Abstract
The innate-like natural killer T (NKT) cells are essential regulators of immunity. These cells comprise at least two distinct subsets and recognize different lipid antigens presented by the MHC class I like molecules CD1d. The CD1d-dependent recognition pathway of NKT cells is highly conserved from mouse to humans. While most type I NKT cells can recognize αGalCer and express a semi-invariant T cell receptor (TCR), a major population of type II NKT cells reactive to sulfatide utilizes an oligoclonal TCR. Furthermore TCR recognition features of NKT subsets are also distinctive with almost parallel as opposed to perpendicular footprints on the CD1d molecules for the type I and type II NKT cells respectively. Here we present a view based upon the recent studies in different clinical and experimental settings that while type I NKT cells are more often pathogenic, they may also be regulatory. On the other hand, sulfatide-reactive type II NKT cells mostly play an inhibitory role in the control of autoimmune and inflammatory diseases. Since the activity and cytokine secretion profiles of NKT cell subsets can be modulated differently by lipid ligands or their analogs, novel immunotherapeutic strategies are being developed for their differential activation for potential intervention in inflammatory diseases.
Keywords: CD1, cancer, glycolipids, NKT cells, sulfatide, Th1/Th17
NKT CELL SUBSETS
The multifaceted immune system orchestrates interactions between cells to eliminate invading pathogens and maintain homeostatic regulation of self-tolerance. It consists both of cells which respond to antigens never before encountered (the adaptive immune response) and cells which, through evolutionary selection, have been preprogrammed to respond to common environmental antigens (the innate immune response). Among the innate like lymphocyte populations of the immune system are natural killer T (NKT) cells, comprised of at least two subsets, which bridge the innate and adaptive immune responses (see Fig. 1) [1–4]. NKT cells play an important modulatory role in autoimmunity, tumor surveillance, and response against invading pathogens. They display T cell receptors characteristic of conventional T cells in addition to cell surface proteins expressed by NK cells, such as CD56/161(humans) and NK1.1 (mice) [2, 3, 5]. Unlike conventional CD4+ T cells, which respond to protein antigens presented by the major histocompatibility complex (MHC) class II molecules, most NKT cells are reactive to lipid antigens presented by CD1d molecules [2, 3, 5]. Several antigen presenting cells (APC) including dendritic cells (DC), macrophages (M(ϕ)), B cells, thymocytes, and hepatocytes express CD1d molecules and can therefore activate NKT cells. Activation of NKT cells results in the rapid release of abundant cytokines of both T helper 1 (Th1) (IFN-γ) and Th2 (IL-4 & IL-13) responses. Interestingly, though murine type I NKT cells can secrete IL-17, these cells in human PBMCs from healthy individuals do not [6].
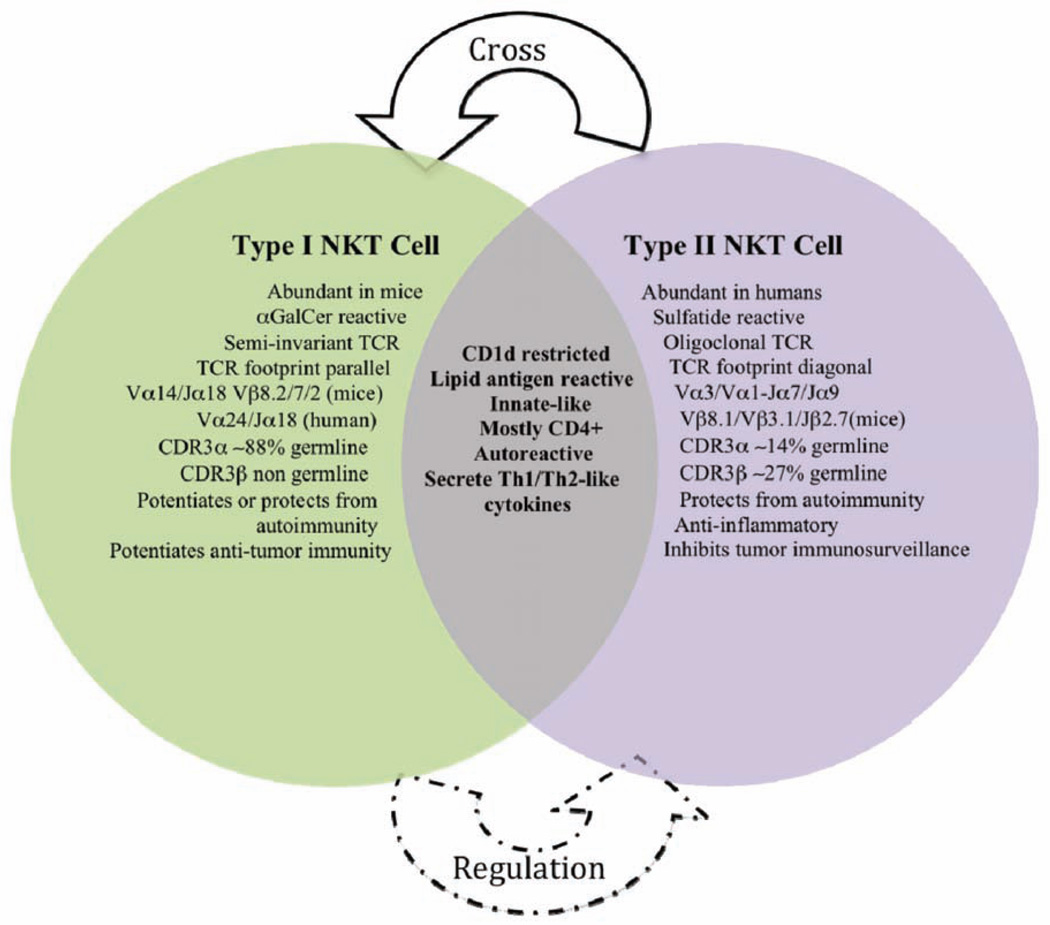
Fig. 1.
A list of major similarities and differences between type I NKT and type II NKT cells.
The more widely studied type I NKT cells, also known as semi-invariant NKT cells, are more prevalent than type II NKT cells in mice than in humans [1, 7], comprising about 50% of murine intrahepatic lymphocytes [8–10]. These cells react strongly to α-galactosylceramide (αGalCer), an exogenous marine derived glycolipid [11–13]. A major difference between the two subsets resides in their TCRs: the invariant TCR is encoded by a predominantly germline Vα gene (75–88%) (Vα14/Jα18 in mice and Vα24/JαQ in humans), as well as more diverse non-germline Vβ chain genes (Vβ8.2/7/2 mice and Vβ11 humans) [1–5, 7, 14–16]. Type I NKT cells respond to α-, as well as, β-linked glycolipids. The semi-invariant TCR binds to CD1d in a parallel configuration, predominantly involving the α chain [2, 3, 5, 15].
We have focused on the biology of type II NKT cells in health and during disease. In humans, type II NKT cells is the predominant subset [1, 17]. A key element in characterizing the physiological function of this NKT cell subset was the identification of one of its naturally occurring ligands. Towards this goal we focused on screening myelin lipids that possess structural characteristics similar to αGalCer, for their ability to stimulate NKT cells. In our initial studies we found that the naturally occurring galactolipid sulfatide was able to stimulate NKT cells in a CD1d-dependent fashion. Upon further characterization we discovered that sulfatide stimulated CD 1 d-restricted NKT cells were not of type I, but a major subset of type II NKT cells [18]. We found that sulfatide-reactive type II NKT cells generally play a protective role in autoimmune diseases by controlling the inflammatory responses mediated by type I NKT cells [19]. In some cases non-sulfatide reactive type II NKT cells may play a pathogenic role such as in ulcerative colitis [20]. Type II NKT cells express oligoclonal TCRs utilizing a limited number of Vα- and Vβ-chains. In contrast to type I NKT cells, only about 14% of the TCR Vα and 13–27% of the Vβ chains in type II NKT cells, are encoded by germline gene segments [19]. Notably, type II NKT TCRs contact the ligand predominantly using the β chain rather then the α chain, suggesting that the TCR Vβ chain is responsible for providing antigen fine specificity [21]. The TCR-binding mechanism of type II NKT cells uses features derived from both the type I NKT cells and conventional T cells [1, 19, 21, 22]. Unlike type I NKT cells, the type II NKT subset does not respond to α-linked glycolipids [21]. The above studies clearly indicate distinctive molecular recognition features of the two NKT cell subsets.
