Summary
I review recent studies that connect development and evolution of skull bones in teleosts. Development uses genetic information to build a structured, modular phenotype, and since selection acts on the phenotype, developmental modularity may influence evolvability. Just how is a complex developing morphology spatially partitioned into modules? Here I briefly examine cellular, molecular genetic, and multivariate statistical approaches to the identification of developmental modules. Furthermore I review our evidence that developmental modularity provides evolutionarily labile regions within the skull and hence potentially biases evolutionary change in a positive manner. This view is rather different from early ones in the field of evolutionary developmental biology, in which developmental constraint due to patterns such as heterochronies were supposed to negatively impact evolution.
Introduction
A key issue in evolutionary developmental biology is how development might contribute to evolvability (Hendrikse et al., 2007). Development is situated between genotype, the ultimate source of phenotypic variation, and phenotype – upon which selection necessarily acts. Hence development would seem to be in a perfect position strategically to be able to bias evolutionary change. That it does so, is a view assumed by many authors, essentially following from the famous ‘spandrals’ paper of Gould and Lewontin (1979). This paper was possibly the first to capture the interest of the scientific community in developmental ‘constraint’, usually defined to mean that certain morphologies are inaccessible – essentially impossible for development to make, whether they would be adaptive or not (reviewed in Olson, 2012). However, Gould, particularly in his later writings (e.g. Gould, 2002) also promoted that constraint can act positively, not just negatively, in evolutionary change.
Just how does development ‘structure the variation upon which selection acts’ (Hallgrímsson et al., 2009), and thus potentially play into evolvability? Heterochrony, a change in developmental timing between ancestor and descendant, is a famous example – often cited as a way that development could constrain evolution. Heterochrony might utilize global timing regulatory mechanisms and if so “may yield extensive consequences for entire organismal phenotypes, as suites of correlated characters change in concert with altered rates of development” (Gould, 2002; p. 1039). As a way to “move sets of characters quickly” heterochrony would be “utilizing constraint as a positive accelerator of evolutionary change” (op. cit.). Here, rather than considering the whole organism level, I take a ‘simpler system’ approach, examining development and evolution of just a single skull bone, the opercle, which along with the subopercle provides a flexible gill cover support in most teleosts. Among teleosts, renowned for their marvelous diversity, the opercle, and neighboring skull bones, come in a great variety of shapes and sizes (Fig. 1). In fact, the observed variety makes one question the notion of impossible morphologies due to constraint. I arrive at a distinctive view than the one championed by Gould, namely we see that heterochrony can have very local effects within the organism. Furthermore, I argue that for heterochrony to be regionally restricted in this manner means that developmental control could be modular rather than global, acting largely separately region-by-region, where the semiautonomous regions are defined to be developmental modules (Klingenberg, 2009, Hallgrímsson et al., 2009). I present this argument by first demonstrating with zebrafish that the pattern of morphological development of the opercle shows modularity. Then, with the same organism, I argue that modularity is also evident in the genetic regulation of opercle morphogenesis. I then turn to stickleback to examine opercle evolution and its developmental basis. The stickleback work reveals heterochrony, and that developmental modularity within the opercle serves to dissociate from one another what otherwise might be a suite of correlated characters evolving together. In this more nuanced view of the role of development in evolution, developmental structuring of variation into modules could provide for evolvability by facilitating very local morphological adaptations in response to selection (Jamniczky et al., 2014).
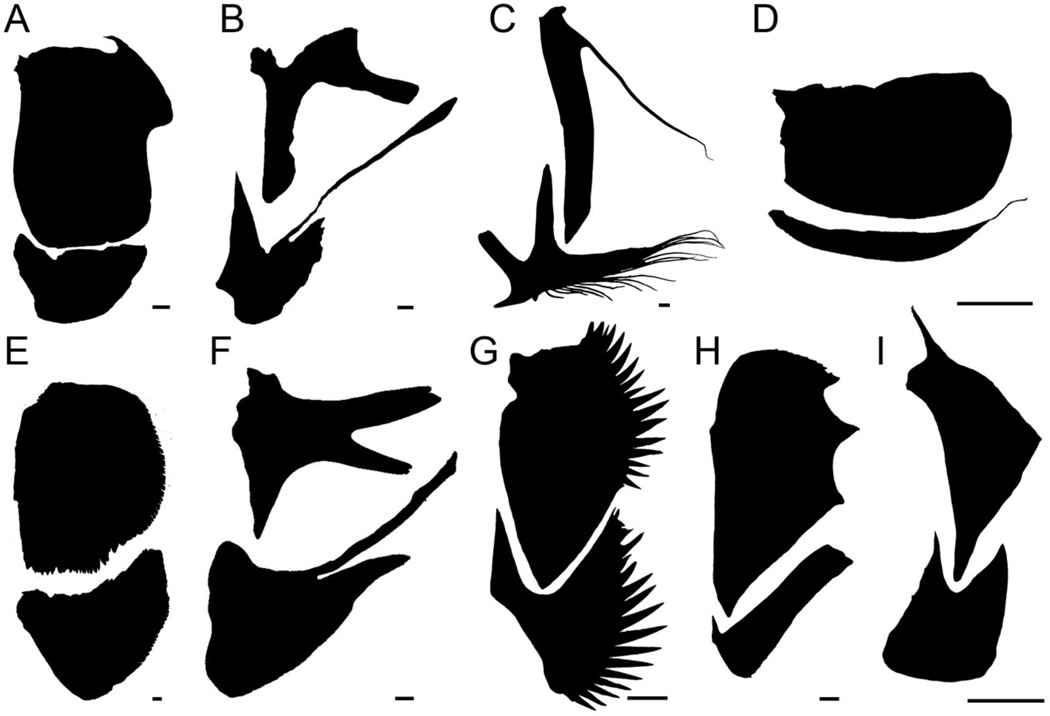
Fig. 1.
