Abstract
Background
The most effective treatment for posttraumatic stress disorder (PTSD) is exposure therapy, which aims to facilitate extinction of conditioned fear. Recent evidence suggests that brain-derived neurotrophic factor (BDNF) facilitates extinction learning. This study assessed whether the Met-66 allele of BDNF, which results in lower activity-dependent secretion, predicts poor response to exposure therapy in PTSD.
Methods
Fifty-five patients with PTSD underwent an 8-week exposure-based cognitive behavior therapy program and provided mouth swabs or saliva to extract genomic DNA to determine their BDNF Val66Met genotype (30 patients with the Val/Val BDNF allele, 25 patients with the Met-66 allele). We examined whether BDNF genotype predicted reduction in PTSD severity following exposure therapy.
Results
Analyses revealed poorer response to exposure therapy in the PTSD patients with the Met-66 allele of BDNF compared with patients with the Val/Val allele. Pretreatment Clinician Administered PTSD Scale severity and BDNF Val66Met polymorphism predicted response to exposure therapy using hierarchical regression.
Conclusions
This study provides the first evidence that the BDNF Val66Met genotype predicts response to cognitive behavior therapy in PTSD and is in accord with evidence that BDNF facilitates extinction learning.
Keywords: BDNF, CBT, fear extinction, fMRI, genetics, posttraumatic stress disorder, treatment predictor
Brain-derived neurotrophic factor (BDNF) is a neurotrophin with a key role in regulating protein synthesis and synaptic plasticity in the brain (1,2). BDNF mediates long-term potentiation in the hippocampus, and is thought to act as a key biological mechanism underlying associative learning and memory consolidation (1,2). Extinction learning is a form of inhibitory associative learning, where a previously feared stimulus (conditioned stimulus) becomes associated with safety signals. Regions of the infralimbic prefrontal (IL PFC) cortex in rats, and ventromedial prefrontal cortex (vmPFC) in humans are critical for retention of extinction learning (2,3). Recall of extinction in humans activates vmPFC and hippocampus (3,4). Consolidation of extinction learning requires plasticity in the IL PFC, which depends at least partially on protein synthesis (5,6).
Recent evidence suggests that BDNF plays a key role in extinction learning (2,7). Rats that fail to learn extinction have reduced BDNF in hippocampal afferents to the IL PFC; however, augmenting BDNF in this network prevents extinction failure (2). Similarly, deletion of hippocampal-dependent BDNF impairs extinction learning in rats (8). BDNF is likely to be responsible for consolidating extinction memory in IL PFC because direct infusion of BDNF into the IL PFC significantly enhances extinction, as demonstrated by reduced conditioned freezing relative to saline-infused rats (2). This effect is apparent on the initial extinction trial, suggesting that extinction training is not an essential mediator of this effect. Indeed, subsequent infusions of BDNF into IL PFC facilitates extinction of conditioned fear responses without any extinction training (2).
A single-nucleotide polymorphism in the gene encoding human BDNF gives rise to a functional variant at codon 66 with the substitution of the amino acid valine (Val) by methionine (Met) (Val66Met:9). The Met allele (Met/Met or Val/Met genotype) has been associated with reduced hippocampal function and poorer episodic memory and results in reduced activity-dependent secretion of BDNF (9). In genetic mouse models, insertion of the Met allele has been shown to decrease the release of BDNF from hippocampal neurons (10) and has been shown to impair extinction learning in both mice and humans (7). A divergent finding was reported by Lonsdorf and colleagues (11), who found that the BDNF Met group displayed reduced extinction learning during the first block of extinction trials, relative to the Val/Val group. However, this may have been due to the poorer extinction learning revealed during fear acquisition in the BDNF Met group in this study. In addition, evidence suggests that BDNF has differential effects on neural plasticity in prelimbic and infralimbic prefrontal regions and amygdala networks during fear expression and extinction (12,13). Importantly, Soliman et al. (7) found that human Met allele carriers displayed reduced activity in vmPFC regions and greater activity in amygdala during extinction learning than those with the Val/Val genotype (7). This suggests that BDNF Met allele carriers fail to recruit inhibitory vmPFC networks involved in extinction learning that act to inhibit amygdala fear-based processing (7). Furthermore, epigenetic upregulation of the BDNF promoter within the PFC, resulting in increased levels of BDNF expression, correlates with fear extinction (14).
