Abstract
Objective
Schizophrenia has been associated with age-related abnormalities, including abnormal glucose tolerance, increased pulse pressure, increased inflammation, abnormal stem cell signalling and shorter telomere length. These metabolic abnormalities as well as other findings suggest schizophrenia and related disorders might be associated with accelerated aging. Testosterone activity has a progressive decline with increasing age.
Methods
We tested the hypothesis that circulating biologically active testosterone is lower in newly diagnosed, antipsychotic-naïve male patients with nonaffective psychosis than in matched control subjects.
Results
Patients (n=33) were matched to control subjects (n=33) for age, gender, body mass index, socioeconomic status of the family of origin, and smoking. The free androgen index (FAI), a measure of biologically active testosterone, was significantly lower in the psychosis group [mean 57.7%, SD=26.1] than in control subjects [71.6%, 27.0; p=0.04], with an effect size of 0.53. Multivariate analysis also supported the findings. In the psychosis group, FAI had a significant negative correlation with the conceptual disorganization item (r=-0.35, p=0.049), but not with reality distortion (r=-0.21; p=0.24), negative symptoms (r=0.004; p=0.98) or depression (r=-0.014; p=0.94).
Conclusion
Lower testosterone is consistent with accelerated aging in nonaffective psychosis, but further testing of this hypothesis is needed.
Keywords: Schizophrenia, sex hormone, androgen, senescence, aging, first episode
1. Introduction
Schizophrenia has 1% lifetime prevalence in the general population, causing long-term disability to the majority of affected persons. The neurodevelopmental hypothesis of schizophrenia indicates that under a background of genetic vulnerability, intra-uterine insults affect brain development leading to the onset of schizophrenia during adulthood (1). However, the mechanism that link early insults with schizophrenia onset remains elusive.
Intra-uterine insults are associated with other medical illnesses having onset in adulthood, which may represent a model for studying schizophrenia. Epidemiological studies have correlated early insults to type 2 diabetes mellitus (T2DM) onset later in life, leading to the so-called “Barker's thrifty phenotype hypothesis” (2). Molecular pathways underlying the mechanisms by which a suboptimal foetal environment leads to increased risk of T2DM in adulthood suggest a role for oxidative damage and the over-activation of the molecular pathways related to aging (3). In our previous work, we have studied schizophrenia and molecular biomarkers of aging, under the theoretical framework of schizophrenia as a disorder of accelerated aging (4). We reported a reduced telomere content (5) (a proxy of telomere length or TL), significantly lower blood levels of SDF-1α (6), a molecule that is critical in maintenance, mobilization and homing of adult stem cells (7) and we also found increased blood levels of the pro-inflammatory cytokine interleukin-6 (IL-6) (8). Our group has reported an increased prevalence of additional age-related features subjects with schizophrenia, which include abnormal glucose tolerance (8-10) and increased pulse pressure (5). In this study, we aim to study another biological marker of the aging phenotype: androgens.
There is a progressive decline of sex hormone levels in normal aging, starting during the early twenties (11). Testosterone, which is the main androgen in males, is secreted almost exclusively by the Leydig cells of the testes and is largely bound to plasma proteins, with 1–2% free in blood, 40–50% loosely bound to albumin, and 50–60% specifically and strongly bound to the sex hormone-binding globulin (SHBG) (11). Free and albumin-bound testosterones form the two pools that are readily available for biological action. In men, total testosterone blood levels decline on the order to 1.6% per year, whereas SHBG increases around 1.2% per year (12), leading to a decline in the availability of biologically active testosterone. There are several ways of measuring biological active testosterone. The free testosterone index, also known as the free androgen index (FAI), is a widely used indicator of androgen activity, above testosterone total level. FAI represents the ratio of total testosterone to SHBG (expressed as a percentage), and therefore quantifies biologically active, or available, testosterone. The amount of testosterone produced by Leydig cells is primarily under the control of LH. Besides aging, other factors influence the FAI. For instance, obesity and a sedentary lifestyle influence androgens by decreasing SHBG levels; on the other hand, nutritional deficiency increases levels of SHBG (13).
