 week, 2 weeks, 1 month, 6 months, and 2 years. The discounting data were reduced by calculating the indifference point, or the average of the last immediate amount chosen during the ascending titration and the .rst immediate amount chosen during the descending titration (Dixon, Marley, & Jacobs, 2003). Indifference points signify the subjective value of a reward when it is delayed by a specific time period. The indifference points were used to compute area under the indifference curve (AUC) values, with AUC ranging from 0 (steepest discounting) to 1 (no discounting) (Myerson, Green, & Warusawitharana, 2001).
Episodic or Control Thinking during the Delay Discounting (DD) Task
Participants were reminded of the future events that corresponded to the time period they were making decisions about prior to each DD trial. They were instructed to think about their future events that corresponded to the delayed time period during the DD task (Peters & Büchel, 2010). For example, when making a choice between $95 now and $100 in 6 months, participants were asked to think about their most vivid event occurring in 6 months (e.g. a birthday party) as they were making the decision. Similarly, in CET condition, participants were instructed to think about story events with the highest imagery during each DD trial. They were also reminded which specific story events to keep in mind prior to each DD trial. As a manipulation check, participants were asked to rate the frequency and vividness of their episodic thoughts during the DD task using a Likert scale anchored by 1= not at all frequent/vivid to 6 = very frequent/vivid.
Measures
Weight and Height
Weight was measured using a digital scale (TANITA Corporation of America Inc., Arlington Heights, IL), and height was measured using a digital stadiometer (Measurement Concept and Quick Medical, North Bend, WA). Height and weight assessments were used to compute BMI (kg/m2). Overweight/obese women had BMI ≥ 25 and ≤ 45.
Demographics
Race/ethnicity, income and educational level were obtained using a standardized questionnaire adapted from the MacArthur’s network for studies on socio-economic status and health (Adler, Epel, Castellazzo, & Ickovics, 2000).
Time perspective
Participants completed the Zimbardo Time Perspective Inventory that assesses four different types of time perspectives; past-negative, past-positive, present-fatalistic, present-hedonistic and future time perspectives (Epel, Bandura, & Zimbardo, 1999; Zimbardo & Boyd, 1999). The ZTPI has been shown to have test-retest reliability ranging between .70 to .80 and significant convergent and discriminant validity (Epel et al., 1999; Zimbardo & Boyd, 1999). Time perspective is associated with risky behaviors and health behaviors (Henson et al., 2006; Keough, Zimbardo, & Boyd, 1999).
The Behavioral Inhibition and Behavioral Approach Scale (BIS/BAS)
The balance of behavioral approach and behavioral avoidance or inhibition system was assessed using the BIS/BAS scales (Carver & White, 1994). This instrument assesses individual differences in behavioral inhibition and in three dimensions of behavioral activation; drive, fun seeking and reward responsiveness. The BIS/BAS has test–retest reliability ranging between .59 to .69 and significant convergent and divergent validity (Carver & White, 1994). Six participants were excluded from analyses using the BIS/BAS scales since they refused to answer some questionnaire items on these subscales.
Analytical Plan
Differences in AUC values between EFT and CET conditions were compared using mixed analysis of covariance (ANCOVA) with weight status (lean vs. overweight/obese) as the between subject variable and condition (EFT vs. CET) and reward amount ($10, $100) as the within subject variables, controlling for differences in vividness of EFT or CET imagery. ZTPI and BIS/BAS scores were included as covariates in the ANCOVA to assess whether they moderated the effect of EFT on DD. Effect sizes (ES) were calculated using Cohen’s d statistic for within subject analyses. Data analyses were completed using SYSTAT version 11 (Systat Software, 2004).