The pathogenic or protective effects of NKT subsets following stimulation in vivo are determined by their cytokine secretion profile, timing of activation, and the lipid antigens involved. Understanding how these subsets’ TCRs bind to their ligands and how they cross-regulate each other should lead to the development of novel strategies of immune regulation for intervention in autoimmune diseases in a human leukocyte antigen (HLA)-independent manner (see Fig. 1). Overwhelming evidence of the regulatory function for both type I and type II NKT cells makes them extremely attractive targets for immunotherapeutic manipulation.
NKT CELLS RECOGNIZE BOTH EXOGENOUS AND ENDOGENOUS LIPIDS
Consistent with the structural and recognition differences between TCRs of the two NKT cell subsets, different lipid antigens have the ability to activate these cells. For example αGalCer, the most affine ligand for type I NKT cells, is not recognized by type II NKT cells. Recognition of autologous lipids rather than microbial lipids is most likely the primary function of type II NKT cells [23]. However type I NKT cells can respond to both endogenous and exogenous lipids. The endogenous lipids include: isoglobotrihexosylceramide (iGb3), βgalactosyl ceramide (βGalCer) and disialoganglioside (GD3) [2, 12, 24, 25]. Exogenous lipids include: αgalactosylceramide (αGalCer), ocglucouronosylceramide (αGluCer), βglucouronosylceramide (βGluCer), αgal-urono-syl-cer, αgluuronosylcer, and αgalactosyldiacyglycerol (αGalDAG) [2, 5, 24, 26]. Type I NKT cells have been identified by utilizing αGalCer/CD1d tetrameric reagents due to their strong binding affinity for CD1d and the semiinvariant TCR [5, 7, 8, 11, 27]. Stimulation of type I NKT cells with αGalCer results in a cytokine burst, TCR down regulation, anergy, and apoptosis [1, 12]. It is likely that the in vivo effects of αGalCer stimulation do not reflect true hysiological responses due to its non-mammalian nature and its extraordinarily high binding affinity. Though self-antigens such as iGb3 can stimulate type I NKT cells [28] they bind to CD1d poorly and are inefficient in comparison to αGalCer. More studies will be necessary to identify a detailed physiological role for type I NKT cells following recognition of self-antigens.
While a major population of type II NKT cells can be identified by sulfatide/CD1d loaded tetramers [18], these cells can recognize several other hydrophobic antigens including lysosulfatide, lysophosphatidylcholine [29], Glc Cer, GalCer [21], and phenyl 2,2,4,6,7-pentamethyldihydro-benzofuran 5-sulfonate (PPBF in humans)[5]. Sulfatide is an endogenous galactosylceramide and is an immunodominant glycolipid enriched in myelin, pancreatic β cells, kidney and liver cells [30]. In fact one fifth of galactolipid in myelin occurs as sulfatide. It is interesting to note that sulfatide binds promiscuously to all CD1 isotypes, some of which can present antigens to conventional T cells. However CD1d presents sulfatide strictly to NKT cells in both humans and in mice [22, 31, 32]. Sulfatide/CD1d tetramers have been utilized to establish proliferative and cytokine responses of type II NKT cells during various experimental autoimmune diseases [7, 19, 21, 31, 33, 34].
THE CYTOKINE SECRETION PROFILE OF CONVENTIONAL MHC-RESTRICTED CD4+ T CELLS IS IMPORTANT IN AUTOIMMUNITY
Conventional CD4+ T cells produce cytokines when activated by endogenous or exogenous protein antigens presented by APCs expressing MHC class II molecules. Self-reactive CD4+ Th1/Th17 cells are the key mediators of autoimmunity (see Fig. 2) [35]. Much like NKT subsets, T helper subsets cross regulate each other to maintain homeostasis in a healthy immune system: for instance Th1 cytokines frequently disrupt the actions of Th2 cytokines and vice versa. Th1 responses are mainly involved in cell mediated inflammatory responses and these cells secrete IL-2, IFN-γ, TNF-β, GM-CSF, and IL-3 while Th2 cell responses are generally protective in autoimmunity by predominantly secreting IL4, IL-5, IL-10, and IL-13 [36]. It is widely accepted that organ specific autoimmune responses result from poorly regulated Th1 responses [36]. Generally protection from autoimmunity is observed when the global cytokine secretion profiles are shifted from a Th1/Th17 bias to a predominately Th2 bias. Th17 cells secrete IL-17 (A/F) and are mostly involved in a pathogenic role during autoimmune diseases [35, 37, 38]. Interestingly, type I NKT cells in healthy humans do not secret IL-17 [6]. However human type I NKT cells can specifically suppress Th17 cells [39]. The method of type I NKT activation with αGalCer is a deciding factor in determining Th1/Th2 balance among MBP reactive T cells and correlates with severity of disease [7]. Although studies have been carried out with type I-deficient Jα18−/− or type I/type II-lacking CD1d−/− mice to examine the influence of NKT cell subsets in experimental autoimmunity, it is not clear whether deficiency of NKT cell subsets alters global T helper subset bias.
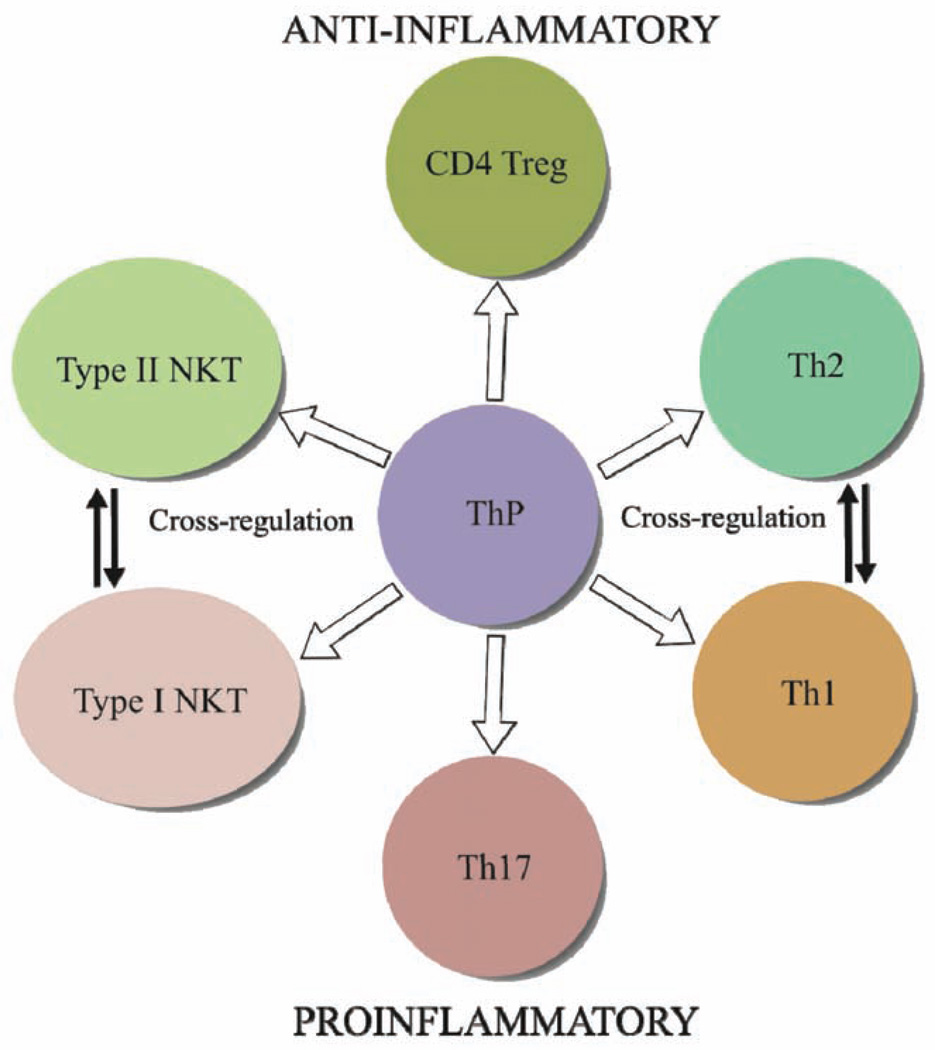
Fig. 2.