Nonaffective psychosis shares genetic, neuropsychological and neuroimaging abnormalities with schizophrenia (14). As a partial test of the hypothesis that schizophrenia and related disorders are associated with an excess of age-related features, we tested the hypothesis that the FAI is lower in newly diagnosed, antipsychotic-naive male patients with nonaffective psychosis (schizophrenia and related conditions) than in matched control subjects.
2. Materials and methods
2.1 Subjects
Psychotic subjects were consecutively recruited at the time of their first clinical contact for non-affective psychotic symptoms at a general academic hospital (the Hospital Clinic of Barcelona), from November 2006 to January 2010. Inclusion criteria for the psychosis group allowed having a maximum cumulative (lifetime) antipsychotic exposure of one week, and no antipsychotic use in the 30 days prior to the study; although in this study all subjects were drug-naïve. The psychosis subjects were allowed to receive anti-anxiety medication (lorazepam) the night before blood was drawn, to a maximum of 3 mg, but not on the day of assessment. Control subjects were recruited using advertisements. All of the subjects were Caucasian residents of Spain except for 2 Asian and 1 North African subjects in the psychosis group.
Inclusion criteria for all subjects were: 1) male, 2) age from 18 to 64 years, 3) no history of diabetes or other serious medical or neurological condition associated with glucose intolerance or insulin resistance (e.g. Cushing's disease), 4) not taking a medication associated with insulin resistance (hydrochlorothiazide, furosemide, ethacrynic acid, metolazone, chlorthalidone, beta blockers, glucocorticoids, phenytoin, nicotinic acid, cyclosporine, pentamidine, or narcotics), 5) no history of cocaine use in the previous 30 days, 6) have not previously received an antipsychotic or antidepressant medication, except having a maximum cumulative (lifetime) antipsychotic exposure of one week, and no antipsychotic use in the 30 days prior to the study (although in this particular study, all subjects were drug-naïve), 7) No primary diagnosis of drug-induced psychosis. Additional exclusion criteria for the control subjects were 1) no lifetime diagnosis of schizophrenia or major depressive disorder, 2) no current diagnosis of adjustment disorder and 3) no first degree relative diagnosed with a psychotic disorder. All subjects gave informed consent for participation in the study, which was conducted under the supervision of the authors' institutional review boards.
The two subject groups came from a larger cohort of 33 male patients and 42 male control subjects who had participated in a metabolic study described elsewhere (8) and in whom testosterone was assayed. From that larger cohort, blind to testosterone blood levels, all 33 subjects from the psychosis group were selected and matched, as a group, to 33 subjects from the control group for age, BMI, smoking, blood cortisol levels and socioeconomic status of the family of origin. A secondary, confirmatory multiple regression analysis was also conducted in which all of the subjects who had been recruited were included (33 and 42 subjects in the two groups, as noted above), Multiple regression models are explained in the statistical analysis section.
2.2 Metabolic and psychiatric assessment
All subjects were given a two-hour, 75 g oral glucose tolerance test (GTT), which began between 8 and 9 AM after an overnight fast. At baseline, total testosterone blood levels (Roche, Elecsys) and sex hormone-binding globulin (SHBG; Roche, Elecsys) were evaluated. The free androgen index (FAI) was calculated accordingly the formula 100 × [testosterone (mmol/L) / SHBG (mmol/L)]. Cortisol was measured using a radioimmunoassay (Immuchem, Ivoz-Ramet, Belgium). Height, weight, and waist and hip circumference, while wearing underwear and without shoes, were recorded between the baseline and two-hour blood samples.
Within 24 hours of the blood test, all subjects were interviewed using the Spanish translation of the Structured Clinical Interview for DSM-IV Axis I Disorders, clinician version (SCID-I). Two psychiatrists (CGR and EFE) interviewed all subjects. They were also administered the Dartmouth Assessment of Lifestyle Inventory (15), which quantifies substance abuse. Socio-economic status (SES) of the family of origin was assessed with the Hollingshead-Redlich scale (16). The Spanish version of the Positive and Negative Syndromes Scale (PANSS) and the Schedule for the Deficit Syndrome (SDS) (17) were administered only to the psychosis group. PANSS factor scores were calculated as follows: reality distortion (the sum of the delusions and hallucinatory behavior item scores), disorganization [the conceptual disorganization item], negative symptoms (the sum of the scores for the seven items of the negative syndrome subscale) and depression (sum of guilt and depression item scores) (18).