Results
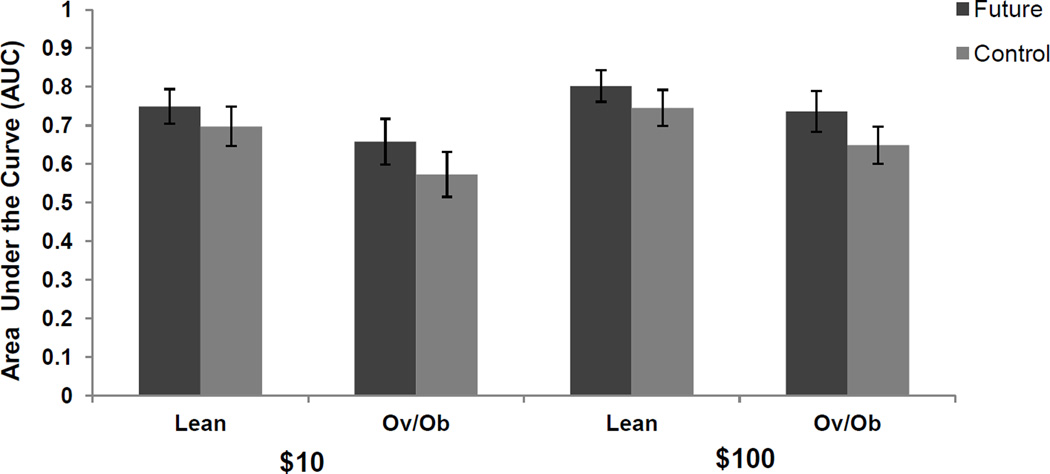
Participants’ rated frequency and vividness of EFT imagery (4.45 ± 0.75) higher than CET imagery (3.95 ± 0.81), (F (1, 46) = 18.36, p < 0.001, ES = 0.61) during the DD task and this difference in imagery was entered as a covariate in future analysis. ANCOVA showed a main effect of condition (F (1, 46) = 8.96, p=0 .004, ES$10=.32, ES$100=.50), controlling for differences in imagery during the DD task. There were higher AUC values in the EFT condition compared to the CET condition for both $10 (0.70 ± 0.26 vs. 0.64 ± 0.27) and $100 (0.77 ± 0.23 vs. 0.70 ± 0.24) rewards (Figure 1). There were no differences between the EFT effect in lean and obese individuals (F (1, 46) =2.18, p = 0.16). For lean participants, the average change in AUC values due to EFT was 0.05 ± 0.15 for $10 compared to 0.08 ± 0.23 in overweight/obese participants. Similarly, the average change in AUC values due to EFT for $100 was 0.06 ± 0.13 in lean participants while it was 0.09 ± 0.15 in overweight/obese participants. The EFT effect size in the lean participants was ES$10=.36, ES$100=.45 while the EFT effect size for obese participants was ES$10=.37, ES$100=.59. We also found that participants discounted smaller rewards ($10) more than they do larger ($100) rewards, F (1, 46) = 13.67, p=0.001 ES = 0.48 (Frederick, Loewenstein, & O'donoghue, 2002).
Figure 1.
Area under the curve (mean ± SEM) of delayed rewards for lean and overweight/obese (OvOb) participants choosing immediate or delayed $10 or $100 rewards.
Though other studies have demonstrated significant difference in impulsivity between lean and obese women (Weller et al., 2008), we did not observe a significant main effect of group (lean vs. overweight/obese) on AUC (F (1, 45) = 2.09, p=.16). As expected the mean AUC values were lower for the overweight/obese women compared to the lean women in the future condition $10future (0.66 ± 0.29 vs. 0.75 ± 0.22), $100future (0.74 ± 0.26 vs.0.80 ± 0.20) as well as the control condition $10control (0.57 ± 0.29 vs. 0.70 ± 0.25), $100control (0.65 ± 0.23 vs. 0.75 ± 0.23).
A one-way ANOVA showed there were no differences in time perspective, behavioral inhibition and behavioral activation between lean and obese participants. The moderation analysis showed that scores on the drive subscale of the BAS scale moderated the EFT effect on DD (F (1, 38) = 6.88, p = 0.012).