A simplified scheme showing the generation of different Th lineages, CD4+ Treg as well as NKT cells from Th precursors (ThP). The development of different subsets is dependent upon the presence of different cytokines as well as engagement of specific transcription factors. The immune modulatory potential of Th subsets is also mediated by differential cytokine secretion profiles. FoxP3+CD4+ Treg cells could secrete IL-10 and TGF-β for anti-inflammatory response. Th1 and Th17 cells play a major role in the development of autoimmune diseases: while Th1 cells secrete IFN-γand TNF-α, Th17 cells secrete IL-17A, IL-17F, and IL-22. On the other hand Th2 cells can play antiinflammatory role by secretion of IL-4, IL-5, IL-10 and IL-13. Interestingly though both NKT subsets can release Th1 and Th2-like cytokines such as IL-4 and IFN-γ, type I NKT cells can generally play an inflammatory role while type II NKT are more often antiinflammatory. Th1 and Th2 cell cross regulate each other much like the NKT cell subsets via secretion of cytokines and modulation of antigen-presenting cells.
INVOLVEMENT AND MODULATION OF TYPE I AND TYPE II NKT CELLS IN AUTOIMMUNITY
Multiple Sclerosis (MS)/Experimental Autoimmune Encephalomyelitis (EAE)
Multiple sclerosis is an autoimmune disease involving inflammation in the central nervous system (CNS) triggered by a MHC-restricted CD4+ T cell mediated response to myelin self-antigens. Inflammation due to activated T cells, M(ϕ) and local phagocytic microglia results in active lesions of the CNS, followed by demyelination of neurons leading to paralysis which can be progressively chronic and debilitating. Activated microglia and M(ϕ) in CNS lesions may engulf myelin debris and present its component lipids to pathogenic T cells [30]. Studies show that type I NKT cells are decreased in the peripheral blood mononuclear cells (PBMC) of MS patients [40, 41], possibly due to apoptosis following activation. However patients in remission have an elevated type I NKT cell count in their PBMCs following treatment with IFN-β [42].
EAE is used as a prototype to study inflammation, demyelination and paralysis in the CNS. EAE is induced by immunization with myelin proteins, e.g., myelin basic protein (MBP), myelin proteolipid protein (PLP) or myelin oligodendrocyte glycoprotein (MOG) along with complete Freund’s adjuvant (CFA). In EAE a cytokine profile of myelin-protein-reactive CD4+ T cells skewed towards Th1/Th17 is pathogenic while a Th2 biased response is generally protective [43]. Under some conditions activation of type I NKT cells with αGalCer or its analogs (OCH) skews the conventional Th cell response towards Th2, resulting protection from EAE [7, 44]. However other conditions of αGalCer administration, e.g., timing of lipid injection and different MHC haplotypes, can bias conventional CD4+ T cells towards Th1/Th17 leading to exacerbation of EAE [7]. IFN-γ secretion may not be always pathogenic as it can also have protective effects on EAE by inducing apoptosis of pathogenic T cells [45]. The ultimate role of type I NKT cells in EAE remains controversial [7, 46–48]. Nevertheless in our laboratory Jα18−/− mice develop less severe EAE than the WT mice, suggesting their disease-potentiating role.
It is noteworthy that sulfatide reactive type II NKT cells but not type I NKT cells are increased several fold within the CNS during EAE, inverting the usual ratio (type II NKT cells 3–4% and type I NKT cells 0.6–0.9%) and affording protection from disease [18, 30]. Furthermore administration of sulfatide to activate type II NKT cells decreases the number of IFN-γ- and IL-17-secreting MBP and PLP-reactive encephalitogenic CD4+ T cells and in turn protects from EAE using a CD1d-dependent regulatory pathway (Maricic et al., manuscript in preparation). This type II NKT-mediated immunoregulatory pathway results in (i) inactivation of type I NKT cells that now behave like regulatory T cells (ii) tolerization of dendritic cells (iii) tolerization of microglia in the CNS and (iv) inhibition of the effector functions of pathogenic conventional CD4+ T cells. Since APCs that activate pathogenic Th1/Th17 in lymphoid organs and in the CNS are tolerized following sulfatide administration, this activation of type II NKT cell is much more potent in regulating autoimmune demyelination than solely the inactivation of type I NKT cells by αGalCer (Maricic et al., manuscript in preparation).
Type 1 Diabetes (T1D)/Non-Obese Diabetic (NOD) Mice
Type I or juvenile onset diabetes is characterized by T cell mediated destruction of insulin producing β cells in the pancreas resulting in elevated glucose levels in the blood [24]. Although initial studies in diabetes patients suggested a reduced number of type I NKT cells in patient PBMCs [49, 50] subsequent studies have failed to confirm this observation [51], thus contributing to the controversy over the correlation between type I NKT population frequency and disease status. Furthermore whether NKT cells in the PBMC of humans and mice are an accurate indication of their activity in the target tissues is also unknown.
Non-obese diabetic mice develop diabetes spontaneously and have fewer thymic and splenic type I NKT cells [24]. Although CD1d deficiency may or may not potentiate diabetes, over expression of CD1d in these animals prevents disease [52–56]. Protection from diabetes by NKT cells is associated with an elevated Th2 cytokine profile in pathogenic islet protein-reactive CD4+ T cells [4, 57, 58] while a Th1 bias correlates with disease severity [13, 59]. In spite of this a Th1 to Th2 cytokine profile shift in conventional CD4+ T cells alone may not be sufficient to prevent T1D in NOD mice [60, 61]. Recently activation of type II NKT cells by sulfatide has been shown to protect NOD mice from T1D [19, 31]. It has also been demonstrated that the C24:0 but not C18:0 sulfatide protects against the transfer of T1D [31]. These data suggest that analogs of sulfatide may have therapeutic value in clinical trials in human subjects at risk for or newly diagnosed with T1D.
Rheumatoid Arthritis (RA)/Collagen-Induced Arthritis (CIA)
Rheumatoid Arthritis is a systemic autoimmune disease in which pathogenic T cells produce proinflammatory cytokines, causing swelling and painful inflammation of the joints. Human PBMCs [41, 62] and synovia [63] display lower levels of NKT cells as well as a Th1 bias during disease [62].