2.3 Statistical analysis
The two matched groups were compared using the non-paired Student's t-test, or the χ2 test for comparisons of proportions. Significance was defined as 2-tailed p<0.05 for all statistical tests, and these were performed using version 16.0 of the Statistical Package for Social Sciences (SPSS) for Windows.
Two separate linear regression analyses were also performed. In the first analysis, the matched sample of 33 patients and controls was included. The second analysis included all of the subjects who had been recruited (i.e., not only those in the two matched groups; n=33 patients and n=42 control subjects). For each analysis, the dependent variable was FAI; the independent variables were diagnosis (patients versus controls as a 0/1 variable), age, BMI, smoking (average number of cigarettes per day), cortisol, and SES. Additional models that included DALI scores for alcohol and illicit drug use and/or fasting glucose blood levels were also performed.
On previous results in factor analytic studies (19) we examined the relationship of domains of psychopathology to FAI using the r Spearman correlation index. Correlation indexes for exploring morphometric and testosterone data were also performed.
3. Results
The two groups were very similar with regard to demographics, BMI, waist to hip ratio, smoking (which in our sample population was correlated with measures of abuse of other drugs, including alcohol; data not shown), SES and cortisol blood levels (Table 1). DSM-IV diagnoses for the 33 subjects were: schizophrenia (n=19; n=17 paranoid subtype and n=2 disorganized subtype), brief psychotic disorder (n=7), schizophreniform disorder (n=3), and psychosis NOS (n=4).
Table 1. Characteristics of the Nonaffective Psychosis and Control Subjects. Matched sample.
| Psychosis (N=33) |
Control (N=33) |
Statistics | p | |
|---|---|---|---|---|
| Mean age [SD] | 27.0 [6.77] | 27.8 [5.19] | t=-0.58 | 0.56 |
| Socio Economic Status [SD]§ | 39.2 [14.95] | 45.5 [14.39] | t=-1.59 | 0.12 |
| Mean body mass index [SD] | 22.5 [4.09] | 23.8 [2.44 | t=-1.56 | 0.12 |
| Mean number cigarettes per day [SD] | 8.3 [9.73] | 5.1 [6.89] | t=1.55 | 0.13 |
| Mean waist/hip ratio [SD] § | 0.85 [0.05] | 0.87 [0.06] | t=-1.39 | 0.17 |
| Fasting glucose [SD] (mg/dL) | 85.4 [7.17] | 86.2 [6.25] | t=-0.77 | 0.64 |
| Two hours glucose during the Glucose Tolerance Test (mg/dL) | 114.6 [34.3] | 83.1 [20.1] | t=4.49 | <0.001 |
| Cortisol blood levels (mg/dL) | 16.3 [5.87] | 18.7 [4.54] | t=-1.859 | 0.07 |
| PANSS – Reality Distortion | 8.73 [2.18] | |||
| PANSS – Negative Symptoms | 23.35 [6.77] | |||
| PANSS – Disorganization | 3.54 [1.44] | |||
| PANSS - Depression | 6.35 [2.50] |
SES were available among 25 subjects in the psychotic group and 31 in the control group. PANSS derived factors are: reality distortion (the sum of the delusions and hallucinatory behavior item scores), disorganization [the conceptual disorganization item], negative symptoms (the sum of the scores for the seven items of the negative syndrome subscale) and depression (sum of item thoughts of guilty and depression)
3.1 Testosterone blood levels
There was a significant difference in the FAI between the two groups (t=-2.123; p=0.04): the psychosis group had a mean FAI [and SD] of 57.7% [26.1], versus 71.6% [27.0] in the control group. This corresponding effect size was 0.52, which represents a medium effect size.
Two other testosterone-related measures, total testosterone and SHBG, did not differ significantly between the two groups. Total testosterone was 22.0 mmol/L [7.7] in the psychosis group and 23.1 [6.4] in the control group (t=-0.63; p=0.53); for SHBG the respective means and standard deviations were 41.7 mmol/L [16.8] and 35.0 [13.3; t=1.73; p=0.09). A comparison of the entire sample (n=33 subjects with psychosis and n=42 controls) yielded similar results (data not shown).