Diversity of teleost fish gill cover-supporting bones. Each pair of bones (opercle upper, subopercle lower, disarticulated by dissection) is from a species of a different teleost family (of which there are more than 400). A. Goldeye Hiodon alosoides, Hiodontidae. B. Tidepool sculpin Oligocottus maculosus, Cottidae. C. Goosefish Lophius americanus, Lophiidae. D. Northern pipefish Syngnathus fuscus, Syngnathidae. E. Pink salmon Oncorhynchus gorbuscha, Salmonidae. F. Toadfish Opsanus tau, Batrachoididae. G. Yellowtail clownfish Amphiprion clarkia, Pomacentridae. H. Alfonsino Beryx decadactylus, Berycidae. I. Spotted puffer Tetraodon sp., Tetraodontidae. Scale bars: 0.2 mm for B and 2 mm for the others.
Modularity in the spatial-temporal pattern of opercle development
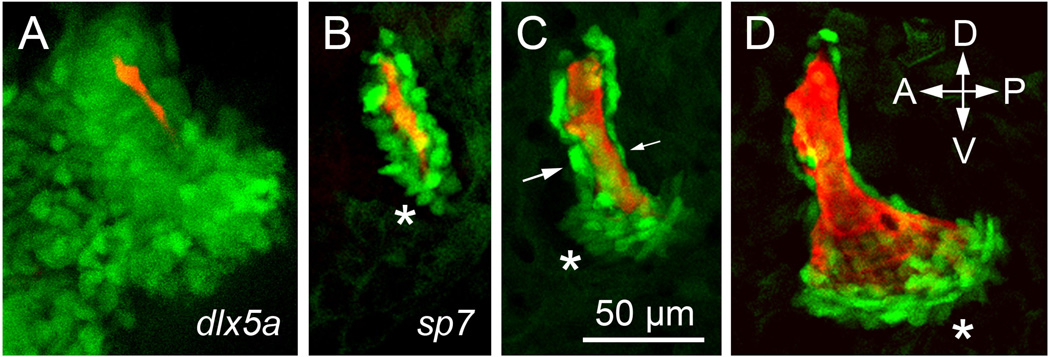
As is well known, early development of skeletal elements – bones and cartilages – occurs within mesenchymal condensations (Hall and Miyake, 2000). But, considering these condensations, just how is patterning achieved so that the element grows, and characteristically changes shape as it grows, to eventually reach the size and shape characterizing the mature morphology? We have followed the developmental trajectory of skeletal element morphogenesis most carefully with the zebrafish opercle. A major part of morphogenetic patterning is in when and where osteoblasts differentiate within the mesenchymal condensation. Here we consider the first two phases of morphogenesis, occurring over just two days (Fig. 2). At such early stages the opercle mesenchymal condensation, visualized in the living larva with transgenic markers, is a multilayered cloud of mostly pre-skeletal cells surrounding the early developing bone (Fig. 2A). In contrast, at the same stage, differentiating matrix-secreting osteoblasts are arranged mostly in a cellular monolayer lining the bone matrix (Kimmel et al., 2010; Fig. 2B). Hence only a small subset of the bone lineage cells of the preskeletal condensation has been selected to differentiate into osteoblasts at this stage. Time-lapse analyses in intact embryos and newly hatched larvae show that when and where the pre-osteoblasts are selected for the transition to the active matrix-secreting osteoblasts is a highly dynamic and apparently exquisitely regulated process. At the onset of phase1, a single osteoblast, or a small cluster of osteoblasts differentiate. The first matrix secreting cells are always located at the site where the opercle will soon make an articulating joint with the hyosymplectic cartilage. Defining phase 1, opercle outgrowth is then linear; new osteoblasts are recruited successively at the ventral end of the cluster, making a ventral growing tip that continues to extend (Fig. 2B, asterisk). Notice that just at the growing tip the labeled cells are present in a small multilayer – this clustering signaling the rapid addition of new cells just at the tip. Within a day, at a rather abrupt transition to phase 2, newly differentiated osteoblasts begin be added to the anterior and posterior surfaces of the growing tip (Fig. 2C, asterisk). The new pattern of addition locally broadens the ventral region, which generates a fan shape (Fig. 2D) as active outgrowth continues. The other, older, regions of the bone, where osteoblasts are monolayered, grow more slowly: Notice in Fig. 2C that the osteoblasts lining the anterior side of the nascent bone are round (large arrow), while those on the posterior side are flattened (small arrow). This difference, (not observed in every instance) signals what we observe to be a second level of local matrix-growth control; the rounded osteoblasts being the more active in bone secretion than the flattened ones (Kimmel et al., 2010). Hence regulation seems to include not only where and when osteoblasts differentiate, but also spatiotemporal control of the level of matrix secretion once they have differentiated.