Collagen-induced arthritis is induced by injection of heterologous type II collagen in adjuvant, which leads to vigorous T and B cell responses. During CIA there is an increase in the activation of Th1 cells expressing proinflammatory cytokines, equivalent to studies in RA patients [64]. The shift from a Th1 to a Th2 cytokine profile results in IL-10 and IL-4 dependent protection. Interestingly, Jα18−/− mice develop less severe CIA which is associated with a Th2 profile (increased IL-10 production) of collagenreactive pathogenic CD4+ T cells [65], suggesting a pathogenic role for type I NKT cells in CIA. These data are consistent with a study that shows anergizing type I NKT cells by administration of αGalCer or its analog OCH results in a shift from Th1 bias to a Th2 bias in collagen-reactive CD4+ T cells, protecting against CIA [44, 65]. Currently the role of type II NKT cells is unknown in CIA, and further investigations are required.
Myasthenia Gravis (MG)/Experimental Autoimmune Myasthenia Gravis (EAMG)
Myasthenia Gravis occurs as a result of antibodies directed at acetylcholine receptors at postsynaptic neuromuscular sites blocking neurostimulation, causing intense muscle weakness and fatigue. This organ specific autoimmune disease displays elevated levels of type I NKT cells in PBMCs, in contrast to the cases of MS and RA [41]. The reason for this difference is unknown. Nevertheless NKT cell levels return to normal levels after treatment [66].
Experimental autoimmune myasthenia gravis is induced following immunizations with heterologous acetylcholine receptors in adjuvant. Interestingly susceptibility to EAMG is unaffected in CD1d−/− mice [55, 67] suggesting that type I NKT cells may not be pathogenic. However αGalCer administration has proven to be protective in mice [67, 68]. Generally, reduced levels of IFN-γ and increased IL-4 secretion protects against EAMG [66]. There is limited knowledge regarding the type II NKT cell role in EAMG, indicating the importance for future studies.
Systemic Lupus Erythmatosus (SLE)
Systemic lupus erythmatosus involves autoantibodies directed against nuclear antigens controlled by auto reactive CD4+ T cells resulting in inflammation of the connective tissues. A reduced number and a suppressed cytokine production capacity of type I NKT cells in PBMC of SLE patients have been observed along with their recovery after treatment [41, 62]. However levels of both conventional Th1 and Th2 cells are increased in the serum of lupus patients correlating with disease activity [69]. During disease exacerbation there is an increase in a conventional Th1 cell response, while during remission there is an increase in Th2 response [70]. Recently IL-17 has been implicated in the pathogenesis of SLE and accordingly serum levels of IL-17 as well as its induction in PBMCs of SLE patients were found to be higher [71, 72].
The role of type I NKT cells in the experimental models of lupus is controversial. This may result from the difference in genetic backgrounds, spontaneous models vs induced models and timing of the administration of αGalCer during disease [73–77]. Diminished type I NKT cell levels and a Th1 bias have been observed in MRL/lpr mice that spontaneously develop lupus-like autoimmunity [78]. One study indicated that CD1d−/− mice do not develop renal disease or skin lesions [79], whereas others suggested that CD1d−/− mice expressed exacerbated lupus skin disorder but not renal symptoms [78]. Consistent with the contributory role of type I NKT cells in spontaneous disease in (NZB x NZW F1) mice, these cells have been shown to promote anti-dsDNA autoantibody production by B cells in vitro as well as in vivo following adoptive transfer [75, 77, 80]. Complicating a simplified hypothesis, while continuous αGalCer injection in younger (4 wk) lupus prone mice partially alleviates SLE symptoms by increasing Th2 bias [59], identical treatment in older mice (8–12 wk) increased a Th1 cytokine profile as well as disease severity [73]. Collectively these studies suggest that type I NKT cells promote production of autoantibodies in spontaneous models of lupus. Our preliminary studies suggest that activation of type II NKT cells following administration of sulfatide significantly prevents lupus nephritis in (NZB X NZW) F1 mice indicating their protective capacity can counteract potentially pathogenic type I NKT cells.
Liver Diseases
A variety of destructive changes in liver following injury or inflammation have now been shown to be the direct result of type I NKT cell activity. In autoimmune hepatitis loss of immune tolerance to hepatocytes leads to parenchyma destruction and inflammation resulting in swelling of the liver. During studies of concanavalin A (ConA)-induced hepatitis Jα18−/− and CD1d−/− mice were found to be resistant to liver damage [34, 81, 82]. Adoptive transfer of type I NKT cells into Jα18−/− mice restored Con-A sensitivity [82, 83]. Accordingly αGalCer administration in wild type mice results in a type I NKT cell-mediated immune cascade and liver damage following ConA challenge [84]. Administration of sulfatide prevents ConA induced hepatitis by activation of type II NKT cells, which in turn leads to an accumulation of anergic type I NKT cells in the liver that is dependent upon IL-12 and MIP-2 [34]. These studies indicate a pathogenic role for type I NKT cells in liver damage induced by the drug.
Primary biliary cirrhosis (PBC) is an organ specific autoimmune disease which develops as the result of antibodies attacking the mitochondrial enzyme pyruvate dehydrogenase complex dihydrolipoyl transacetylase, leading to inflammation and swelling of bile ducts in the liver. This blocks the flow of bile, damages liver cells, and leads to liver scarring or cirrhosis. Type I NKT cells and CD1d expression are increased in liver but decreased in PBMC during disease progression [85, 86]. Th2 cells protect from autoimmune cholangitis while proinflammatory Th1 cells potentiates disease. Jα18−/− mice are resistant to disease and CD1d−/− mice have a reduced incidence of cholangitis, indicating a pathogenic role for type I NKT cells [29, 87]. Stimulation of type I NKT cells with αGalCer results in disease exacerbation associated with the Th1 cytokine release [24, 29, 87, 88].
Ischemia reperfusion injury (IRI) occurs when type I NKT cells secrete pro inflammatory cytokines (TNF-α and IFN-γ) within 1 to 6 hours following transplant, resection surgeries, liver trauma, or hepatic shock. This is accompanied by an increase in liver enzymes in serum and infiltration of neutrophils causing injury to hepatocytes [89]. Consistent with the pathogenic role of type I NKT cells in liver diseases, Jα18−/− mice do not develop IRI [89]. Sulfatide-mediated activation of type II NKT cells induces anergy in type I NKT cells leading to decreased levels of IFN-γ+ and a significantly reduced recruitment of myeloid cells and liver injury [89]. These studies suggest that pretreatment with sulfatide may be therapeutic for improving the outcome of liver transplants and reducing incidence of IRI. Collectively, the studies carried out in hepatitis, PBC and IRI indicate that while type I NKT cells are pathogenic type II NKT cells are protective in liver injury and may be manipulated to alleviate autoimmune or inflammatory liver diseases.
NKT CELL SUBSETS IN TUMOR IMMUNOSURVEILLANCE
Decreased frequency of type I NKT cells [90] and deficiency in IFN-γ secretion [91] have been reported in some but not all cases of cancer. Type I NKT cells appear to protect against tumors while activation of type II NKT cells may increase tumor susceptibility [33]. Type I NKT cells protect against tumors by promoting proinflammatory responses, which are generally pathogenic in autoimmunity. Activation of type I NKT cells by αGalCer has been proven to be effective against several tumors such as carcinomas, sarcomas, melanomas and thymomas. Anti-tumor activity of type I NKT cells is dependent upon their IFN-γ secretion [92], which in turn results in IL-12 secretion by DCs [78] and activation of antitumor CD8+ cytotoxic T cells as well as NK cells. In cancer patients type I NKT cells generate reduced levels of IFN-γ [41, 91, 93], corresponding to the observation that low levels of type I NKT cells in the PBMCs of cancer patients usually indicate a poor clinical outcome [94]. Although type II NKT cells exhibit a protective role in autoimmune diseases, they can promote tumor growth during immunosurveillance [33, 95, 96]. Activation of type II NKT cells with sulfatide results in anergizing type I NKT cell activity and accordingly enhances tumor growth [95]. Jα18−/− and CD1d−/− mice are more susceptible to the growth and frequency of tumors, which improve after type I NKT cells are replaced [97]. When type I and type II NKT cells are stimulated simultaneously, type I NKT-mediated effects are suppressed by type II NKT cells [33]. In some cases a Th2 profile by type I NKT cells can induce suppression of anti-tumor immunity as well [96]. It is likely that the levels of CD1d and lipid antigen expression by tumors play a crucial role in immunosurveillance. Generally the presence of type I NKT cells in experimental models has been shown to be important for an effective anti-tumor immune response. Surprisingly we have found that some of the high CD1d-exprssing tumor cell lines implanted into type I NKT-deficient mice do not form tumors whereas CD1d-negative tumor lines form tumors rapidly in comparison to NKT-sufficient animals (Manuscript in preparation). These data suggest that the tumor microenvironment, CD1d expression, lipid antigen presentation and subsequent activation of type I or type II NKT cells all play an important role in tumor development. This is particularly relevant to organ-specific autoimmune diseases where local tissue environment, differential lipid availability and CD1d expression could engage different NKT cell subsets with variable consequences.
