1.0 Introduction
Patients with schizophrenia have shown hyperprolactinemia secondary to the use of antipsychotics, which block dopamine D2 receptors (Kapur et al., 2002). However, studies of drug-naïve patients have also found increased prolactin concentrations (Aston et al., 2010; Lee and Kim, 2006; Segal et al., 2007). A limitation of these studies of antipsychotic-naïve patients is that potentially confounding variables have received little attention.
In addition, an issue of mechanism has not been examined. In contrast to other pituitary hormones that are largely controlled by releasing factors, prolactin secretion is controlled mainly by an inhibiting factor, dopamine (Engler et al., 2009). However, prolactin is also partially regulated by thyrotropin-releasing hormone (TRH) through a extracellular signal-regulated kinase (Oride et al., 2009) and ghrelin, a peptide hormone involved in metabolic homeostasis, (Messini et al., 2010) which stimulates prolactin secretion by a direct action on the pituitary somatomammotroph cells. Prolactin release is also activated during the stress response (Jaroenporn et al., 2007).
We tested the hypothesis that there is an abnormal regulation of prolactin levels in patients with a first episode of nonaffective psychosis prior the use of antipsychotic treatment, and that this could not be attributed to increased concentrations of TSH, ghrelin, or cortisol, or to potentially confounding variables such as age, gender, body mass index (BMI) and smoking.
2.0 Material and Methods
2.1 Subjects
Patients (N=33) with nonaffective psychosis were recruited at the time of their first contact with psychiatric services in a general academic hospital (Hospital Clinic of Barcelona), and were assessed very early in this hospital stay. The first episode psychosis patients met criteria for schizophrenia, schizophreniform disorder, brief psychotic disorder, delusional disorder, or psychosis not otherwise specified. In both groups, at least 75% of the patients were of European Caucasian origin. The psychosis group had a maximum cumulative (lifetime) antipsychotic exposure of 1 week, and no antipsychotic use in the 30 days prior to the study. Control subjects, who were recruited using advertisements, were matched to the patients on the basis of age, gender, body mass index, smoking (which was highly correlated with all drug abuse; data not shown), and socioeconomic status of the family of origin. The matching was done on a group (e.g group mean) rather than individual by individual basis.
Patients were allowed to receive anti-anxiety medication (lorazepam) the night before blood was drawn (but not at any other time prior to blood sampling), to a maximum of 3 mg. In schizophrenia patients the mean effect of 4 mg lorazepam intramuscularly on prolactin levels has been reported to be 3.8 (SD=0.9) μg/l, reaching peak levels in 4 hr (Harvey et al., 2004); 46 % of the patients in the current study had taken lorazepam; these patients took the lorazepam at least 8 hours before blood was drawn.
Additional inclusion and exclusion criteria for all subjects were: 1) age from 18 to 64 years, 2) no history of diabetes or other serious medical or neurological condition associated with glucose intolerance or insulin resistance (e.g. Cushing’s disease), 3) not taking a medication associated with insulin resistance (hydrochlorothiazide, furosemide, ethacrynic acid, metolazone, chlorthalidone, beta blockers, glucocorticoids, phenytoin, nicotinic acid, cyclosporine, pentamidine, or narcotics), and 4) no history of cocaine use in the previous 30 days. Exclusion criteria for the control subjects were no lifetime diagnosis of schizophrenia or major depressive disorder, no current diagnosis of adjustment disorder.
All subjects gave informed consent for participation in the study, which was conducted under the supervision of the authors’ respective ethics committees, and came from a larger study of metabolic abnormalities and glucose dysregulation in neuropsychiatric disorders (Fernandez-Egea et al., 2009a; Fernandez-Egea et al., 2009b; Fernandez-Egea et al., 2011)
2.2 Assessments
All subjects were interviewed using the Spanish translation of the Structured Clinical Interview for DSM-IV (SCID; First and Spitzer, 1999). They were also administered the Dartmouth Assessment of Lifestyle Inventory, which quantifies substance abuse (Rosenberg et al., 1998). Psychopathology was assessed by applying the Positive and Negative Syndrome Scale Spanish version (Peralta and Cuesta, 1994) and the Calgary Depression Scale for Schizophrenia, Spanish version (Sarro et al., 2004). All subjects underwent a blood sampling, which began between 8 and 9 AM after being at rest for at least 15 minutes and an overnight fast. Serum prolactin levels were measured using a chemiluminiscence immunoassay (ADVIA Centaur Bayer, Tarrytown, NY). Inter- and intra-assay coefficients of variation were 4.9% and 4.1% respectively. The sensitivity of the assay was 0.3 ng/mL. Prolactin normal ranges were for male (2–15 ng/mL) and for female (5.4–20 ng/mL).