Fig. 2.
Early morphogenesis of the zebrafish opercle. Live confocal imaging in Danio rerio larvae with bone matrix vitally stained with Alizarin Red, and transgenic labeling (green) of associated cells. A. Ectomesenchyme of the skeletal condensation labeled by expression of dlx5a:eGFP at 3 days post fertilization (dpf). Original image by Sawyer Watkins. B-D. Osteoblasts labeled labeled transgenically by expression of sp7:eGFP at 3, 4 and 5 dpf. Asterisks: local expansion of osteoblast numbers at the actively growing ventral bone edge. Arrows in C: cells of different shapes line the anterior and posterior edges of the bone. Imaging by Tyler Huycke, from the zebrafish developmental ‘FishFace’ atlas https://www.facebase.org/fishface/home.
The conclusion from these analyses, reinforced by examining phases after the early period described here (Kimmel et al., 2010), is that the developmental trajectory of bone formation consists of a stereotyped sequence of seemingly compartmental steps. We have interpreted the compartments – discrete regions within the single forming bone – to be developmental modules, where modules are defined as semiautonomous units of morphological patterning (Wagner, 1996; Wagner and Altenberg, 1996; Klingenberg, 2008, Hallgrímsson et al., 2009). Here the modules are specific local regions of outgrowth of a single bone, and sequence of bone addition is reminiscent of other developmentally modular processes. For example, the mammalian mandible also shows modular patterning (Klingenberg, 2009 and references therein). Trunk somites, perhaps the clearest example of developmental modules in vertebrates, develop in a spatial-temporal sequence. Under control of a cycling gene network, somites appear sequentially along the body axis (Pourquié, 2011; Naiche et al., 2011). Similarly, a tetrapod limb is sequentially patterned in a compartment-like manner (stylopod, zeugopod, autopod), exhibits sequential outgrowth along the limb proximodistal axis (Shubin et al., 1997), and has a covariance structure indicating modularity (Young and Hallgrímsson (2005). However, in the case of the opercle, in contrast to somites and vertebrate limbs, opercle developmental modules are not organized along any single axis. Furthermore, the opercular modules are not evident in the eventual morphology of the bone in the way somites and limb compartments are: Indeed, the early opercle modules are completely obscured by later ones as bone grows outward, appositionally, from its edges. The ‘overwriting’ of developmentally early modules by later ones supports theoretical argument for complexity in developmental modular organization, encapsulated in a ‘palimpsest’ hypothesis of development (Hallgrímsson et al., 2009). Hence it is clear that developmental analyses, not just adult morphological analyses, are necessary for understanding developmental modular patterning (Hallgrímsson et al., 2009).
Modularity in the molecular genetic control of opercle development
Mutational analyses with zebrafish are beginning to reveal the way that genes are brought into play to control the local regulation of opercle osteoblast appearances and activities. By the definition that modules are semiautonomous, we would predict that sets of genetic regulatory mechanisms underlying the special features of individual modules are, at least to some extent, distinctive from one another. Thus different combinations of Hox genes function in different blocks of somites and in different limb compartments (Carroll, 1995; Shubin et al., 1997). Our analyses of how the early developmental modules of the opercle can be perturbed by mutations also reveal such genetic independence.
In our clearest example, mutational analysis shows that the signaling protein Indian hedgehog-a (Ihha) functions as an activator of opercle development. The onset of this control begins at the initiation of phase 2, this timing itself suggesting modularity (Huycke et al., 2012). Moreover, the ihha mutant phenotype is highly positionally specific; ventral outgrowth is markedly reduced whereas, for example, elongation of the dorsal edge is unaffected (Fig 3A, B). This specificity illustrates a key feature of modular patterning – dissociability (Needham, 1933; Raff and Raff, 2000): A module is broken and the observable effect is localized to that module. In consequence, development of one region of the bone accordingly is dissociated from that of other regions with which it is correlated in the wild type. Time-lapse and marker analyses show the cellular defect: Ventral opercle osteoblasts normally express ihha specially, and signaling is required just at this region for pre-osteoblasts to divide at their normal levels. Hence in the ihha mutant, pre-osteoblast cell division and, as a consequence, local osteoblast recruitments are both reduced (Huycke et al., 2012). Thus fewer cells are locally available to undergo osteoblast differentiation and contribute to outgrowth of the opercle ventral posterior edge.