NKT CELLS IN PATHOGENESIS OF ASTHMA
Bronchial asthma is a complex disorder associated with wheezing, shortness of breath and airway obstruction as a result of airway hypersensitivity (AHR). Allergic asthma, a most common form of asthma is associated with the presence of allergen-specific Th2 cells secreting IL-4, IL-5 and IL-13 leading to an increase in airway eosinophils, basophils, mast cells and B cells producing IgE. In addition to Th2 cells, recent studies have also suggested the involvement of Th17 and type I NKT cells in asthma [3, 98, 99]. Although controversial, several studies have indicated the presence or increased levels of type I NKT cells in bronchoalveolar lavage fluid or in endobronchial biopsy tissues from patients with asthma [3, 98, 99]. These findings are consistent with several studies in experimental models of asthma showing the requirement of type I NKT cells for the development of AHR. Thus allergen-, ozone-, and virusinduced AHR fails to develop in type I NKT-deficient Jα18−/−or NKT-deficient CD1d−/− mice [83, 100, 101]. Additionally, activation of type I NKT cells by αGalCer results in severe AHR independent of conventional CD4+ T cells in mice [102] as well as in monkeys [103]. Collectively studies in experimental asthma clearly suggest a contributory role of type I NKT cells. However more functional studies are needed in humans to clarify their pathogenic role in asthma. Consistent with a regulatory role for type II NKT cells, a recent study in a murine model of asthma has shown that activation of type II NKT cells by sulfatide or their adoptive transfer results in reduced inflammatory cellular infiltrate in the lung and decreased levels of IL-4/IL-5 and antigenreactive IgE in the lung [104]. Additionally similar to other experimental models, this is associated with the inactivation of type I NKT cells following sulfatide administration.
CROSS-IMMUNOREGULATORY PATHWAY(S) FOLLOWING ACTIVATION OF NKT CELLS
A novel regulatory mechanism is orchestrated following activation of type II NKT cells with sulfatide resulting in protection from autoimmunity and inflammatory tissue damage. This unique pathway involves cross regulation of type I NKT cells and inhibition of pathogenic Th1/Th17 cells through tolerization of cDCs. It has been shown to be effective in the control of EAE [18], diabetes [31], liver diseases [34] and lupus (Halder, unpublished data). Interestingly, while activation of type I NKT cells predominantly activates hepatic conventional DC (cDC), sulfatide-mediated activation of type II NKT cells predominantly activates hepatic plasmacytoid DC (pDC). Additionally, type II NKT-DC interactions result in a rapid (within hours) recruitment of type I NKT cells into liver in IL-12 and MIP2-dependent fashion. However recruited type I NKT cells are not activated, do not secrete cytokines and become anergic. Thus anergy in type I NKT cells leads to reduced levels of IFN-γ followed by reduced recruitment of myeloid cells and NK cells and protection from liver damage [89]. Furthermore tolerized cDC further inhibit conventional pathogenic CD4+ effector T cells mediating autoimmunity [18]. Thus adoptive transfer of cDCs from sulfatide-treated but not control mice into naïve recipients leads to prevention of inflammatory damage. Furthermore, sulfatide reactive type II NKT cell activation also causes tolerization of tissueresident APC, such as microglia in the CNS and thereby impairing the development of pathogenic Th1 and Th17 cells [18]. A recent study has suggested that the ICOS and PD-1 ligand pathways are required for regulation of diabetes in NOD mice by CD4+ type II NKT cells [105]. Interestingly, a similar pathway is also involved in regulation by Treg cells [106, 107]. Since activation of sulfatide reactive type II NKT cells inhibits the effector functions of pathogenic conventional Th1/Th17 cells in the peripheral organs as well as in the affected tissues such as CNS and liver, targeting these cells leads to a broader therapeutic response than targeting type I NKT cells alone for intervention in autoimmune disease.
Although some studies suggest that type I NKT cells could potentially cross-regulate type II NKT activity [95], more data are needed to clarify mechanisms of regulation. It is clear that activation of type I NKT cells with αGalCer leads to a cascade of events modulating activity of several cells types, including dendritic cells, B cells, NK cells and neutrophils [2, 108]. Therefore it is likely that sulfatidemediated anergy induction in type I NKT cells also results in modulation of the activity of these other cell types. As mentioned above, our data clearly indicate a significant alteration in the activity of DC populations following sulfatide-mediated activation of type II NKT cells. Currently studies are in progress to investigate the involvement of other cellular players following type II NKT activation in the presence and absence of type I NKT cells.
IS THE ANALYSIS OF NKT CELLS IN PBMC IN HUMANS REFLECTIVE OF THEIR BEHAVIOR IN TISSUES?
The frequency and cytokine secretion profiles of NKT cells in PBMCs of patients with autoimmune diseases and the role of NKT subsets in immune regulation in human inflammatory conditions are being investigated, but correlations are not yet clear [40, 41, 62, 83]. Dissimilar to mice, there is immense variability in NKT cell frequency in PBMC within healthy individuals (0.01% to 3%) [2, 3, 5, 60, 109], but studies of twins and congenic mice indicate NKT cell number is genetically regulated [4]. At a minimum we should expect to address whether healthy individuals with diminished NKT cell frequency are at a greater risk for disease. This implies carrying out population studies over time. The frequency of NKT cells in murine PBMCs is unrelated to their number in the thymus or spleen [110]. At present it is not clear whether the frequency of NKT cells in the PBMCs can provide an accurate indication of systemic or organ-specific NKT cell pools in humans [111], and studying this presents obvious difficulties. Independent of PBMC NKT cell number, two further types of systematic studies could provide an appreciation of the role of NKT cells in human diseases. First, it is possible that NKT cell defects are caused by polymorphisms in molecules essential for NKT development, such as the SLAM [112] and PLZF [113] pathways. Therefore attention should be paid to polymorphisms of these molecules in various human conditions. Second, longitudinal analysis in humans with a particular disease is essential for observing changes in NKT cell number and cytokine secretion patterns during progression [111] in order to assess their possible role. Correlating the frequency of NKT cells, their cytokine patterns and disease would provide a beginning for understanding their etiological link to autoimmunity.