Posttraumatic stress disorder (PTSD) is conceptualized as failure of fear extinction learning, and patients with this disorder display reduced activity in vmPFC regions implicated in extinction (15–17). Exposure therapy for PTSD aims to facilitate extinction learning (16), and successful exposure therapy in PTSD has been associated with increased activity in vmPFC networks involved in extinction and reduced activity in amygdala response to fearful faces (17), as well as size of the rostral anterior cingulate cortex (which is implicated in extinction learning (18). The only previous study of genetic predictors of exposure therapy in patients with PTSD found that the low-expression allele of the serotonin transporter gene was associated with poor treatment response (19). Carriers of this allele have been shown to acquire conditioned startle responses more readily (20) and display decoupling of the amygdala-prefrontal network, which is implicated in extinction learning (21). This finding suggests that genetic vulnerability to enhanced conditioning or impaired extinction may influence response to exposure therapy. On the basis of the role that BDNF plays in extinction, we examined the capacity of the BDNF Val66Met polymorphism (mediating activity-dependent BDNF secretion) to predict the response to exposure therapy in PTSD. Specifically, given evidence that BDNF facilitates extinction learning, we hypothesized that the BDNF Met allele would predict poorer response to exposure therapy in PTSD.
Methods and Materials
Participants
A subset of the current sample has been reported in a previous study examining the serotonin transporter gene in relation to response to exposure therapy in PTSD (19). Eighty-two civilians with PTSD (24 who had survived motor vehicle accidents, 31 who had survived physical assaults) who were of white European ancestry and had completed an 8-week exposure-based cognitive behavior therapy (CBT) program at the Traumatic Stress Clinic (University of New South Wales, Australia) between January 2003 and August 2006 (19) were approached to participate in the study. Fifty-five participants agreed to participate and provided DNA suitable for BDNF genotyping. Eighteen participants had comorbid major depression (8 in the Val-Val group, 10 in the Met group), and 5 reported comorbid substance abuse. There were no significant differences in age, gender, time since trauma, comorbid disorders, PTSD severity, or treatment response between those who participated or those who refused to participate in the study. Inclusion criteria were a DSM-IV diagnosis of PTSD and age between 18 and 65 years. Exclusion criteria included active suicidal intent or psychosis, severe traumatic brain injury, personality disorder, taking psychotropic medication, or cognitive impairment that prevented engaging in CBT. Before obtaining informed consent, participants were informed that the study was testing the relationship between genetics and recovery from trauma, and participants were reimbursed $20. After complete description of the study to the subjects, written informed consent was obtained.
Procedure
Diagnoses of PTSD were made by master’s level clinical psychologists with the Clinician Administered PTSD Scale (CAPS) (23). Participants then completed 8 weekly 90-minute individual CBT sessions. A subset of these participants were drawn from an arm of a previously reported randomized controlled trial of CBT that comprised imaginal exposure combined with in vivo exposure and cognitive restructuring (22). The first session comprised psychoeducation, the subsequent six sessions comprised imaginal and in vivo exposure and cognitive restructuring, and the final session comprised relapse prevention. Treatment response was quantified using the CAPS at posttreatment (within 2 weeks of therapy cessation) by a clinical psychologist independent from the study.