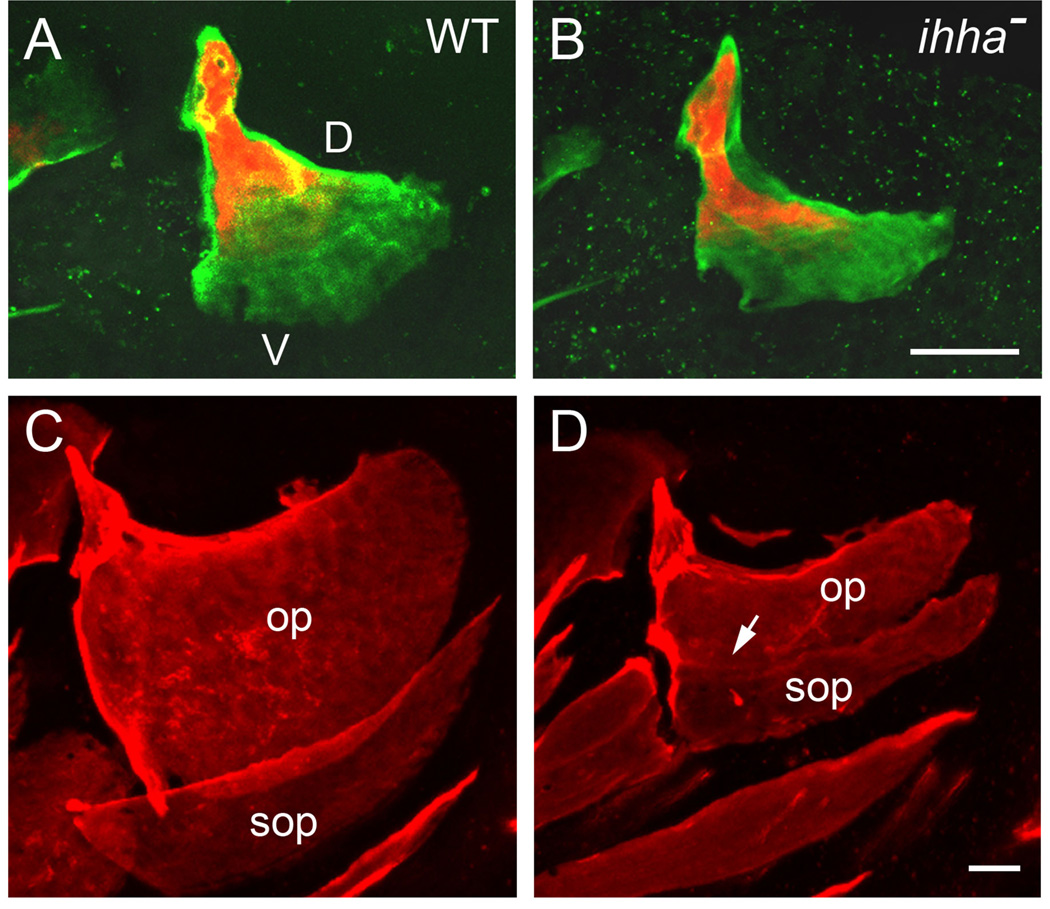
Fig. 3.
Opercle outgrowth and the formation of the opercle-subopercle articulation are specifically dependent on Ihha signaling in zebrafish. Live confocal imaging of intact larvae. A, B: Ventral (V; toward the bottom of the figure) outgrowth is specifically reduced in the ihha mutant (B) as compared with WT (A). In contrast, elongation of the dorsal (D, upper) bone edge appears normal. Two-color labeling of the growing bone matrix, first with Alizarin Red at 5 dpf, and then with calcein at 8 dpf to show where matrix was deposited between the two stages. C, D: The opercle (op) and subopercle (sop) fuse together in mutants (D, arrow), at the location where they normally form an overlapping articulation (C). Also note that both bones are reduced in size. Alizarin Red labeling at 14 dpf. Images from Huycke et al., 2012.
A further change characterizes the ventral opercle edge in ihha mutants. Normally this edge forms the prominent articulation with the subopercle (examples in Fig. 1), a bone that develops several days after the opercle in zebrafish (see the FishFace Atlas: https://www.facebase.org/fishface/home). In ihha mutants, when the subopercle develops, the two bones fuse together where they normally would articulate (Fig. 3B, C; arrow in C). Hence, loss of a single signaling molecule results in two phenotypic changes that at first glance would appear to be rather distinctive from one another. An early and continuing reduced rate of bone growth, and a later bone fusion both involve the same local region of skeletal tissue. A possible explanation to relate these two phenotypes comes from the work of Willems et al., (2012). Based on their studies of bone fusion between normally articulating vertebral centra, induced by lowering sp7 function in medaka, these authors suggested that the borders of the developing centra require a special function of sp7-expressing osteoblasts, and these cells are missing in the medaka with lowered sp7. Extending this idea, we can propose that normally the cells recruited to the opercle ventral-posterior edge include a special type of ‘joint osteoblast’ required for the articulation at the bone border. Joint osteoblasts would be preferentially lost in the mutant, accounting for reduced (but not completely missing) cell recruitment, and accounting for loss of the opercle-subopercle joint. In support, a transgenic marker trps1:EGFP is normally expressed at high levels in osteoblasts at the opercle ventral edge but expression is reduced in mutants – and trps1 is known to be associated with joint identity (Talbot et al., 2010; Michikami et al., 2012; Nichols et al., 2013). Whether this model of a joint ‘module’ is valid requires further study, as would be worthwhile for the understanding mechanisms of joint patterning. Furthermore, because fusion of bones provides a way that bones can be lost during evolution (Patterson 1997), the question is also of interest with respect to reduction of bones in the skull during evolution.