THE PATH FROM MURINE STUDIES TO POTENTIAL INTERVENTIONS IN HUMAN DISEASE
The CD1d dependent antigen recognition pathway is highly conserved from mice to humans and several key features of NKT cell subsets are shared between them. Although studies in mice have mostly used NKT cells from the thymus, spleen or liver the systemic results of their manipulation indicate that clinical studies based upon them are warranted. Therefore discoveries in experimental models can be translated into the clinical setting [1, 114] allowing the application of murine studies to humans. It is fortunate that type II NKT cells occur more frequently relative to type I in humans, facilitating additional studies to further characterize them using appropriate lipid ligands. A detailed characterization of type II NKT cells and their ligands in humans is important for their appropriate manipulation in disease conditions. A path to the manipulation of type I NKT cells has already been opened by phase I/II clinical trials with αGalCer in humans for their anti-tumor effects [115–118]. Other analogs of αGalCer that are able to skew conventional CD4+ T cell responses more towards either a Th1- or a Th2-like profile will make their way into clinical studies. In the near future it is possible that we should be able to differentially activate or inhibit type I and type II NKT cells for the development of novel immunotherapeutic in autoimmune diseases.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health (R01CA100660, R01AA020864 and R01AI05227), Juvenile Diabetes Research Foundation (24-2007-362) and Multiple Sclerosis National Research Institute to V.K. Authors would like to thank other members of the laboratory for their help and support.
ABBREVIATIONS
- NKT
Natural killer T cell
- MHC
Major histocompatibility complex
- DC
Dendritic cell
- M(ϕ)
Macrophage
- Th
T helper cell
- APC
Antigen presenting cell
- αGalCer
α-Galactosylceramide
- PBMC
Peripheral blood mononuclear cells
- CNS
Central nervous system
- MS
Multiple sclerosis
- EAE
Experimental autoimmune encephalomyelitis
- T1D
Type 1 diabetes
- NOD
Non-obese diabetic mice
- RA
Rheumatoid arthritis
- CIA
Collagen induced arthritis
- MG
Myasthenia gravis
- EAMG
Experimental autoimmune myasthenia gravis
- SLE
Systemic lupus erythmatosus
- IRI
Ischemic reperfusion injury
- PBC
Primary biliary cirrhosis
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Arrenberg P, Halder R, Kumar V. Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. J Cell Physiol. 2009;218(2):246–250. doi: 10.1002/jcp.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114(10):1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11(3):197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 5.Godfrey DI, Pellicci DG, Patel O, Kjer-Nielsen L, McCluskey J, Rossjohn J. Antigen recognition by CD1d-restricted NKT T cell receptors. Semin Immunol. 2009;22(2):61–67. doi: 10.1016/j.smim.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Snyder-Cappione JE, Tincati C, Eccles-James IG, et al. A comprehensive ex vivo functional analysis of human NKT cells reveals production of MIP1-alpha and MIP1-beta, a lack of IL-17, and a Th1-bias in males. PLoS One. 2010;5(11):e15412. doi: 10.1371/journal.pone.0015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahng AW, Maricic I, Pedersen B, et al. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194(12):1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000;191(11):1895–1903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu YH, Jayawardena J, Weiss A, et al. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J Exp Med. 1999;189(1):103–110. doi: 10.1084/jem.189.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuda JL, Naidenko OV, Gapin L, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192(5):741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowe NY, Uldrich AP, Kyparissoudis K, et al. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J Immunol. 2003;171(8):4020–4027. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 12.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 13.Wilson SB, Delovitch TL. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol. 2003;3(3):211–222. doi: 10.1038/nri1028. [DOI] [PubMed] [Google Scholar]
- 14.Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J Exp Med. 1995;182(4):993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4(3):231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 16.Park SH, Weiss A, Benlagha K, Kyin T, Teyton L, Bendelac A. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J Exp Med. 2001;193(8):893–904. doi: 10.1084/jem.193.8.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Exley MA, Tahir SM, Cheng O, et al. A major fraction of human bone marrow lymphocytes are Th2-like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J Immunol. 2001;167(10):5531–5534. doi: 10.4049/jimmunol.167.10.5531. [DOI] [PubMed] [Google Scholar]
- 18.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199(7):947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arrenberg P, Halder R, Dai Y, Maricic I, Kumar V. Oligoclonality and innate-like features in the TCR repertoire of type II NKT cells reactive to a beta-linked self-glycolipid. Proc Natl Acad Sci USA. 2010;107(24):10984–10989. doi: 10.1073/pnas.1000576107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuss IJ, Heller F, Boirivant M, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113(10):1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girardi E, Maricic I, Wang J, et al. Type II natural killer T cells use features of both innate-like and conventional T cells to recognize sulfatide self antigens. Nat Immunol. 2012;13(9):851–856. doi: 10.1038/ni.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zajonc DM, Maricic I, Wu D, et al. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J Exp Med. 2005;202(11):1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinkai K, Locksley RM. CD1, tuberculosis, and the evolution of major histocompatibility complex molecules. J Exp Med. 2000;191(6):907–914. doi: 10.1084/jem.191.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu L, Van Kaer L. Natural killer T cells and autoimmune disease. Curr Mol Med. 2009;9(1):4–14. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- 25.Ortaldo JR, Young HA, Winkler-Pickett RT, Bere EW, Jr, Murphy WJ, Wiltrout RH. Dissociation of NKT stimulation, cytokine induction, and NK activation in vivo by the use of distinct TCR-binding ceramides. J Immunol. 2004;172(2):943–953. doi: 10.4049/jimmunol.172.2.943. [DOI] [PubMed] [Google Scholar]
- 26.Rhost S, Lofbom L, Rynmark BM, et al. Identification of novel glycolipid ligands activating a sulfatide reactive, CD1d-restricted, type II natural killer T lymphocyte. Eur J Immunol. 2012 doi: 10.1002/eji.201142350. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Hayakawa Y, Rovero S, Forni G, Smyth MJ. Alphagalactosylceramide (KRN7000) suppression of chemical- and oncogene-dependent carcinogenesis. Proc Natl Acad Sci USA. 2003;100(16):9464–9469. doi: 10.1073/pnas.1630663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou D, Mattner J, Cantu C, 3rd, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306(5702):1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 29.Chang DH, Deng H, Matthews P, et al. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood. 2008;112(4):1308–1316. doi: 10.1182/blood-2008-04-149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halder RC, Jahng A, Maricic I, Kumar V. Mini review: immune response to myelin-derived sulfatide and CNS-demyelination. Neurochem Res. 2007;32(2):257–262. doi: 10.1007/s11064-006-9145-4. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian L, Blumenfeld H, Tohn R, et al. NKT cells stimulated by long Fatty acyl chain sulfatides significantly reduces the incidence of type 1 diabetes in nonobese diabetic mice. PLoS One. 2012;7(5):e37771. doi: 10.1371/journal.pone.0037771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shamshiev A, Donda A, Prigozy TI, et al. The alphabeta T cell response to self-glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity. 2000;13(2):255–264. doi: 10.1016/s1074-7613(00)00025-x. [DOI] [PubMed] [Google Scholar]
- 33.Ambrosino E, Terabe M, Halder RC, et al. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179(8):5126–5136. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- 34.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117(8):2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters A, Lee Y, Kuchroo VK. The many faces of Th17 cells. Curr Opin Immunol. 2011;23(6):702–706. doi: 10.1016/j.coi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charlton B, Lafferty KJ. The Th1/Th2 balance in autoimmunity. Curr Opin Immunol. 1995;7(6):793–798. doi: 10.1016/0952-7915(95)80050-6. [DOI] [PubMed] [Google Scholar]