3.2. Multiple variant analyses
In order to confirm our results, a linear regression analysis was performed with the matched sample. FAI was used as the dependent variable and age, smoking habit (average number of cigarettes per day), diagnosis, SES, cortisol and BMI as the independent variables. This model explained 23% of the variance (r2 = 0.23) and showed that diagnosis and age were significant predictors of FAI (Table 2). The linear regression model using the whole sample yielded similar results (data not shown). Other models that included DALI scores for alcohol and illicit drug also yielded similar results (data not shown). An additional multiple regression analysis was performed with fasting glucose as a covariate, which was not a significant predictor of FAI (p=0.926).
Table 2.
Association Between Nonaffective Psychosis and Free Androgen Index (%): Confirmatory Multiple Regression using the matched sample.
| Standardized Coefficients | t | Significance | |
|---|---|---|---|
| Group (psychosis/control) | 0.31 | 2.22 | 0.03 |
| Number of Cigarettes (average per day) | 0.150 | 1.12 | 0.27 |
| Age (in years) | -0.37 | -2.82 | 0.007 |
| Body Mass Index (in Kg/m2) | 0.16 | 1.23 | 0.22 |
| Cortisol (mg/dL) | 0.04 | 0.35 | 0.65 |
| Socio Economic Status | 0.06 | 0.45 | 0.65 |
| Constant | 2.22 | 0.04 |
3.3. Analysis of potential confounding factors
There is biological plausibility for a correlation between fasting glucose levels and FAI. There was no significant correlation between FAI and fasting glucose in the entire sample (r=0.10; p=0.40), or in the psychosis (r=-0.04; p=0.83) or control (r=0.24; p=0.18) groups, separately. BMI did not correlate with either total testosterone blood levels or FAI in the entire sample (r=-0.08; p=0.50 and r=-0.19; p=0.10, respectively) or in the psychosis (r=-0.06; p=0.72 and r=-0.22; p=0.21, respectively) or control (r=-0.09; p=0.63 and r=-0.22, p=0.22) groups, separately. We did not find a correlation between FAI and waist to hip ratio (WHR) (r=0.08, p=0.55) in the entire sample, or after stratifying by groups (psychosis, r=0.08, p=0.72; controls r=-0.04, p=0.82). Total testosterone was not correlated with WHR in the psychosis group (r=-0.23; p=0.30), but was significantly correlated with WHR the total sample (r=-0.31; p=0.01) and in controls (r=-40; p=0.02).
3.4. FAI versus age
Figure 1 plots FAI versus age in the two groups. Spearman's rho correlation coefficient was significant for the entire sample (r=-0.23; p=0.047), and within psychosis (r=-.40: p=0.03). By contrast, in control subjects, FAI was not correlated with age either in the matched sample (n=33, r=-0.198; p=0.214) or in the entire sample (n=42, r=-0.17; p=-0.33). Total testosterone was also significantly negatively correlated with age (r=-0.29, p=0.02). SHBG was not correlated with age in the whole sample (r=0.40; p=0.70).
Figure 1. Individual FAI scores and subjects' age.