Discussion
This experiment replicated the effect of EFT reducing DD (Daniel et al., in press) using a within-subject design. Despite the suggested neurophysiological differences between lean and obese individuals that relate to the EFT mechanism, we found no differences in the effectiveness of EFT between lean and obese individuals. Given that EFT works with overweight/obese women as well as normal weight women, and DD is associated with higher energy intake, obesity and less success in obesity treatment (Appelhans et al., 2012; Appelhans et al., 2011; Best et al., 2012; Francis & Susman, 2009; Weller et al., 2008), including EFT as a component of obesity treatment may reduce impulsive decision making associated with obesity and weight gain.
It may not be surprising that EFT may work better for larger than smaller magnitude future rewards, as individuals discount the future less as the reward magnitude increases (Kirby & Maraković, 1996). However, as the rate of discounting decreases across participants at higher reward magnitudes, there might be less variability in DD, which would make it more difficult to detect the EFT effect at very large delayed reward magnitudes. This suggests there may be a “sweet spot” in terms of the reward magnitude that is most influenced by EFT. To the extent that there are differences in delay discounting between lean and obese women, this could account for the trend of greater effects of EFT in the obese. As we showed in the current study, the effect size for $10 was about equal for lean and overweight/obese women (0.35 vs. 0.37), but the differences in the effects size at $100 were more discrepant (0.45 vs. 0.59).
Though other studies have demonstrated significant difference in impulsivity between lean and obese women (Davis et al., 2010; Weller et al., 2008), we did not observe a significant main effect of group (lean vs. overweight/obese) on AUC. However, as expected the mean AUC values were lower for the overweight/obese women compared to the lean women in both the future and the control conditions. While we were initially surprised to not find this effect, it may be due to the fact that the delay discounting task was modified so that people engaged in either EFT or CET while they were engaged in the task. Thus, we do not have a measure of delay discounting that is equivalent to the measure used in the two studies that have shown this effect (Davis et al., 2010; Weller et al., 2008). We measured delay discounting in the traditional manner in a sample of 199 women of varying BMI levels in a recently completed study, and showed a very significant relationship between BMI and delay discounting, so the differences in the task is the most likely explanation for the failure to find significant between group differences.
This study compared the impact of EFT on discounting of money not food and delaying gratification for food may be more difficult for obese individuals. The rate of discounting differs by reinforcer type with steeper discounting of consumable rewards e.g. drug of abuse, cigarette, alcohol and food than non-consumable rewards (Bickel et al., 1999; Odum & Rainaud, 2003; Petry, 2001). However, research suggests that individuals discount primary rewards like food in a similar pattern to monetary rewards. For example, a study by McClure and colleagues observed that thirsty individuals prefer smaller but more immediate amounts of fluids (water and juice) than larger amounts of fluid delayed by 5 minutes (McClure, Ericson, Laibson, Loewenstein, & Cohen, 2007). Thus, we expect that even with primary rewards like food, EFT would increase the ability to delay gratification. This is supported by the results of our previous study, in which we demonstrated that EFT significantly reduced energy intake of high energy dense foods in overweight/obese women during a tempting laboratory situation (Daniel et al., in press). Since EFT impacted both DD of money and food intake, we assume the discounting of monetary rewards reflects the ability to delay gratification in general. Therefore, the results would likely be similar if the reinforcer in the delay discounting task was a primary reward like food.
We found that the EFT effect was moderated by scores on the drive subscale of the BIS/BAS such that individuals who were more responsive to the EFT effect also had lower scores on the drive subscale of the BIS/BAS. The drive subscale reflects persistent pursuit of desired goals (Carver & White, 1994) which suggests that for these individuals EFT may be cuing a temporal re-orientation that improves inter-temporal decision making in favor of longer term goals. Goal directed behavior is coordinated by the prefrontal cortex and EFT increases activation in this region during decision making (Peters & Büchel, 2010). Thus the greater EFT responsiveness may reflects greater prefrontal cortex activation in a sub-population that may typically show suboptimal activation during decision making. Indeed research shows that higher behavioral activation scores are associated with greater left prefrontal activation implying that less activation is linked to lower behavioral activation (Coan & Allen, 2003; Sutton & Davidson, 1997).