- 37.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 38.Marwaha AK, Leung NJ, McMurchy AN, Levings MK. TH17 cells in autoimmunity and immunodeficiency: protective or pathogenic? Front Immunol. 2012;3:129. doi: 10.3389/fimmu.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uemura Y, Liu TY, Narita Y, et al. Cytokine-dependent modification of IL-12p70 and IL-23 balance in dendritic cells by ligand activation of Valpha24 invariant NKT cells. J Immunol. 2009;183(1):201–208. doi: 10.4049/jimmunol.0900873. [DOI] [PubMed] [Google Scholar]
- 40.Illes Z, Kondo T, Newcombe J, Oka N, Tabira T, Yamamura T. Differential expression of NK T cell V alpha 24J alpha Q invariant TCR chain in the lesions of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J Immunol. 2000;164(8):4375–4381. doi: 10.4049/jimmunol.164.8.4375. [DOI] [PubMed] [Google Scholar]
- 41.van der Vliet HJ, von Blomberg BM, Nishi N, et al. Circulating V(alpha24+) Vbeta11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin Immunol. 2001;100(2):144–148. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- 42.Gigli G, Caielli S, Cutuli D, Falcone M. Innate immunity modulates autoimmunity: type 1 interferon-beta treatment in multiple sclerosis promotes growth and function of regulatory invariant natural killer T cells through dendritic cell maturation. Immunology. 2007;122(3):409–417. doi: 10.1111/j.1365-2567.2007.02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413(6855):531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 45.Furlan R, Bergami A, Cantarella D, et al. Activation of invariant NKT cells by alphaGalCer administration protects mice from MOG35-55-induced EAE: critical roles for administration route and IFN-gamma. Eur J Immunol. 2003;33(7):1830–1838. doi: 10.1002/eji.200323885. [DOI] [PubMed] [Google Scholar]
- 46.Mars LT, Laloux V, Goude K, et al. Cutting edge: V alpha 14-J alpha 281 NKT cells naturally regulate experimental autoimmune encephalomyelitis in nonobese diabetic mice. J Immunol. 2002;168(12):6007–6011. doi: 10.4049/jimmunol.168.12.6007. [DOI] [PubMed] [Google Scholar]
- 47.Singh AK, Wilson MT, Hong S, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194(12):1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teige A, Teige I, Lavasani S, et al. CD1-dependent regulation of chronic central nervous system inflammation in experimental autoimmune encephalomyelitis. J Immunol. 2004;172(1):186–194. doi: 10.4049/jimmunol.172.1.186. [DOI] [PubMed] [Google Scholar]
- 49.Kukreja A, Cost G, Marker J, et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109(1):131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson SB, Kent SC, Patton KT, et al. Extreme Th1 bias of invariant Valpha24JalphaQ T cells in type 1 diabetes. Nature. 1998;391(6663):177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 51.Oikawa Y, Shimada A, Yamada S, et al. High frequency of valpha24(+) vbeta11(+) T-cells observed in type 1 diabetes. Diabetes Care. 2002;25(10):1818–1823. doi: 10.2337/diacare.25.10.1818. [DOI] [PubMed] [Google Scholar]
- 52.Alyanakian MA, Grela F, Aumeunier A, et al. Transforming growth factor-beta and natural killer T-cells are involved in the protective effect of a bacterial extract on type 1 diabetes. Diabetes. 2006;55(1):179–185. [PubMed] [Google Scholar]
- 53.Falcone M, Yeung B, Tucker L, Rodriguez E, Sarvetnick N. A defect in interleukin 12-induced activation and interferon gamma secretion of peripheral natural killer T cells in nonobese diabetic mice suggests new pathogenic mechanisms for insulin-dependent diabetes mellitus. J Exp Med. 1999;190(7):963–972. doi: 10.1084/jem.190.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong S, Wilson MT, Serizawa I, et al. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7(9):1052–1056. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 55.Shi FD, Flodstrom M, Balasa B, et al. Germ line deletion of the CD1 locus exacerbates diabetes in the NOD mouse. Proc Natl Acad Sci USA. 2001;98(12):6777–6782. doi: 10.1073/pnas.121169698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang B, Geng YB, Wang CR. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J Exp Med. 2001;194(3):313–320. doi: 10.1084/jem.194.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cardell SL. The natural killer T lymphocyte: a player in the complex regulation of autoimmune diabetes in non-obese diabetic mice. Clin Exp Immunol. 2006;143(2):194–202. doi: 10.1111/j.1365-2249.2005.02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammond KJ, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG. alpha/beta-T cell receptor (TCR)+CD4-CD8-(NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med. 1998;187(7):1047–1056. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caielli S, Sorini C, Falcone M. The dangerous liaison between iNKT cells and dendritic cells: does it prevent or promote autoimmune diseases? Autoimmunity. 2010;44(1):11–22. doi: 10.3109/08916931003782130. [DOI] [PubMed] [Google Scholar]
- 60.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195(5):637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pakala SV, Kurrer MO, Katz JD. T helper 2 (Th2) T cells induce acute pancreatitis and diabetes in immune-compromised nonobese diabetic (NOD) mice. J Exp Med. 1997;186(2):299–306. doi: 10.1084/jem.186.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kojo S, Adachi Y, Keino H, Taniguchi M, Sumida T. Dysfunction of T cell receptor AV24AJ18+, BV11+ double-negative regulatory natural killer T cells in autoimmune diseases. Arthritis Rheum. 2001;44(5):1127–1138. doi: 10.1002/1529-0131(200105)44:5<1127::AID-ANR194>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 63.Maeda T, Keino H, Asahara H, Taniguchi M, Nishioka K, Sumida T. Decreased TCR AV24AJ18+ double-negative T cells in rheumatoid synovium. Rheumatology (Oxford) 1999;38(2):186–188. doi: 10.1093/rheumatology/38.2.186. [DOI] [PubMed] [Google Scholar]
- 64.Luross JA, Williams NA. The genetic and immunopathological processes underlying collagen-induced arthritis. Immunology. 2001;103(4):407–416. doi: 10.1046/j.1365-2567.2001.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiba A, Oki S, Miyamoto K, Hashimoto H, Yamamura T, Miyake S. Suppression of collagen-induced arthritis by natural killer T cell activation with OCH, a sphingosine-truncated analog of alpha-galactosylceramide. Arthritis Rheum. 2004;50(1):305–313. doi: 10.1002/art.11489. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki S, Utsugisawa K, Iwasa K, et al. Autoimmunity to endoplasmic reticulum chaperone GRP94 in myasthenia gravis. J Neuroimmunol. 2011;237(1–2):87–92. doi: 10.1016/j.jneuroim.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 67.Liu R, La Cava A, Bai XF, et al. Cooperation of invariant NKT cells and CD4+CD25+ T regulatory cells in the prevention of autoimmune myasthenia. J Immunol. 2005;175(12):7898–7904. doi: 10.4049/jimmunol.175.12.7898. [DOI] [PubMed] [Google Scholar]
- 68.Beaudoin L, Laloux V, Novak J, Lucas B, Lehuen A. NKT cells inhibit the onset of diabetes by impairing the development of pathogenic T cells specific for pancreatic beta cells. Immunity. 2002;17(6):725–736. doi: 10.1016/s1074-7613(02)00473-9. [DOI] [PubMed] [Google Scholar]
- 69.Gigante A, Gasperini ML, Afeltra A, et al. Cytokines expression in SLE nephritis. Eur Rev Med Pharmacol Sci. 2011;15(1):15–24. [PubMed] [Google Scholar]
- 70.Akahoshi M, Nakashima H, Tanaka Y, et al. Th1/Th2 balance of peripheral T helper cells in systemic lupus erythematosus. Arthritis Rheum. 1999;42(8):1644–1648. doi: 10.1002/1529-0131(199908)42:8<1644::AID-ANR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 71.Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127(3):385–393. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 72.Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9(8):589–593. doi: 10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- 73.Chuang YP, Wang CH, Wang NC, Chang DM, Sytwu HK. Modulatory function of invariant natural killer T cells in systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:478429. doi: 10.1155/2012/478429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh AK, Yang JQ, Parekh VV, et al. The natural killer T cell ligand alpha-galactosylceramide prevents or promotes pristaneinduced lupus in mice. Eur J Immunol. 2005;35(4):1143–1154. doi: 10.1002/eji.200425861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takahashi T, Strober S. Natural killer T cells and innate immune B cells from lupus-prone NZB/W mice interact to generate IgM and IgG autoantibodies. Eur J Immunol. 2008;38(1):156–165. doi: 10.1002/eji.200737656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wermeling F, Lind SM, Jordo ED, Cardell SL, Karlsson MC. Invariant NKT cells limit activation of autoreactive CD1d-positive B cells. J Exp Med. 2010;207(5):943–952. doi: 10.1084/jem.20091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeng D, Dick M, Cheng L, et al. Subsets of transgenic T cells that recognize CD1 induce or prevent murine lupus: role of cytokines. J Exp Med. 1998;187(4):525–536. doi: 10.1084/jem.187.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang YF, Tomura M, Ono S, Hamaoka T, Fujiwara H. Requirement for IFN-gamma in IL-12 production induced by collaboration between v(alpha)14(+) NKT cells and antigenpresenting cells. Int Immunol. 2000;12(12):1669–1675. doi: 10.1093/intimm/12.12.1669. [DOI] [PubMed] [Google Scholar]