2.3 Statistical Analysis
In our sample of 33 patients with psychosis and 37 control subjects for whom data were available for all of these variables, we examined the univariate correlation between prolactin concentrations and age, smoking (number of cigarettes per day), socioeconomic status of the family of origin, and body mass index. Gender was not examined, as prolactin concentrations are known in larger populations to differ between men and women. Of these, only age and smoking had a correlation with a p < .20. Based on these results, blind to prolactin value, subjects with psychosis and healthy controls were matched on age, gender, and smoking. Matching was done on a group (e.g. group mean) rather than an individual basis. In the matched groups, prolactin, ghrelin, TSH, and cortisol were examined using the t-test. Because it was not normally distributed, we examined log-transformed prolactin in all analyses. The matched groups had 33 psychosis and 33 control subjects.
In order to assess the robustness of our results from the matched sample, we also conducted a confirmatory analysis in the larger, unmatched sample (33 psychosis subjects and 37 control subjects), using logistic regression, in which the dependent variable was diagnosis (as a 0/1 variable), and the independent variables were log-transformed prolactin, age, gender, and smoking.
All analyses were performed using SPSS (Statistical Package for Social Sciences) for Windows, version 17.0.
3.0 Results
Among the patients, the DSM-IV diagnoses included 18 with schizophrenia, 9 with brief psychotic disorder, 3 with schizophreniform disorder and 3 with psychosis not otherwise specified.
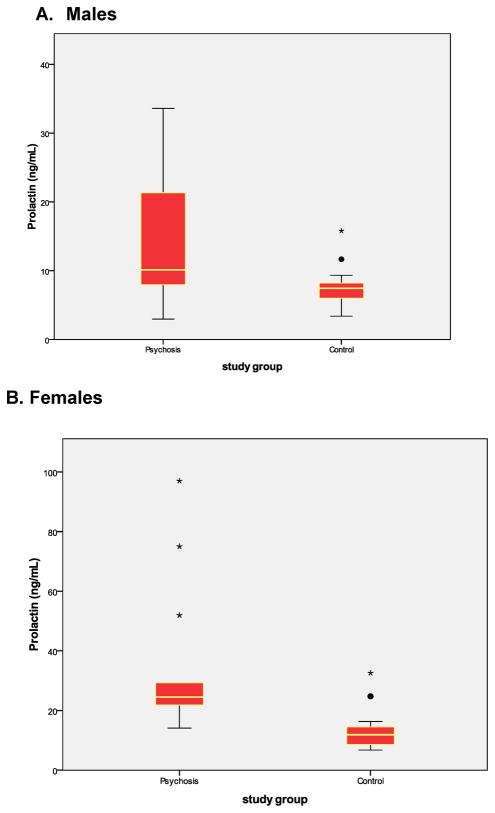
The two matched groups were very similar with regard to age, gender, BMI, smoking, (Table 1). In the matched groups, patients had significantly higher prolactin values, but did not differ with regard to TSH, ghrelin, or cortisol (Table 1). The two groups also differed significantly when divided by gender (Table 2 and Figure).
Table 1.
Clinical and Demographic Characteristics of the Patient and Control Subjects
| Patients (N = 33) | Control subjects (N=33) | p value | |
|---|---|---|---|
|
|
|||
| Mean age | 28.6 (7.1) | 26.8 (5.5) | 0.26 |
| Male/female (% male) | 20/13 (61%) | 21/12 (64%) | 0.80 |
| Mean cigarettes/day | 6.2 (8.0) | 3.7 (6.3) | 0.46 |
| Mean prolactin (ng/mL) | 22.9 (19.4) | 9.8 (5.8) | 0.001 |
| Mean TSH (mUI/L) | 1.8 (1.0) | 1.8 (0.9) | 0.84 |
| Mean ghrelin (pg/mL)* | 1082 (516) | 1156 (304) | 0.54 |
| Mean cortisol (μg/dL)** | 17.6 (6.2) | 19.1 (6.2) | 0.33 |
| Mean BMI*** | 21.8 (4.3) | 23.1(2.9) | 0.17 |
For ghrelin, N=25 for patients and N=28 for control subjects.
For cortisol, N = 33 for patients and N=32 for control subjects.
For BMI, N=30 for patients and N=33 for control subjects.
Table 2.