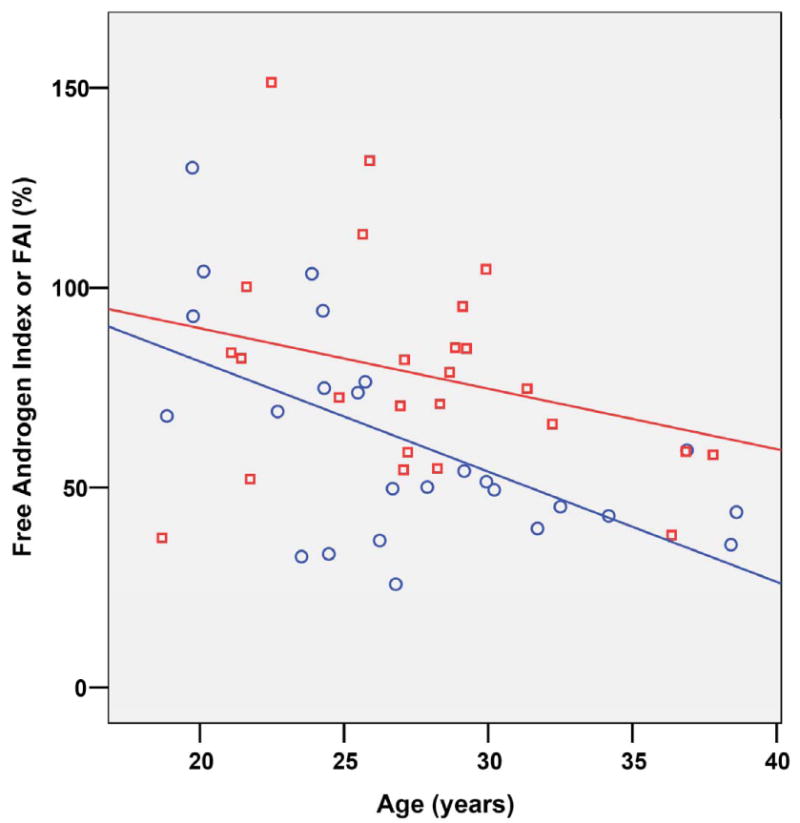
Red squares marks healthy controls subjects whereas blue diamonds marks patients diagnosed with non affective psychosis. Lines marks the r lineal adjustment for each subsample.
In the psychosis group, FAI had a significant negative correlation with the conceptual disorganization item (r=-0.35, p=0.049), but not with reality distortion (r=-0.21; p=0.24), negative symptoms (r=-0.004; p=0.98) or depression (r=-0.01; p=0.94).
4. Discussion
In this study, newly diagnosed antipsychotic-naïve men with schizophrenia and related disorders had a lower concentrations of biologically active testosterone, as measured by FAI, than matched control subjects. These differences could not be attributed to confounding by BMI, age, psychotropic medications, cortisol, fasting glucose blood levels, socioeconomic status or smoking. In our primary analysis, the study sample was chosen from a larger dataset and some subjects were excluded; however, a multiple regression analysis in both the matched and the entire sample showed a similar pattern of results. We believe this is the first report of reduced biologically active testosterone in newly diagnosed, antipsychotic-naïve patients with nonaffective psychosis compared to matched control subjects.
FAI is widely used as an approach for measuring active testosterone, and decreases with aging (12). FAI is a better measure of testosterone activity than is direct total testosterone blood levels or SHGB, as the fraction actually available for biological action may vary with the tissue as well as other factors, including fat mass, loss of muscle and bone mass, or insulin resistance (13). As expected, total testosterone was lower in the psychotic group (although not statistically significant) and SHBG was higher in the psychotic group (p=0.08, no statistically significant). Lack of statistical difference might be due to relatively small sample size. In our sample, FAI and total testosterone did not correlate with BMI, either in the whole sample or after splitting by group. We found a correlation between waist to hip ratio only in control subjects and total testosterone (not FAI) which is reported in the literature (20). Psychotic subjects did not show this correlation, supporting the view that they present a different metabolic pattern.
Our results are consistent with previous reports of reduced total and free testosterone blood levels in patients with schizophrenia (21-29). However, in these other studies, the schizophrenia subjects had been chronically exposed to antipsychotic medications. Since antipsychotics increase the risk of diabetes, and diabetes is associated with low testosterone concentrations (30, 31), these drugs are an important potentially confounding variable. One study did include several drug-naïve subjects, but they were not evaluated as an independent subgroup (24), while another study included only drug naïve patients, but there was no control group (25).
Some previous studies (21, 22, 26, 28) focused on the potential relationship between low testosterone levels and negative symptoms, which have led to three treatment trials with testosterone supplementation, targeting negative symptoms (32, 33). Despite promising initial results (32, 33), a recent meta-analysis indicates an overall lack of efficacy of testosterone for negative symptoms (34). We did not replicate the association between the severity of negative symptoms and testosterone. Instead, we found a negative correlation between FAI and disorganization. Perhaps some of the reports of an association between negative symptoms and low testosterone were due to secondary negative symptoms caused by disorganization, as previous studies did not consider disorganization separately. In turn, as all our cases were acutely psychotic, this may have masked primary negative symptoms. A few reports have also been published regarding depressive symptoms and lower testosterone levels in both young and old adults with depression (35). We did not find a correlation between depression and FAI in our study; however, few of our patients would have met criteria for a depressive episode. As both diabetes (36) and low testosterone (35) have been previously linked to depression the relationship of testosterone to depression in patients with psychosis deserves further exploration.