- 79.Chan G, Hanke T, Fischer KD. Vav-1 regulates NK T cell development and NK cell cytotoxicity. Eur J Immunol. 2001;31(8):2403–2410. doi: 10.1002/1521-4141(200108)31:8<2403::aid-immu2403>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 80.Forestier C, Molano A, Im JS, et al. Expansion and hyperactivity of CD1d-restricted NKT cells during the progression of systemic lupus erythematosus in (New Zealand Black x New Zealand White)F1 mice. J Immunol. 2005;175(2):763–770. doi: 10.4049/jimmunol.175.2.763. [DOI] [PubMed] [Google Scholar]
- 81.Diao H, Kon S, Iwabuchi K, et al. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity. 2004;21(4):539–550. doi: 10.1016/j.immuni.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 82.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci USA. 2000;97(10):5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akbari O, Stock P, Meyer E, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9(5):582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 84.Osman Y, Kawamura T, Naito T, et al. Activation of hepatic NKT cells and subsequent liver injury following administration of alphagalactosylceramide. Eur J Immunol. 2000;30(7):1919–1928. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 85.Tsuneyama K, Yasoshima M, Harada K, Hiramatsu K, Gershwin ME, Nakanuma Y. Increased CD1d expression on small bile duct epithelium and epithelioid granuloma in livers in primary biliary cirrhosis. Hepatology. 1998;28(3):620–623. doi: 10.1002/hep.510280303. [DOI] [PubMed] [Google Scholar]
- 86.Kita H, Naidenko OV, Kronenberg M, et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123(4):1031–1043. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- 87.Chuang YH, Lian ZX, Yang GX, et al. Natural killer T cells exacerbate liver injury in a transforming growth factor beta receptor II dominant-negative mouse model of primary biliary cirrhosis. Hepatology. 2008;47(2):571–580. doi: 10.1002/hep.22052. [DOI] [PubMed] [Google Scholar]
- 88.Wu SJ, Yang YH, Tsuneyama K, et al. Innate immunity and primary biliary cirrhosis: activated invariant natural killer T cells exacerbate murine autoimmune cholangitis and fibrosis. Hepatology. 2010;53(3):915–925. doi: 10.1002/hep.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arrenberg P, Maricic I, Kumar V. Sulfatide-mediated activation of type II natural killer T cells prevents hepatic ischemic reperfusion injury in mice. Gastroenterology. 2010;140(2):646–655. doi: 10.1053/j.gastro.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Motohashi S, Kobayashi S, Ito T, et al. Preserved IFN-alpha production of circulating Valpha24 NKT cells in primary lung cancer patients. Int J Cancer. 2002;102(2):159–165. doi: 10.1002/ijc.10678. [DOI] [PubMed] [Google Scholar]
- 91.Dhodapkar MV, Geller MD, Chang DH, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197(12):1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kitamura H, Iwakabe K, Yahata T, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189(7):1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tahir SM, Cheng O, Shaulov A, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167(7):4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 94.Molling JW, Langius JA, Langendijk JA, et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25(7):862–868. doi: 10.1200/JCO.2006.08.5787. [DOI] [PubMed] [Google Scholar]
- 95.Berzofsky JA, Terabe M. NKT cells in tumor immunity: opposing subsets define a new immunoregulatory axis. J Immunol. 2008;180(6):3627–3635. doi: 10.4049/jimmunol.180.6.3627. [DOI] [PubMed] [Google Scholar]
- 96.Renukaradhya GJ, Sriram V, Du W, Gervay-Hague J, Van Kaer L, Brutkiewicz RR. Inhibition of antitumor immunity by invariant natural killer T cells in a T-cell lymphoma model in vivo. Int J Cancer. 2006;118(12):3045–3053. doi: 10.1002/ijc.21764. [DOI] [PubMed] [Google Scholar]
- 97.Smyth MJ, Thia KY, Street SE, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191(4):661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iwamura C, Nakayama T. Role of NKT cells in allergic asthma. Curr Opin Immunol. 2010;22(6):807–813. doi: 10.1016/j.coi.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 99.Umetsu DT, Dekruyff RH. Natural killer T cells are important in the pathogenesis of asthma: the many pathways to asthma. J Allergy Clin Immunol. 2010;125(5):975–979. doi: 10.1016/j.jaci.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim EY, Battaile JT, Patel AC, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14(6):633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pichavant M, Goya S, Meyer EH, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008;205(2):385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meyer EH, Goya S, Akbari O, et al. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci USA. 2006;103(8):2782–2787. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matangkasombut P, Pichavant M, Yasumi T, et al. Direct activation of natural killer T cells induces airway hyperreactivity in nonhuman primates. J Allergy Clin Immunol. 2008;121(5):1287–1289. doi: 10.1016/j.jaci.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang G, Nie H, Yang J, et al. Sulfatide-activated type II NKT cells prevent allergic airway inflammation by inhibiting type I NKT cell function in a mouse model of asthma. Am J Physiol Lung Cell Mol Physiol. 2011;301(6):L975–L984. doi: 10.1152/ajplung.00114.2011. [DOI] [PubMed] [Google Scholar]
- 105.Kadri N, Korpos E, Gupta S, et al. CD4(+) type II NKT cells mediate ICOS and programmed death-1-dependent regulation of type 1 diabetes. J Immunol. 2012;188(7):3138–3149. doi: 10.4049/jimmunol.1101390. [DOI] [PubMed] [Google Scholar]
- 106.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharma MD, Baban B, Chandler P, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117(9):2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9(1):28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 109.Chan AC, Serwecinska L, Cochrane A, Harrison LC, Godfrey DI, Berzins SP. Immune characterization of an individual with an exceptionally high natural killer T cell frequency and her immediate family. Clin Exp Immunol. 2009;156(2):238–245. doi: 10.1111/j.1365-2249.2009.03888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Berzins SP, Kyparissoudis K, Pellicci DG, et al. Systemic NKT cell deficiency in NOD mice is not detected in peripheral blood: implications for human studies. Immunol Cell Biol. 2004;82(3):247–252. doi: 10.1046/j.1440-1711.2004.01238.x. [DOI] [PubMed] [Google Scholar]
- 111.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11(2):131–142. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 112.Borowski C, Bendelac A. Signaling for NKT cell development: the SAP-FynT connection. J Exp Med. 2005;201(6):833–836. doi: 10.1084/jem.20050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Savage AK, Constantinides MG, Han J, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29(3):391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 115.Giaccone G, Punt CJ, Ando Y, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8(12):3702–3709. [PubMed] [Google Scholar]
- 116.Nieda M, Okai M, Tazbirkova A, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103(2):383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 117.Chang DH, Osman K, Connolly J, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201(9):1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ishikawa A, Motohashi S, Ishikawa E, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11(5):1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]