Prolactin in Male vs. Female Subjects
| Male | Female | |
|---|---|---|
|
|
||
| Psychosis subjects | 15.1 (9.1) (N=20) | 35.1 (24.7) (N=13) |
| Control subjects | 7.6 (2.7) (N=21) | 13.6 (7.7) (N=12) |
| P value | 0.002 | 0.002 |
Figure. Distribution of Prolactin Concentrations in Males and Females.
The colored boxes include the middle 50% of each group. The horizontal line within the colored box represents the median. The data between the colored box and the tails contain the upper and lower quartiles, excluding outliers, which are represented by stars and filled circles.
Within the patient group, there was no significant difference between those who received and those who did not receive lorazepam the night before the blood sampling (respective means were 20.0 (11.1) vs. 25.4 (24.4), p = 0.43).
In the logistic regression analysis with the larger sample, prolactin values were significantly greater in the psychosis group, after controlling for the variance accounted for by gender, age, and smoking (p < .001; other data not shown).
The pattern of results was the same when the patients who did versus those who did not receive lorazepam were examined separately (table not shown).
4.0 DISCUSSION
We found that newly diagnosed antipsychotic-naive patients with schizophrenia and related disorders had higher prolactin concentrations than did matched controls subjects. These differences could not be attributed to gender, age, smoking, socioeconomic status of the family of origin, or BMI. In addition, the two subject groups did not differ with regard to concentrations of TSH, ghrelin, or cortisol.
Our study had a limited sample size. However, a small sample size is more likely to have biased the results toward a negative finding because of insufficient statistical power than to create a false-positive result. In addition, the effect size for the prolactin difference was approximately 1.0 standard deviations, which is greater than the usual value for a “large” effect size. In contrast, the TSH, ghrelin, and cortisol effect sizes were 0.24 or smaller, and the largest of these (for cortisol) was in the direction opposite what would be expected with an increased prolactin concentration. The administration of lorazepam did not appear to limit the validity of our results, as there was no significant difference in prolactin concentrations between those who did and those who did not receive lorazepam. As noted above, any prolactin effect is very small and resolves within eight hours, which is longer than the period of time between administration and blood sampling. Moreover, the group that received lorazepam had slightly (but not significantly) lower prolactin concentrations, a result that would have biased our results towards a falsely negative rather than a falsely positive study.
Others have also reported hyperprolactinemia in newly diagnosed, antipsychotic-naïve patients (Aston et al., 2010; Lee and Kim, 2006; Segal et al., 2007), although the patient and control subjects were less extensively matched on potentially confounding variables such as smoking (Mackin et al., 2011). Our findings suggest that hyperprolactinemia is not due to stress associated with the onset of psychosis, as cortisol levels were similar in both groups, but due to a pre-existing vulnerability. This pre-existing vulnerability of schizophrenia has been described previously in other metabolic areas that contribute also to the secondary side effects of the antipsychotic treatments (Fernandez-Egea et al., 2009a; Fernandez-Egea et al., 2009b; Fernandez-Egea et al., 2011). Nor does the increase appear to be due to the effects of TSH or ghrelin, which also impact prolactin release. Although the mechanism of this increase in prolactin remains unclear, prolactin-releasing peptide (PrRP), a hypothalamic releasing factor in mammals, might account for some of these findings, as its disturbances lead to metabolic disorders (Onaka et al., 2010) similar to those found in first episode psychosis. Another possible mechanism is increased inflammation, which has been reported in antipsychotic-naïve patients with schizophrenia and increases prolactin concentrations (Friedrich et al., 2011; Miller et al., 2011)
A dopaminergic abnormality in the pituitary might also contribute to this abnormality (Engler et al., 2009) Serotonin also helps control prolactin release (Duval et al., 2003) and a serotonergic abnormality might contribute to increased prolactin concentrations as well.
Hyperprolactinemia is related to several adverse clinical effects, galactorrhea, amenorrhea, menstrual irregularities in women, erectile and ejaculatory dysfunction, altered spermatogenesis, reduced muscle mass in men, an increased risk of osteoporosis, and hypogonadism and decreased libido in both sexes (Haddad and Wieck, 2004; Zhang-Wong and Seeman, 2002). Sexual side effects interfere with treatment adherence (Lieberman et al., 2005). Another important consequence of increasing prolactin concentrations is an increased risk of osteopenia, osteoporosis and hip fractures (Stubbs, 2009). Better understanding of the causes of this problem, and clinical management when it appears, would help patients with schizophrenia.