Heterochrony without constraint in stickleback opercle evolution
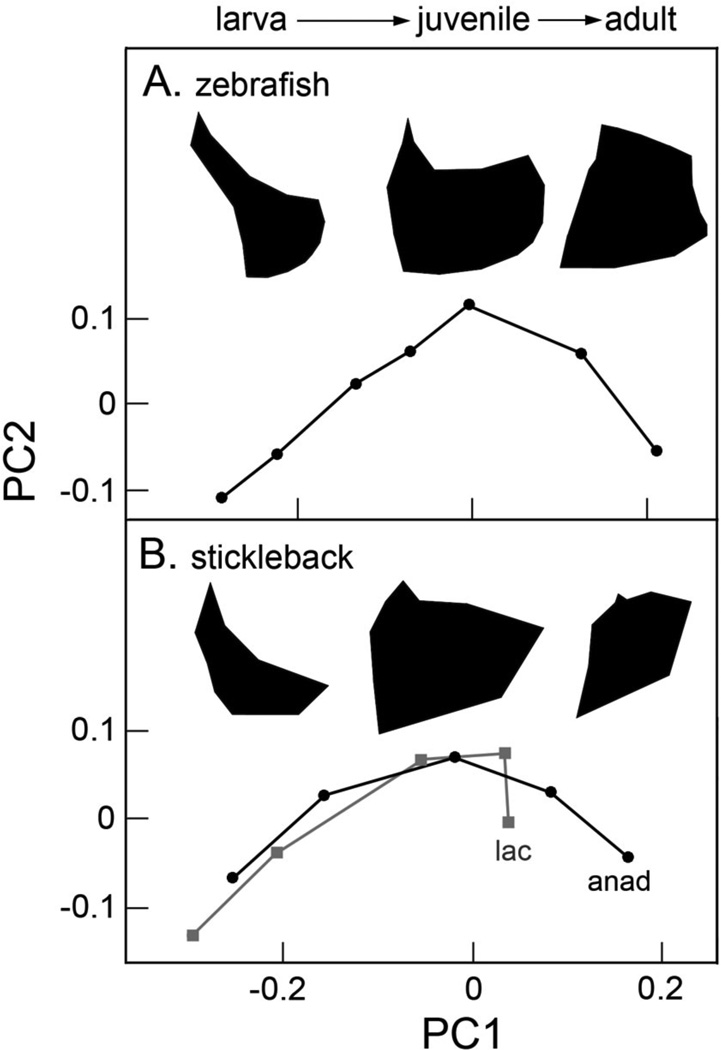
Just as zebrafish provides an important system for developmental genetics, threespine stickleback has many positive attributes for understanding evolution and evolutionary developmental biology (Cresko et al., 2007; Hohenlohe et al., 2010; Jones et al., 2012). Even though stickleback and zebrafish are only very distantly related among teleost fish, understanding coming from zebrafish can provide a cell-molecular framework for understanding how development has evolved in stickleback. Aspects of opercle morphological development are similar between the two, likely evolutionarily conserved. Thus, ‘shape spaces’ based on independent principal component analyses (PCAs) of developing bone shapes in the two species, look strikingly similar to one another (Fig. 4). PC1 scores increase throughout development for both zebrafish and oceanic (anadromous) stickleback, and PC2 scores for both first rise during larval development and then fall again by the adult stage. Intriguingly, aspects of shape changes captured by PC1 and PC2 in the two independent analyses roughly match up (see also the silhouettes accompanying the plots in Fig. 4). It seems likely that with such similarity in developmental changes in morphology, the two species also share cellular and genetic mechanisms of developmental patterning.
Fig. 4.
Zebrafish (A) and anadromous (anad; the oceanic ancestral morph) threespine stickleback (B) opercles undergo similar shape transitions during development. Lacustrine stickleback (lac, Gasterosteus aculeatus) slow down the ancestral trajectory. Silhouettes illustrate the changing bone shapes along the course of the trajectory. Not shown is that bones dramatically grow in size as they develop. Data from Kimmel et al., 2010 (A); and 2012b (B).