Genetic Analysis
Genomic DNA was extracted from mouth swabs or via saliva samples. The collection methodology shifted from mouth swabs to saliva samples during the course of the study because of the higher and more reliable yield of DNA provided by saliva samples. Genomic DNA was extracted from mouth swabs using a standard proteinase K digestion and chloroform extraction procedure, and saliva samples were purified using the Oragene DNA collection kit (DNA Genotek, Ottawa, Canada). The BDNF Val66Met genotypes were determined using primer extension followed by mass spectrometry analysis on the Sequenom MassARRAY system (Sequenom, San Diego, California) by the Australian Genome Research Facility (http://www.agrf.org.au). The genotype frequencies of the 55 participants were 54.5% Val/Val (n = 30), 40% Val/Met (n = 22), and 5.5% Met/Met (n = 3), which were in Hardy-Weinberg equilibrium (χ2 = .16, p> .05). We grouped Met allele carriers (Val/Met and Met/Met genotypes) together for analyses because the rarity of the Met/Met genotype prevents meaningful analysis. Posttreatment, all 55 patients had completed the full treatment with 30 BDNF Val/Val homozygotes and 25 BDNF Met allele carriers.
Statistical Analysis
To examine the impact of BDNF genotype on response to exposure treatment, a repeated-measures analysis of covariance (ANCOVA) was conducted on total CAPS scores with BDNF Group (Val/Val, Met) as the between factor, and Time (pretreatment, posttreatment) as the within factor, and 5HTT serotonergic tranporter genotype as a covariate. To determine if BDNF genotype could predict response to exposure therapy, a hierarchical multiple regression was conducted with CAPS posttreatment data as the dependent variable, and age, pretreatment PTSD severity (pretreatment CAPS scores), previous psychiatric history, 5HTT serotonergic transporter genotype, and BDNF genotype entered as predictors (in that order). Age, psychiatric history, and 5HTT transporter genotype were included as predictors because these variables may mediate the effects of BDNF and response to exposure treatment (19,24,25). An alpha value of p < .05 was used for all analyses.
Results
Demographic and Clinical Data
Demographic and clinical data are presented in Table 1. There were no significant differences between the Val/Val and Met carrier groups on age, time posttrauma, pretreatment depression scores, pretreatment PTSD severity, trauma type, or gender distribution. There were no significant differences in the distribution of 5HTT serotonergic transporter genotypes across the groups.
Table 1.
Demographic and Clinical Data for Participants with the BDNF Val/Val (n = 30) and BDNF Met (n = 25) genotypes
| Variable | Val/Val | Met Carriera | Fb | p |
|---|---|---|---|---|
| Age | 49 (15) | 44 (13) | 1.8 | .19 |
| Months Posttrauma | 48 (110) | 70 (112) | .55 | .46 |
| Pretreatment BDI | 22 (11) | 25 (9) | .97 | .33 |
| Pretreatment CAPS | 62 (15) | 63 (17) | .01 | .99 |
| Posttreatment CAPS | 24 (20) | 37.6 (27) | 4.5 | .038c |
| Trauma Type | 14 MVA 16 Assault |
15 MVA 10 Assault |
3.3 | .19 |
| Gender | 18 M/12 F | 17 M/8 F | .38 | .58 |
| 5HTT Genotype | 24 S/6 L | 17 S/8 L | 1.04 | .36 |
Quantitative variables are mean values, with standard deviation indicated in parentheses.
5HHT genotype, genotype on the serotonin transporter gene; BDI, Beck Depression Inventory; BDNF, brain-derived neurotrophic factor; CAPS, Clinician Administered PTSD Scale; F, female; L, long allele on the serotonin transporter gene; M, male; MVA, motor vehicle accident; S, short allele on the serotonin transporter gene.
The Met carrier group comprises those subjects with Val/Met or Met/Met genotypes.
t statistic or χ2 statistic, as appropriate.
Significant predictor.