Acknowledgments
Supported in part by grant RO1 DK069265 from the National Institute of Diabetes and Digestive and Kidney Diseases (Dr. Kirkpatrick), NARSAD (Dr. Fernandez-Egea) and by the Government of Catalonia, Comissionat per Universitats i Recerca del Departament d’Innovació, Universitats i Empresa (DIUE) 2009SGR1295 (Dr. Bernardo). The views stated in this article represent those of the authors and are not official statements of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Role of the funding source
This work was supported by grant RO1 DK069265 from the National Institute of Diabetes and Digestive and Kidney Diseases (Dr. Kirkpatrick), NARSAD (Dr. Fernandez-Egea) the Government of Catalonia, Comissionat per Universitats i Recerca del Departament d’Innovació, Universitats I Empresa (DIUE) 2009SGR1295 (Dr. Bernardo) and Fondo de Investigación Sanitaria (registered number PI080055) of the Ministerio de Ciencia e Innovación (Dr. Parellada). The views stated in this article represent those of the authors and are not official statements of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Footnotes
Conflict of Interest
Drs. Garcia-Rizo, Fernandez-Egea, Oliveira and Ms. Justicia have nothing to disclose.; Dr. Parellada has provided consulting services to Janssen-Cilag, Johnson&Johnson and GlaxoSmithKline and he is speaker/advisory board member for Janssen-Cilag. Dr. Bernardo received consultant fees from Bristol-Meyer-Squibb and Wyeth, and honoraria from Janssen-Cilag, Eli Lilly, Pfizer, Synthelab, Glaxo Smith Kline and Astra-Zeneca. Dr. Kirkpatrick received consulting fees from Eli Lilly, Cephalon, Abbott, Boehringer Ingelheim, and Sunovion.
Contributors
Drs. Kirkpatrick, Fernandez-Egea, Parellada and Bernardo designed the study and contributed to analyses and manuscript preparation. Ms Azucena Justicia, Dra. Cristina Oliveira and Dr. Garcia-Rizo were involved in data collection, interpretation, and manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aston J, Rechsteiner E, Bull N, Borgwardt S, Gschwandtner U, Riecher-Rossler A. Hyperprolactinaemia in early psychosis-Not only due to antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Duval F, Mokrani MC, Monreal J, Bailey P, Valdebenito M, Crocq MA, Macher JP. Dopamine and serotonin function in untreated schizophrenia: clinical correlates of the apomorphine and d-fenfluramine tests. Psychoneuroendocrinology. 2003;28:627–642. doi: 10.1016/s0306-4530(02)00047-1. [DOI] [PubMed] [Google Scholar]
- Engler H, Doenlen R, Riether C, Engler A, Niemi MB, Besedovsky HO, del Rey A, Pacheco-Lopez G, Feldon J, Schedlowski M. Time-dependent alterations of peripheral immune parameters after nigrostriatal dopamine depletion in a rat model of Parkinson’s disease. Brain Behav Immun. 2009;23:518–526. doi: 10.1016/j.bbi.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Fernandez-Egea E, Bernardo M, Donner T, Conget I, Parellada E, Justicia A, Esmatjes E, Garcia-Rizo C, Kirkpatrick B. Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. Br J Psychiatry. 2009a;194:434–438. doi: 10.1192/bjp.bp.108.052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Egea E, Bernardo M, Heaphy CM, Griffith JK, Parellada E, Esmatjes E, Conget I, Nguyen L, George V, Stoppler H, Kirkpatrick B. Telomere length and pulse pressure in newly diagnosed, antipsychotic-naive patients with nonaffective psychosis. Schizophr Bull. 2009b;35:437–442. doi: 10.1093/schbul/sbn169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Egea E, Miller B, Garcia-Rizo C, Bernardo M, Kirkpatrick B. Metabolic Effects of Olanzapine in Patients With Newly Diagnosed Psychosis. J Clin Psychopharmacol. 2011;31:154–159. doi: 10.1097/JCP.0b013e31820fcea3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich N, Schneider HJ, Spielhagen C, Markus MR, Haring R, Grabe HJ, Buchfelder M, Wallaschofski H, Nauck M. The association of serum prolactin concentration with inflammatory biomarkers - Cross-sectional findings from the population-based Study of Health in Pomerania. Clin Endocrinol (Oxf) 2011 doi: 10.1111/j.1365-2265.2011.04075.x. [DOI] [PubMed] [Google Scholar]
- Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64:2291–2314. doi: 10.2165/00003495-200464200-00003. [DOI] [PubMed] [Google Scholar]
- Harvey AT, Flockhart D, Gorski JC, Greenblatt DJ, Burke M, Werder S, Preskorn SH. Intramuscular haloperidol or lorazepam and QT intervals in schizophrenia. J Clin Pharmacol. 2004;44:1173–1184. doi: 10.1177/0091270004267807. [DOI] [PubMed] [Google Scholar]
- Helena CV, Cristancho-Gordo R, Gonzalez-Iglesias AE, Tabak J, Bertram R, Freeman ME. Systemic oxytocin induces a prolactin secretory rhythm via the pelvic nerve in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2011 doi: 10.1152/ajpregu.00176.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaroenporn S, Nagaoka K, Kasahara C, Ohta R, Watanabe G, Taya K. Physiological roles of prolactin in the adrenocortical response to acute restraint stress. Endocr J. 2007;54:703–711. doi: 10.1507/endocrj.k07-003. [DOI] [PubMed] [Google Scholar]
- Kapur S, Langlois X, Vinken P, Megens AA, De Coster R, Andrews JS. The differential effects of atypical antipsychotics on prolactin elevation are explained by their differential blood-brain disposition: a pharmacological analysis in rats. J Pharmacol Exp Ther. 2002;302:1129–1134. doi: 10.1124/jpet.102.035303. [DOI] [PubMed] [Google Scholar]
- Lee BH, Kim YK. The relationship between prolactin response and clinical efficacy of risperidone in acute psychotic inpatients. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:658–662. doi: 10.1016/j.pnpbp.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Mackin P, Waton A, Nulkar A, Watkinson HM. Prolactin and smoking status in antipsychotic-treated patients. J Psychopharmacol. 2011 doi: 10.1177/0269881110379289. [DOI] [PubMed] [Google Scholar]
- Messini CI, Dafopoulos K, Chalvatzas N, Georgoulias P, Anifandis G, Messinis IE. Effect of ghrelin and thyrotropin-releasing hormone on prolactin secretion in normal women. Horm Metab Res. 2010;42:204–208. doi: 10.1055/s-0029-1241197. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-Analysis of Cytokine Alterations in Schizophrenia: Clinical Status and Antipsychotic Effects. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaka T, Takayanagi Y, Leng G. Metabolic and stress-related roles of prolactin-releasing peptide. Trends Endocrinol Metab. 2010;21:287–293. doi: 10.1016/j.tem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Oride A, Kanasaki H, Purwana IN, Miyazaki K. Possible involvement of mitogen-activated protein kinase phosphatase-1 (MKP-1) in thyrotropin-releasing hormone (TRH)-induced prolactin gene expression. Biochem Biophys Res Commun. 2009;382:663–667. doi: 10.1016/j.bbrc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ. Psychometric properties of the positive and negative syndrome scale (PANSS) in schizophrenia. Psychiatry Res. 1994;53:31–40. doi: 10.1016/0165-1781(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg SD, Drake RE, Wolford GL, Mueser KT, Oxman TE, Vidaver RM, Carrieri KL, Luckoor R. Dartmouth Assessment of Lifestyle Instrument (DALI): a substance use disorder screen for people with severe mental illness. Am J Psychiatry. 1998;155:232–238. doi: 10.1176/ajp.155.2.232. [DOI] [PubMed] [Google Scholar]
- Sarro S, Duenas RM, Ramirez N, Arranz B, Martinez R, Sanchez JM, Gonzalez JM, Salo L, Miralles L, San L. Cross-cultural adaptation and validation of the Spanish version of the Calgary Depression Scale for Schizophrenia. Schizophr Res. 2004;68:349–356. doi: 10.1016/S0920-9964(02)00490-5. [DOI] [PubMed] [Google Scholar]
- Segal M, Avital A, Berstein S, Derevenski A, Sandbank S, Weizman A. Prolactin and estradiol serum levels in unmedicated male paranoid schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:378–382. doi: 10.1016/j.pnpbp.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Stubbs B. Antipsychotic-induced hyperprolactinaemia in patients with schizophrenia: considerations in relation to bone mineral density. J Psychiatr Ment Health Nurs. 2009;16:838–842. doi: 10.1111/j.1365-2850.2009.01472.x. [DOI] [PubMed] [Google Scholar]
- Zhang-Wong JH, Seeman MV. Antipsychotic drugs, menstrual regularity and osteoporosis risk. Arch Womens Ment Health. 2002;5:93–98. doi: 10.1007/s00737-002-0002-4. [DOI] [PubMed] [Google Scholar]