Over at least the past 10 Myr oceanic sticklebacks have repeatedly and independently invaded fresh water, evolving new morphologies in the new habitats (Bell and Foster, 1994). Many of the changes occur in parallel, i.e., in the same direction independently in different freshwater populations. Parallel evolution is usefully for understanding the change in that it provides natural replicates of an evolutionary ‘experiment’. Opercle morphology is included among these features that evolve in parallel (Kimmel et al., 2012a). Essentially the entire sequence of morphological development of the opercle changes between the oceanic and lacustrine forms. The lacustrine form first shows modest developmental delay along both PC1 and PC2 (Kimmel et al., 2012b; Fig. 4B). The difference between the oceanic and lacustrine forms becomes strikingly prominent at the juvenile stage: Shape development of the lacustrine bone along PC1 is rather abruptly truncated. These findings, as well as accompanying analyses, show that the primary basis of the evolutionary change in development between oceanic and lacustrine stickleback is heterochrony. Particularly with respect to the PC1 axis, the findings perfectly fit the definition of paedomorphosis (literally ‘juvenilized form’): “A descendant is paedomorphic if its later ontogenetic stages retain characteristics from earlier stages of an ancestor. The direction of evolutionary change observed in mature stages is therefore opposite to the direction of ontogenetic change” (Klingenberg 1998), p. 84). Following from this definition, the PC1 axis in an independent analysis characterizing evolutionary change among adult oceanic and lacustrine stickleback is quantitatively identical to the PC1 axis characterizing the ancestral (oceanic) developmental trajectory shown in Fig. 4B (Kimmel et al., 2012b).
Our finding that the lacustrine stickleback opercle is paedomorphic has immediate relevance to the long-standing issue in evolutionary developmental biology raised at the outset of this paper: does a heterochronic pattern of evolutionary change show that development is constraining the course of evolution (e.g. Gould and Lewontin 1979; Gould, 1988). As stated by Raff; “…given that developmental constraints are those attributes of the existing pattern of development that limit or channel further evolutionary change, heterochrony is the chief agent of change arising from within the internal architecture of ontogeny” (Raff, 1996, p. 259, emphasis added, and note that Raff’s context was a criticism of over-enthusiastic interpretation that heterochrony is necessarily constraining). We carried out a quantitative genetic analysis to attempt to directly assess the role of constraint in stickleback opercle evolution. We characterized the genetic variance-covariance matrix G in the same ancestral stickleback population we used in the developmental analysis. We expected that constraint by internal architecture should be revealed by comparing the leading eigenvectors of the ancestral G matrix with the phenotypic vector describing the direction of evolutionary change toward the lake morphology: The constraint hypothesis predicts that evolution should have occurred along one of these genetic “lines of least resistance” (Stebbins, 1974; Schluter, 1996). However, our analysis provided no convincing evidence that parallel evolution in opercle shape was constrained to occur along any of the leading G eigenvectors, and we concluded that the change in morphology was due to selection in the lacustrine environment, rather than developmental constraint (Kimmel et al., 2012a).
Correlation between developmental modularity and evolutionary change
How can we reconcile the results of our developmental analysis, showing heterochronic change that could be expected to be channeling (constraining), and our genetic analysis arguing against the same thing? I argue, along with Raff (1996), that interpretation that heterochrony is necessarily constraining is overly narrow, and that resolution of the apparent conflict comes from backing away from such an interpretation. Figure 4B shows that cessation of shape development of the lacustrine opercle the juvenile stage is with respect to PC1 only. Shape development along PC2 continues, and as for oceanic fish, the adult lacustrine opercle is narrower than that of the juvenile. These data reveal dissociation between the evolutionary changes along the two PC axes. As pointed out above for the zebrafish ihha mutant analysis, dissociation is a hallmark of modular patterning. We infer that a ‘smart’ modular style of development can mitigate any potentially constraining influence of heterochrony. Heterochrony may rarely, if ever, be ‘pure’ – with all temporal aspects of development changed (truncated, in the case of paedomorphosis) simultaneously and coordinately. Rather, as we see for the opercle in our stickleback study, heterochrony more generally may be regionally dissociated (Mitteroecker et al., 2004).
We hypothesized, and provided evidence to support that developmental modularity is presenting a dissociable region within the opercle that facilitates adaptive evolutionary change (Kimmel et al., 2012b). Here we demonstrate the basics of the modularity test, and extend the analysis presented in that paper by including the adjacent bone, the subopercle, which also prominently evolves between oceanic and lacustrine stickleback (Jamniczky et al., 2014). Our logic for such analyses includes the key proposition that if developmental modularity influences how the skeleton changes during evolution, then, turning around the relationship between modularity and evolution, the evolutionary change might predict the nature of the modular patterning. In other words, evolution might show us specifically where the boundaries between developmental modules are located.