Activation in the left PFC has also been suggested to mediate individual differences in delay discounting (Shamosh et al., 2008). The prefrontal cortex may use information conveyed by the episodic memory network or hippocampus to better represent the undiscounted value of future rewards (Benoit et al., 2011). Discounting the future may also occur as a function of a cognitive search that determines lower subjective values for delayed outcomes that are difficult to access (Kurth-Nelson, Bickel, & Redish, 2012). According to this theory, episodically imagined future rewards are easier to access after pre-experiencing of future situations, resulting in less discounting of the future. Thus, if obese individuals engage in maladaptive health behaviors due to a preference for immediate gratification of unhealthy foods or sedentary activities, EFT during health decision making may enhance their ability to delay gratification and show restraint for future health gains.
Most previous studies on the EFT effect on DD compared EFT to either non-episodic thinking or to no thinking at all (Benoit et al., 2011; Peters & Büchel, 2010). This study compared EFT to episodic imagery to control for the autobiographical details of events during EFT thinking. However, EFT was associated with more vivid images than the control task during DD, suggesting that episodic imagery may require greater personal salience to be equal in vividness to EFT. If individuals typically use past experiences to construct their imagination of future event (Addis, Wong, & Schacter, 2007; Schacter et al., 2007; Szpunar & McDermott, 2008), then the lack of personal experience with the story events used in the control task may have made imagery more difficult for participants. In ongoing research from our laboratory, we have used episodic memory for recent events as the control, and found significant differences in DD between EFT and current episodic thinking groups, matched for vividness ratings.
Translation of basic science research into behavioral interventions for obesity requires developing technologies that impact behavioral processes such as DD to influence eating behavior. This study and our previous EFT study (Daniel et al., in press) are the first steps in developing a behavioral technology to reduce DD to influence obesogenic behaviors. Reliably reducing discounting of the future may play an important complementary role to other effective components of behavioral interventions for the next generation of evidence based obesity treatments. This study also contributes to the growing evidence that our adaptive ability to engage in prospective thinking can help us combat our often maladaptive tendency to discount larger future rewards for smaller immediate rewards.
Table 1.
Descriptive Data of Individual Differences Variables
| Mean (SD) | ||
|---|---|---|
| Lean | Overweight/Obese | |
| Age | 23.59 (3.50) | 26.22 (5.86) |
| BMI | 22.31 (1.79) | 31.33 (4.67) |
| Zimbardo Time Perspective (ZTPI) | ||
| Past Negative | 3.10 (0.76) | 3.15 (0.59) |
| Present Hedonistic | 3.36 (0.42) | 3.33 (0.52) |
| Future | 3.70 (0.43) | 3.62 (0.47) |
| Past Positive | 3.69 (0.45) | 3.80 (0.64) |
| Present Fatalistic | 2.63 (0.59) | 2.64 (0.57) |
| Behavioral Inhibition and Behavioral Activation Scale (BIS/BAS) | ||
| BIS | 12.54 (3.54) | 14.21 (4.84) |
| BAS Drive | 8.71 (1.92) | 9.13 (2.40) |
| BAS Fun Seeking | 8.29 (2.07) | 8.74 (2.70) |
| BAS Reward Responsiveness | 7.04 (1.90) | 7.08 (1.44) |
| N(%) | ||
| Race | ||
| Caucasian | 14 (29.17) | 18 (37.50) |
| Non-Caucasian | 10 (20.83) | 6 (12.50) |
| Education Completed | ||
| High School/Vocational | 11 (22.92) | 10 (20.83) |
| College or graduate | 13 (27.08) | 14 (29.17) |
Highlights.
Episodic future thinking (EFT) during decision making reduces delay discounting (DD).
We conducted an experiment to compare the efficacy of EFT between lean and overweight/obese women.
We examined time perspective, behavioral inhibition or behavioral activation as moderators.
Result showed EFT reduced DD similarly for lean and overweight/obese individuals.
The EFT effect was moderated by behavioral activation.