Treatment Response
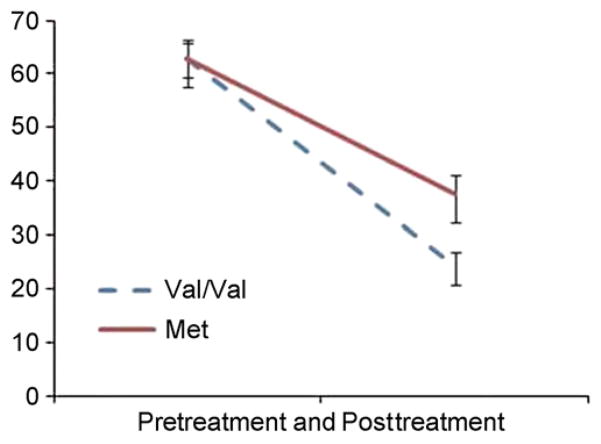
Table 1 presents the mean PTSD severity (total CAPS scores) pretreatment and posttreatment, and Figure 1 illustrates the impact of BDNF genotype on treatment response. Figure 1 reveals that the BDNF Met group showed a reduced response to exposure treatment with a 40% reduction in CAPS score posttreatment, whereas the Val/Val group showed a 62% reduction in CAPS score. A repeated measures ANCOVA (controlling for 5HTT genotype) revealed a significant interaction between BDNF and Time [F(1,52) = 7.7, p = .008, η2p = .13] and a significant effect of time [F(1,52) = 9.8, p = .003], but no significant main effect of BDNF [F(1,52) = 3.8, p = .06]. Post hoc analyses of the BDNF × Time interaction revealed there were no significant differences between Val/Val and Met groups pretreatment, but the Met group had significantly higher CAPS scores than the Val/Val group at posttreatment.
Figure 1.
Effect of brain-derived neurotrophic factor genotype on response to exposure treatment in posttraumatic stress disorder: pre-treatment and posttreatment scores on the Clinician Administered PTSD Scale for the Val/Val (n = 30) and Met (n = 25) groups. Error bars show SEM.
Hierarchical Multiple Regression
Findings from the hierarchical multiple regression are presented in Table 2. Age was a significant predictor of posttreatment PTSD scores, whereas pretreatment CAPS severity, 5HTT genotype, and previous psychiatric history were nonsignificant predictors. BDNF genotype was a highly significant predictor of posttreatment PTSD scores after controlling for pretreatment PTSD severity.
Table 2.
Hierarchical Multiple Regression Predicting Total CAPS Scores at Posttreatment
| Predictor | Beta | t | p |
|---|---|---|---|
| Age | .243 | 1.91 | .04a |
| Psychiatric History | .215 | 1.75 | .17 |
| Pretreatment CAPS Total | .251 | 2 | .08 |
| 5HTT Genotype | .04 | .25 | .801 |
| BDNF Genotype | .43 | 3.2 | .003a |
5HTT serotonin transporter short allele carriers coded as 1, long allele carriers coded as 2. Brain-derived neurotrophic factor (BDNF) Val/Val carriers coded as 1, Met carriers as 0.
CAPS, Clinician Administered PTSD Scale.
Significant predictors (p < .05).
Discussion
PTSD participants with the BDNF Met-66 low activity-dependent secretion allele displayed poorer response to exposure therapy than the Val/Val genotype group. The BDNF genotype was found to be a significant predictor of response to exposure therapy, above and beyond the effect of pretreatment PTSD severity, premorbid psychiatric history, and age. The regression analysis revealed that BDNF genotype contributed 14% of the variance in treatment response after controlling for these other significant predictors. One explanation for this finding is that lower levels of BDNF predict poorer extinction learning in the context of exposure therapy and hence predict the efficacy of an extinction-based therapy. However, an important alternative explanation to consider is that BDNF interacts with the pathogenesis of PTSD to yield a disease subtype that is differentially responsive to subsequent exposure-based treatment. This latter possibility should receive additional investigation given that insufficient extinction may be critical to the pathogenesis of PTSD. To our knowledge, this is the first study to show that the functional BDNF Val66Met polymorphism predicts response to exposure-based therapy. Our finding is in accord with previous evidence in animals that BDNF depletion impairs fear extinction (8) and that BDNF infusion into IL PFC facilitates fear extinction (2). It is also consistent with recent evidence in healthy control participants that the BDNF Met allele impairs extinction in a fear conditioning/extinction paradigm (7).