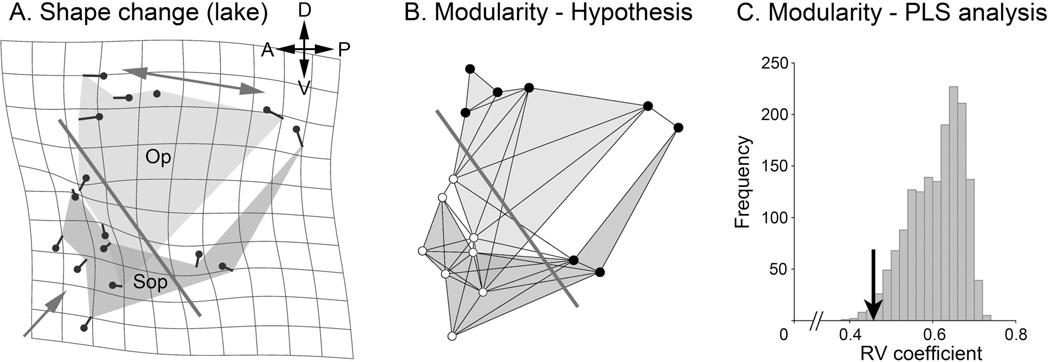
Examining landmark displacements in descendent lacustrine stickleback evolving from ancestral oceanic fish (Fig. 5A) reveals that dorsal region of the opercle-subopercle two-bone configuration shows a different shape deformation than the ventral region. Dorsally the bone pair widens along the anterior-posterior axis (two-headed arrow). Ventrally the configuration is compressed along the dorsal-ventral axis (single-headed arrow). We can construct a boundary line passing obliquely across the configuration (A, bold line) that separates the two regions. Here, within the boundary region, the shape change is prominent. The figure includes a D’arcy Thompson style shape transformation grid (in gray), where deformation of the grid from rectangularity directly shows the deformation of the two-bone configuration. This presentation emphasizes the prominence of the deformation where the boundary is drawn. By the proposal above that developmental modularity is presenting an evolutionarily dissociable region within the configuration, we can hypothesize that this boundary separating regions of different shape deformation (in Fig. 5A) also separates dorsal and ventral developmental modules (Fig. 5B, bold line). I test this hypothesis by the elegant method of Klingenberg (2009). This method uses partial least squares analysis to examine the covariation structure of the two-bone configuration. The test reveals that the covariation between the two blocks of landmarks separated by our hypothetical boundary is among the very lowest of other possible partitions of the configuration (Fig. 5C, arrow). Hence the test supports that the dorsal and ventral blocks are developmental modules. This is because by definition modules are semi-autonomous, and hence would exhibit only low covariance between them.
Fig. 5.
Evolutionary change (A) predicts developmental modularity (B, C) in stickleback. A. Average Procrustes shape change of the opercle (Op) subopercle (Sop) two-bone configuration in two Alaskan lacustrine populations (Boot and Bear Paw Lakes) as compared with an oceanic population (ancestral, Rabbit Slough). The small lollypop-like symbols represent landmarks along the bone edges. The directions and lengths of the tails of these symbols indicate the local bone shape changes in the lake populations. As explained in the text, the large single-headed and double-headed arrows show different shape changes in the dorsal and ventral regions of the configuration. The bold, diagonally oriented line separates these two regions. B. This bold line is hypothesized to be a module boundary between dorsal (upper, 8 dark landmarks) and ventral (lower, 8 white landmarks) modules. The finer lines show proposed landmark connectivities within the configuration, following Klingenberg, 2009. C. Partial least squares (PLS, Klingenberg, 2009) analysis reveals that the RV (multivariate correlation) coefficient of the proposed module pair (arrow, RV=0.46) is among the lowest of all possible subdivisions (n= 1587) that separate the configuration into two blocks of 8 contiguous landmarks each. Only 15 subdivisions yielded a lower RV coefficient (Proportion lower=0.009). Original analysis, dataset from Jamniczky et al., 2014).
Conclusion
Our work have shown that evolutionary change can predict developmental modularity, and I take this correlation to support the hypothesis that modularity has biased evolution to utilize a dissociable region within the opercle-subopercle pair. Jamniczky et al. reached the same conclusion in a recent related study in which we included two additional neighboring bones in the skeletal configuration, the preopercle and the interopercle, to make four bones in all (Jamniczky et al., 2014). In that study we discovered another module boundary – passing across these other two bones in a similar manner to the way the boundary recovered here passes through both the opercle and subopercle. Hence, our finding of modularity associated with evolutionary change may be generalizable. We also found what we believe to be the same boundary recovered here in our examination of the opercle by itself (Kimmel el al., 2012b). This boundary partitions the opercle into dorsal and ventral regions. Hence I suggest that the different evolutionary shape changes in the two regions, the stretching out of the dorsal region and the compression of the ventral region, is due to correlational selection – selection for favorable character combinations (Sinervo and Svensson, 2002) – facilitated by the bone’s underlying integration structure (Jamniczky et al., 2014). Furthermore, heterochrony in a modular system (hence ‘regionally dissociated heterochrony’, Mitteroecker et al. 2004) may provide a positive influence on the course of evolution, a role rather different than imagined the early discussions of developmental constraint (Olson, 2012). In fact, such a positive influence is suggested by the finding of repeated parallel evolution of the opercle to a similar new morphology and likely by similar developmental change, in geographically disparate populations (Kimmel et al., 2012a).
The hypothesis that correlational selection has biased evolutionary change between the oceanic and lacustrine populations makes it understandable why our study of the G matrix (Kimmel et al., 2012a; reviewed above) yielded no support for ancestral genetic covariance structure driving or biasing evolution. Simply put, the most favorable combination of traits would differ in the two environments. Hence a genetic architecture selected in an oceanic population (where we had measured G) would not be optimal in freshwater. In accord with the supposition that correlational selection can reorient the leading eigenvectors of the G matrix (Roff and Fairbairn, 2012), it would of interest to now examine G in a derived, lacustrine population: one might expect G to have evolved to result in a new matchup between the lacustrine phenotypic and and genetic covariance structure.