Acknowledgements
We appreciate Shirin Aghazadeh, Travis Stewart and David Lowry for assisting with data entry, and Katelyn Carr and Henry Lin for feedback on study design. This research was funded in part by Grant 1U01 DK088380 from the National Institute of Diabetes and Digestive and Kidney Diseases to Dr. Epstein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychology. 2000;19(6):586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, Waring ME, Schneider KL, Pagoto SL, DeBiasse MA, Whited MC, Lynch EB. Delay discounting and intake of ready-to-eat and away-from-home foods in overweight and obese women. Appetite. 2012;59:576–584. doi: 10.1016/j.appet.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R. Inhibiting food reward: delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity. 2011;19(11):2175–2182. doi: 10.1038/oby.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atance CM, O'Neill DK. Episodic future thinking. Trends in Cognitive Sciences. 2001;5(12):533–539. doi: 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. The Journal of Neuroscience. 2011;31(18):6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Theim KR, Gredysa DM, Stein RI, Welch RR, Saelens BE, . . Wilfley DE. Behavioral economic predictors of overweight children's weight loss. Journal of Consulting and Clinical Psychology. 2012;80:1086–1096. doi: 10.1037/a0029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction. 2001;96(1):73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146(4):447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Björntorp P. "Portal" adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis, Thrombosis, and Vascular Biology. 1990;10(4):493–496. [PubMed] [Google Scholar]
- Björntorp P. Obesity and adipose tissue distribution as risk factors for the development of disease. Transfusion Medicine and Hemotherapy. 2009;17(1):24–27. doi: 10.1159/000222436. [DOI] [PubMed] [Google Scholar]
- Boyer P. Evolutionary economics of mental time travel? Trends in Cognitive Sciences. 2008;12(6):219–224. doi: 10.1016/j.tics.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Bruehl H, Wolf OT, Convit A. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology. 2009;34(6):815–821. doi: 10.1016/j.psyneuen.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–319. [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40(1):106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Collodi C. Pinocchio. Hertforshire, Great Britain: Wordsworth Editions Limited; 1995. [Google Scholar]
- Corvi AP, Juergensen J, Weaver JS, Demaree HA. Subjective time perception and behavioral activation system strength predict delay of gratification ability. Motivation and Emotion. 2012;36(4):483–490. [Google Scholar]
- D’Argembeau A, Van der Linden M. Phenomenal characteristics associated with projecting oneself back into the past and forward into the future: Influence of valence and temporal distance. Consciousness and Cognition. 2004;13(4):844–858. doi: 10.1016/j.concog.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Daniel TO, Stanton CM, Epstein LH. The future is now: Reducing impulsivity and energy intake using episodic future thinking. Psychological Science. doi: 10.1177/0956797613488780. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Patte K, Curtis C, Reid C. Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite. 2010;54(1):208–213. doi: 10.1016/j.appet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C. From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite. 2007;48(1):12–19. doi: 10.1016/j.appet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Dixon MR, Marley J, Jacobs EA. Delay discounting by pathological gamblers. Journal of Applied Behavior Analysis. 2003;36(4):449–458. doi: 10.1901/jaba.2003.36-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Bandura A, Zimbardo PG. Escaping Homelessness: The Influences of Self-Efficacy and Time Perspective on Coping With Homelessness. Journal of Applied Social Psychology. 1999;29(3):575–596. [Google Scholar]
- Francis LA, Susman EJ. Self-regulation and rapid weight gain in children from age 3 to 12 years. Archives of Pediatrics & Adolescent Medicine. 2009;163(4):297–302. doi: 10.1001/archpediatrics.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick S, Loewenstein G, O'donoghue T. Time discounting and time preference: A critical review. Journal of Economic Literature. 2002;40(2):351–401. [Google Scholar]