Our study presents some limitations, as a comprehensive assessment of the hypothalamic-pituitary-gonadal (HPG) axis was not available. Therefore, blood levels of testosterone regulators such as LH, FSH or estradiol were not assessed. However, our study was a hypothesis-driven analysis of a well-known index (FAI) than decreases with age, rather than an analysis of the HPG axis. Direct measure of Free Testosterone was not available at the time of the study. However, as mentioned earlier, FAI is a widely used measure for the biologically active testosterone. Cortisol blood levels were non-significantly lower in the psychotic group compared to the control group. However, the absolute blood levels of cortisol in the psychotic group weren't low (16.2 mg/dL) and the lack of difference is due to the control group having a moderate-high level of cortisol. This rules out hypercortisolemia as a confounding factor of our findings. An intrinsic limitation of the study is related to the cross-sectional design, and therefore further studies should assess the fluctuation of FAI over time or whether these abnormalities precede the onset of the disorder. Additionally, FAI was not correlated with age in the control group. We consider that a likely explanation is the restricted variance in age controls and the relative small numbers. Another limitation of the study relies on the lack of the level of physical activity assessment, as it may have an influence over testosterone levels. However, both groups were quite young. The psychotic group had a recent onset of their psychosis, and therefore is more likely that the impact of drug-induced sedation or lack of activity were not present. In any case, future studies should consider more carefully the degree of physical activity.
Our findings of decreased testosterone activity are consistent with other evidence for accelerated aging in schizophrenia reported by our group and others (4, 5, 8, 37). As a group, people with schizophrenia suffer premature death compared to the general population (38). No doubt, medication side effects, an increased suicide rate, poor health care, and poor health habits all make substantial contributions to this increased mortality. However, the existence of these factors does not exclude the possibility of a pre-existing vulnerability to other medical comorbidity, which needs further study. Should accelerated aging be substantiated, targeting aging pathways might become a new treatment approach to some of the metabolic problems found in schizophrenia.
Abbreviations
- FAI
free androgen index
- SHBG
sex hormone-binding globulin
- PANSS
positive and negative syndrome scale
- GTT
Glucose tolerance test
- SES
socio-economic status
- BMI
body mass index
- WHR
waist to hip ratio
Footnotes
Declaration of interest: Supported in part by grant RO1 DK069265 from the National Institute of Diabetes and Digestive and Kidney Diseases (Dr. Kirkpatrick), NARSAD (Dr. Fernandez-Egea) and by the Government of Catalonia, Comissionat per Universitats I Recerca del Departament d'Innovació, Universitats I Empresa (DIUE) 2009SGR1295, and by the Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red de Salud Mental, CIBERSAM (Dr. Bernardo). The views stated in this article represent those of the authors and are not official statements of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Drs. Fernandez-Egea, Garcia-Rizo and Miller and Ms. Azucena Justicia have no conflict of interest to disclose. Dr. Bernardo received consultant fees from Bristol-Meyer-Squibb and Wyeth. He also received honoraria from Janssen-Cilag, Eli Lilly Company, Pfizer, Synthelabo, Glaxo Smith Kline and Astra-Zeneca. Dr. Parellada received research grants and consultant fees from Janssen-Cilag and GlaxoSmithKline, and served on the speakers/advisory boards for Janssen-Cilag. Dr. Kirkpatrick received consulting and/or speaking fees from Pfizer, Organon, AstraZeneca, Wyeth, Lilly, Bristol Myers Squibb, Cephalon, and Solvay.