Successful response to exposure therapy is associated with increased functional brain activity in ventromedial prefrontal regions (18). Availability of BDNF in hippocampal projections to the IL medial PFC is critical for extinction memories (2), and BDNF Met carriers display reduced activity in ventromedial prefrontal regions and greater amygdala activity during extinction learning (7). This suggests that lower levels of BDNF in hippocampal and ventromedial prefrontal networks (resulting from the impaired activity-dependent secretion of the BDNF Met allele) result in reduced protein synthesis and neural plasticity in these pathways. Consequently, there is expected to be relatively less change in ventromedial prefrontal activity in response to exposure-based therapy in PTSD patients with the Met allele. To test this hypothesis, future studies should examine functional brain responses associated with exposure-based therapy in PTSD patients when stratified by BDNF Val66Met genotype.
We note that there is a need to consider the potential interaction between BDNF and other functional polymorphisms that have been associated with extinction learning. Although our previous study of genetic prediction of CBT in PTSD found that the low-expression allele of the serotonin transporter gene predicted poorer response to exposure treatment (19), we did not find an interactive effect of the serotonin transporter and BDNF genotype on treatment response. However, we examined the impact of BDNF immediately posttreatment to capture the most direct measure of extinction learning. Because the effect of the serotonin transporter gene was found at 6-month follow-up, the relationship between the serotonin transporter gene and BDNF should be examined at longer time frames posttreatment. The COMTval158 is a methylation enzyme metabolizing mono-aminergic neurotransmitter and has been linked to impoverished extinction learning (20). Of relevance to the current study is evidence that the met/met genotype of COMT is associated with PTSD severity in the context of low-intensity traumatic experience (26). A recent study of exposure therapy for patients with panic disorder found that patients with the Met/Met genotype responded more poorly to therapy than those with at least one Val allele (27). This finding is relevant in light of proposals that panic disorder and PTSD may be mediated by comparable neural circuitry that implicates conditioning and extinction networks (28). Furthermore, findings from this study and our current study support a widely held, but largely untested, hypothesis that extinction is necessary for CBT exposure treatment. Finally, BDNF binds to specific high-affinity TrkB receptors; modulation of TrkB activation has been shown to block the consolidation of fear extinction (29), and the TrkB-NTRk2 polymorphism has been associated with stress-related mood disorders (30,31) and obsessive-compulsive disorder (32). Taken together, these convergent findings suggest that future studies need to identify interactions of genotypes that are implicated in extinction learning to determine the role that these interactions play in how people respond to therapies that are based on extinction learning and retention.
Despite the independent contribution of BDNF genotype to treatment response, this finding needs important qualification. The small samples sizes in this study highlight the need for replication of the finding in larger groups. Future research should also examine genetic predictors of treatment response in relation to nontreatment control conditions to rule out any genetic influences on spontaneous remission. We lacked the sample size necessary to study any interaction of BDNF Val66Met genotype with other potential genetic predictors, as well as other potential factors, such as gender. Our sample was also limited to European patients (future studies should use genetic confirmation of ancestry), and it remains to be seen whether this pattern is observed in other ethnic groups. We also did not operationally measure extinction learning or retention, and thus the extent to which we can attribute the differential response to exposure therapy to extinction remains speculative.
These limitations notwithstanding, this preliminary study presents novel evidence that response to exposure therapy in PTSD is influenced by BDNF genotype. This finding converges with animal and human evidence revealing that BDNF facilitates extinction learning. It confirms predictions of Soliman et al. (7) that the BDNF Val66Met polymorphism influences the efficacy of extinction-based exposure therapy. This study highlights the importance of identifying specific genotypes as potential predictor variables in clinical trials of exposure therapy in PTSD. Evidence that genotypes influence response to CBT may provide a platform for the eventual application of personalized medicine to the treatment of PTSD.
Acknowledgments
This work was supported by a National Health Medical Research Council Program Grant (400403) to RAB, a National Health Medical Research Council Enabling grant (No. 480184) to PRS, and a University of New South Wales Vice-Chancellors Post-doctoral Fellowship to CD-S. We thank Kerrie Pierce for DNA sample preparation.