- Henson JM, Carey MP, Carey KB, Maisto SA. Associations among health behaviors and time perspective in young adults: Model testing with boot-strapping replication. Journal of Behavioral Medicine. 2006;29(2):127–137. doi: 10.1007/s10865-005-9027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO, Melanson EL, Wyatt HT. Dietary fat intake and regulation of energy balance: implications for obesity. The Journal of Nutrition. 2000;130(2):284S–288S. [PubMed] [Google Scholar]
- Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behavioural Brain Research. 2007;182(1):57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keough KA, Zimbardo PG, Boyd JN. Who's smoking, drinking, and using drugs? Time perspective as a predictor of substance use. Basic and Applied Social Psychology. 1999;21(2):149–164. [Google Scholar]
- Kirby KN, Maraković NN. Delay-discounting pr obabilistic rewards: Rates decrease as amounts increase. Psychonomic Bulletin & Review. 1996;3(1):100–104. doi: 10.3758/BF03210748. [DOI] [PubMed] [Google Scholar]
- Kollins SH. Delay discounting is associated with substance use in college students. Addictive Behaviors. 2003;28(6):1167–1173. doi: 10.1016/s0306-4603(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Kurth-Nelson Z, Bickel W, Redish AD. A theoretical account of cognitive effects in delay discounting. European Journal of Neuroscience. 2012;35(7):1052–1064. doi: 10.1111/j.1460-9568.2012.08058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod AK, Pankhania B, Lee M, Mitchell D. BRIEF COMMUNICATION Parasuicide, depression and the anticipation of positive and negative future experiences. Psychological Medicine. 1997;27(04):973–977. doi: 10.1017/s003329179600459x. [DOI] [PubMed] [Google Scholar]
- MacLeod AK, Salaminiou E. Reduced positive future-thinking in depression: Cognitive and affective factors. Cognition & Emotion. 2001;15(1):99–107. [Google Scholar]
- Madden GJ, Francisco MT, Brewer AT, Stein JS. Delay discounting and gambling. Behavioural Processes. 2011;87(1):43–49. doi: 10.1016/j.beproc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. The Journal of Neuroscience. 2007;27(21):5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76(2):235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum, Rainaud Discounting of delayed hypothetical money, alcohol, and food. Behavioural Processes. 2003;64(3):305–313. doi: 10.1016/s0376-6357(03)00145-1. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66(1):138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154(3):243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93(5):729–738. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, . . Thompson PM. Brain structure and obesity. Human Brain Mapping. 2010;31(3):353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BY, Dearing KK, Epstein LH. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite. 2010;55(3):420–425. doi: 10.1016/j.appet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nature Reviews Neuroscience. 2007;8(9):657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR, Conway AR, . . Gray JR. Individual differences in delay discounting relation to intelligence, working memory, and anterior prefrontal cortex. Psychological Science. 2008;19(9):904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8(3):204–210. [Google Scholar]
- Szpunar KK, McDermott KB. Episodic future thought and its relation to remembering: Evidence from ratings of subjective experience. Consciousness and Cognition. 2008;17(1):330–334. doi: 10.1016/j.concog.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Ursache A, Wedin W, Tirsi A, Convit A. Preliminary evidence for obesity and elevations in fasting insulin mediating associations between cortisol awakening response and hippocampal volumes and frontal atrophy. Psychoneuroendocrinology. 2012;37(8):1270–1276. doi: 10.1016/j.psyneuen.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, Ma Y. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity. 2008;17(1):60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and Clinical Psychopharmacology. 1998;6(3):292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Weller RE, Cook EW, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51(3):563–569. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Willeumier KC, Taylor DV, Amen DG. Elevated BMI is associated with decreased blood flow in the prefrontal cortex using SPECT imaging in healthy adults. Obesity. 2011;19(5):1095–1097. doi: 10.1038/oby.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Wuppermann A. Do They Know What is at Risk? Health Risk Perception Among The Obese. Health Economics. 2013 doi: 10.1002/hec.2933. [DOI] [PubMed] [Google Scholar]
- Zimbardo PG, Boyd JN. Putting time in perspective: A valid, reliable individual-differences metric. Journal of Personality and Social Psychology. 1999;77(6):1271–1288. [Google Scholar]