References
- 1.Picchioni MM, Murray RM. Schizophrenia. BMJ. 2007;335(7610):91–5. doi: 10.1136/bmj.39227.616447.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Chen JH, Hales CN, Ozanne SE. DNA damage, cellular senescence and organismal ageing: causal or correlative? Nucleic Acids Res. 2007;35(22):7417–28. doi: 10.1093/nar/gkm681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophrenia bulletin. 2008;34(6):1024–32. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Egea E, Bernardo M, Heaphy CM, Griffith JK, Parellada E, Esmatjes E, Conget I, Nguyen L, George V, Stöppler H, Kirkpatrick B. Telomere length and pulse pressure in newly diagnosed, antipsychotic-naive patients with nonaffective psychosis. Schizophrenia bulletin. 2009;35(2):437–42. doi: 10.1093/schbul/sbn169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Egea E, Bruna A, Garcia-Rizo C, Bernardo M, Kirkpatrick B. Stem cell signaling in newly diagnosed, antipsychotic-naive subjects with nonaffective psychosis. Mol Psychiatry. 2009;14(11):989–91. doi: 10.1038/mp.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20(11):1915–24. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Egea E, Bernardo M, Donner T, Conget I, Parellada E, Justicia A, Esmatjes E, Garcia-Rizo C, Kirkpatrick B. Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. The British journal of psychiatry. 2009;194(5):434–8. doi: 10.1192/bjp.bp.108.052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Egea E, Bernardo M, Parellada E, Justicia A, Garcia-Rizo C, Esmatjes E, Conget I, Kirkpatrick B. Glucose abnormalities in the siblings of people with schizophrenia. Schizophr Res. 2008;103(1-3):110–3. doi: 10.1016/j.schres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Egea E, Miller B, Bernardo M, Donner T, Kirkpatrick B. Parental history of type 2 diabetes in patients with nonaffective psychosis. Schizophr Res. 2008;98(1-3):302–6. doi: 10.1016/j.schres.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26(6):833–76. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 12.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 13.Simm A, Nass N, Bartling B, Hofmann B, Silber RE, Navarrete Santos A. Potential biomarkers of ageing. Biol Chem. 2008;389(3):257–65. doi: 10.1515/BC.2008.034. [DOI] [PubMed] [Google Scholar]
- 14.Lichtermann D, Karbe E, Maier W. The genetic epidemiology of schizophrenia and of schizophrenia spectrum disorders. Eur Arch Psychiatry Clin Neurosci. 2000;250(6):304–10. doi: 10.1007/s004060070005. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg SD, Drake RE, Wolford GL, Mueser KT, Oxman TE, Vidaver RM, Carrieri KL, Luckoor R. Dartmouth Assessment of Lifestyle Instrument (DALI): a substance use disorder screen for people with severe mental illness. The American journal of psychiatry. 1998;155(2):232–8. doi: 10.1176/ajp.155.2.232. [DOI] [PubMed] [Google Scholar]
- 16.Hollishead A, Rendlich S. Social Class and Mental Illness. New York: 1958. [Google Scholar]
- 17.Bernardo M, Fernandez-Egea E, Torras A, Gutierrez F, Ahuir M, Arango C. Adaptation and validation into Spanish of Schedule for the Deficit Syndrome. Med Clin (Barc) 2007;129(3):91–3. doi: 10.1157/13107363. [DOI] [PubMed] [Google Scholar]
- 18.Cuesta MJ, Peralta V. Psychopathological dimensions in schizophrenia. Schizophrenia bulletin. 1995;21(3):473–82. doi: 10.1093/schbul/21.3.473. [DOI] [PubMed] [Google Scholar]
- 19.Levine SZ, Rabinowitz J. Revisiting the 5 dimensions of the Positive and Negative Syndrome Scale. Journal of clinical psychopharmacology. 2007;27(5):431–6. doi: 10.1097/jcp/.0b013e31814cfabd. [DOI] [PubMed] [Google Scholar]
- 20.Tang YJ, Lee WJ, Chen YT, Liu PH, Lee MC, Sheu WHH. Serum testosterone level and related metabolic factors in men over 70 years old. J Endocrinol Invest. 2007;30(6):451–8. doi: 10.1007/BF03346327. [DOI] [PubMed] [Google Scholar]