RAB, KLF, CD-S, and PRS report holding grants from the National Health and Medical Research Council and Australian Research Council. PRS reports paid speaker fees from Janssen unrelated to this study and GJQ report no biomedical financial interests or potential conflicts of interest.
References
- 1.Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdala-dependent learning. Neuroscientist. 2005;11:323. doi: 10.1177/1073858404272255. [DOI] [PubMed] [Google Scholar]
- 2.Peters J, Dieppa-Perea L, Melendex LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan AM, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sotres-Bayon F, Diaz-Mataix L, Bush DE, LeDoux JE. Dissociable roles for the ventromedial prefrontal cortex and amygdala in fear extinction: NR2B contribution. Cereb Cort. 2009;19:474–482. doi: 10.1093/cercor/bhn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZY, Jing DQ, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lonsdorf TB, Weike AI, Golkar A, Schalling M, Hamm AO, Ohman A. Amygdala-dependent fear conditioning in humans is modulated by the BDNFval66met polymorphism. Behav Neurosci. 2010;124:9–15. doi: 10.1037/a0018261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci U S A. 2010;107:2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal responses to overtly fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 16.Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- 17.Felmingham KL, Kemp AH, Williams LM, Das P, Hughes G, Peduto A, Bryant RA. Anterior cingulate and amygdala changes after cognitive behavioural therapy in posttraumatic stress disorder. Psychol Sci. 2007;18:127–129. doi: 10.1111/j.1467-9280.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 18.Bryant RA, Felmingham KL, Whitford T, Kemp AH, Hughes G, Peduto A, Williams LM. Rostral anterior cingulate volume predicts treatment response to cognitive behavior therapy in posttraumatic stress disorder. J Psychiatry Neurosci. 2008;33:142–146. [PMC free article] [PubMed] [Google Scholar]
- 19.Bryant RA, Felmingham KL, Falconer EM, PeBenito L, Dobson-Stone C, Pierce KD, Schofield PR. Preliminary evidence of the short allele of the serotonin transporter gene predicting poor response to cognitive behavior therapy in posttraumatic stress disorder. Biol Psychiatry. 2010;67:1217–1219. doi: 10.1016/j.biopsych.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Ohman A. Genetic gating of human fear learning and extinction: Possible implications for gene–environmental interactions in anxiety disorders. Psychol Sci. 2009;20:198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 21.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachna BS, et al. 5-HTTLPR polymorphism impacts human cingulate–amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 22.Bryant RA, Moulds ML, Guthrie RM, Dang ST, Mastodomenico J, Nixon RD, et al. A randomized controlled trial of exposure therapy and cognitive restructuring for posttraumatic stress disorder. J Consult Clin Psychol. 2008;76:695–703. doi: 10.1037/a0012616. [DOI] [PubMed] [Google Scholar]
- 23.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Klauminzer G, Charney DS. A clinician rating scale for assessing current and lifetime PTSD: The CAPS-1. Behav Ther. 1990;13:187–188. [Google Scholar]
- 24.Toth E, Gerstner R, Wilf-Rakoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- 25.Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci. 2011;31:4475–4483. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guthrie RM, Bryant RA. Extinction learning before trauma and subsequent posttraumatic stress. Psychosom Med. 2007;68:307–311. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- 27.Kolassa IT, Kolassa S, Ertl V, Papassotiropoulos A, De Quervain DJ. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val(158)Met polymorphism. Biol Psychiatry. 2010;67:304–308. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Lonsdorf TB, Ruck C, Bergstrom J, Andersson G, Ohman A, Lindesfors N, Schalling M. The COMTval158met polymorphism is associated with symptom relief during exposure-based cognitive-behavioral treatment in panic disorder. BMC Psychiatry. 2010;10:99. doi: 10.1186/1471-244X-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohli MA, Salyakina D, Pfennig A, Lucae S, Horstmann S, Menke A, et al. Association of genetic variants in the neurotrophic receptor encoding gene NTRK2 and a lifetime history of suicide attempts in depressed patients. Arch Gen Psychiatry. 2010;67:348–359. doi: 10.1001/archgenpsychiatry.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]