- 21.Ko YH, Jung SW, Joe SH, Lee CH, Jung HG, Jung IK, Kim SH, Lee MS. Association between serum testosterone levels and the severity of negative symptoms in male patients with chronic schizophrenia. Psychoneuroendocrinology. 2007;32(4):385–91. doi: 10.1016/j.psyneuen.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Akhondzadeh S, Rezaei F, Larijani B, Nejatisafa AA, Kashani L, Abbasi SH. Correlation between testosterone, gonadotropins and prolactin and severity of negative symptoms in male patients with chronic schizophrenia. Schizophr Res. 2006;84(2-3):405–10. doi: 10.1016/j.schres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Huber TJ, Tettenborn C, Leifke E, Emrich HM. Sex hormones in psychotic men. Psychoneuroendocrinology. 2005;30(1):111–4. doi: 10.1016/j.psyneuen.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Ozcan ME, Banoglu R. Gonadal hormones in schizophrenia and mood disorders. Eur Arch Psychiatry Clin Neurosci. 2003;253(4):193–6. doi: 10.1007/s00406-003-0424-7. [DOI] [PubMed] [Google Scholar]
- 25.Cesková E, Prikryl R, Kaspárek T. Testosterone in first-episode schizophrenia. Neuro Endocrinol Lett. 2007;28(6):811–4. [PubMed] [Google Scholar]
- 26.Goyal RO, Sagar R, Ammini AC, Khurana ML, Alias AG. Negative correlation between negative symptoms of schizophrenia and testosterone levels. Ann N Y Acad Sci. 2004;1032:291–4. doi: 10.1196/annals.1314.042. [DOI] [PubMed] [Google Scholar]
- 27.Räsänen P, Hakko H, Visuri S, Paanila J, Kapanen P, Suomela T, Tiihonen J. Serum testosterone levels, mental disorders and criminal behaviour. Acta psychiatrica Scandinavica. 1999;99(5):348–52. doi: 10.1111/j.1600-0447.1999.tb07240.x. [DOI] [PubMed] [Google Scholar]
- 28.Shirayama Y, Hashimoto K, Suzuki Y, Higuchi T. Correlation of plasma neurosteroid levels to the severity of negative symptoms in male patients with schizophrenia. Schizophr Res. 2002;58(1):69–74. doi: 10.1016/s0920-9964(01)00367-x. [DOI] [PubMed] [Google Scholar]
- 29.Taherianfard M, Shariaty M. Evaluation of serum steroid hormones in schizophrenic patients. Indian journal of medical sciences. 2004;58(1):3–9. [PubMed] [Google Scholar]
- 30.Saad F, Gooren L. The role of testosterone in the metabolic syndrome: a review. J Steroid Biochem Mol Biol. 2009;114(1-2):40–3. doi: 10.1016/j.jsbmb.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Goncharov NP, Katsya GV, Chagina NA, Gooren LJ. Three definitions of metabolic syndrome applied to a sample of young obese men and their relation with plasma testosterone. The aging male : the official journal of the International Society for the Study of the Aging Male. 2008;11(3):118–22. doi: 10.1080/13685530802204629. [DOI] [PubMed] [Google Scholar]
- 32.Nachshoni T, Ebert T, Abramovitch Y, Assael-Amir M, Kotler M, Maayan R, Weizman A, Strous RD. Improvement of extrapyramidal symptoms following dehydroepiandrosterone (DHEA) administration in antipsychotic treated schizophrenia patients: a randomized, double-blind placebo controlled trial. Schizophr Res. 2005;79(2-3):251–6. doi: 10.1016/j.schres.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 33.Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, Weizman A. Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Arch Gen Psychiatry. 2003;60(2):133–41. doi: 10.1001/archpsyc.60.2.133. [DOI] [PubMed] [Google Scholar]
- 34.Elias A, Kumar A. Testosterone for schizophrenia. Cochrane database of systematic reviews (Online) 2007;(3) doi: 10.1002/14651858.CD006197.pub2. CD006197. [DOI] [PubMed] [Google Scholar]
- 35.Almeida OP, Yeap BB, Hankey GJ, Jamrozik K, Flicker L. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch Gen Psychiatry. 2008;65(3):283–9. doi: 10.1001/archgenpsychiatry.2007.33. [DOI] [PubMed] [Google Scholar]
- 36.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31(12):2383–90. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang B, Chang WI, Lanigan CM, Dean B, Sutcliffe JG, Thomas EA. Normal human aging and early-stage schizophrenia share common molecular profiles. Aging Cell. 2009;8(3):339–42. doi: 10.1111/j.1474-9726.2009.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123–31. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]