The work summarized here argues against an extreme view of a negative role of development in evolution (e.g. Rasskin-Gutman, 2005). Since the time of the spandrals paper (Gould and Lewontin, 1979), negative constraint has been rather generally accepted (Wilkins, 2002), even borrowed to explain features of human cultural evolution (Hauser, 2009). However, our findings suggest that caution is warranted. To illustrate, consider the influential heterochrony paper of Alberch and Alberch (1981) coming from the same period as spandrals. Alberch and Alberch (1981) make a strong case for paedomorphosis underlying salamander miniaturization. However, without any direct evidence they claimed that certain “derived features, in particular foot morphology, are merely correlated with small body size and do not necessarily have any adaptive significance” (Alberch and Alberch 1981; p. 258). Furthermore, they stated that such correlation “illustrates the importance of developmental constraints in limiting the capacity of adaptation” (op. cit.). But regionally dissociated heterochrony would be expected to disrupt nonadaptive correlations. Critical evidence is required to support assertion that development negatively constrains certain phenotypic changes to just ‘come along for the ride’ during evolution.
Looking forward, the time seems ripe to provide cellular and molecular genetic foundations for the roles of modularity in evolutionary change. We note that the ihha-dependent ventral opercle module discovered by mutational analysis in zebrafish (Huycke et al., 2012) matches in position the ventral opercle module showing paedomorphic evolution in stickleback (Kimmel et al., 2012b). Furthermore, in zebrafish ihha mutants the rate of cell division of ventral opercle pre-osteoblasts is lowered, locally reducing osteoblast recruitment and slowing the rate of bone growth. One could imagine that the same change in stickleback could be the mechanism at least in part underlying paedomorphosis, as might be examined by comparing cell division rates and osteoblast recruitment to the ventral region of the opercle in oceanic and lacustrine sticklebacks. Quantitative trait mapping and genomic analyses, including genome-wide association analyses in stickleback should let one query what genes might have involved in the evolutionary change, as in studies of body armor and body pigment evolution in this species (Jones et al., 2012). Supporting views expressed by Raff and Wray (1989), our work emphasizes the value of examining details of ontogenetic change occurring at a very local tissue level (vs. a global or whole-body level) for more fully understanding the role of development in evolution.
Acknowledgements
Important imaginative and intellectual contributions to the findings and ideas presented here came from past and present members of my laboratory, and from William Cresko, Paul Hohenlohe, Katrina McGuigan, Patrick Phillips, Benedikt Hallgrímsson and Heather Jamniczky. Tyler Huycke and Sawyer Watkins produced the lovely confocal images, and James T. Nichols provided helpful comments on the manuscript. Funding was from the NIH (grants RO1 DE013834 and PO1HD022486) and NSF (grant IOS-0818738).
References
- Alberch P, Alberch J. Heterochronic mechanisms of morphological diversification and evolutionary change in the neotropical salamander, Bolitoglossa occidentalis (Amphibia: Plethodontidae) J. Morphol. 1981;167:249–264. doi: 10.1002/jmor.1051670208. [DOI] [PubMed] [Google Scholar]
- Bell MA, Foster SA, editors. The Evolutionary Biology of the Threespine Stickleback. Oxford: Oxford University Press; 1994. p. 569. [Google Scholar]
- Carroll SB. Homeotic genes and the evolution of arthropods and chordates. Nature. 1995;376:479–485. doi: 10.1038/376479a0. [DOI] [PubMed] [Google Scholar]
- Cresko W, McGuigan K, Phillips P. Studies of threespine stickleback developmental evolution: progress and promise. Genetica. 2007;129:105–126. doi: 10.1007/s10709-006-0036-z. [DOI] [PubMed] [Google Scholar]
- Gould SJ. The uses of heterochrony. In: McKinney ML, editor. Heterochrony in Evolution: A Multidisciplinary Approach. New York: Plenum Press; 1988. pp. 1–13. [Google Scholar]
- Gould SJ. The Structure of Evolutionary Theory. Cambridge MA: Belknap Press; 2002. p. 1431. [Google Scholar]
- Gould SJ, Lewontin R. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptionist programme. Proc. R. Soc. Lond. B. 1979;205:581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. BioEssays. 2000;22:138–147. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hallgrímsson B, Jamniczky H, Young NM, Rolian C, Parsons TE, Boughner JC, Marcucio RS. Deciphering the palimpsest: Studying the relationship between morphological integration and phenotypic covariation. Evol. Bio. 2009;36:355–376. doi: 10.1007/s11692-009-9076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MD. The possibility of impossible cultures. Nature. 2009;460:190–196. doi: 10.1038/460190a. [DOI] [PubMed] [Google Scholar]
- Hendrikse JL, Parsons TE, Hallgrímsson B. Evolvability as the proper focus of evolutionary developmental biology. Evol. Dev. 2007;9:393–401. doi: 10.1111/j.1525-142X.2007.00176.x. [DOI] [PubMed] [Google Scholar]
- Hohenlohe PA, Bassham S, Etter PD, Stiffler N, Johnson EA, Cresko WA. Population genomics of parallel adaptation in threespine stickleback using sequenced RAD tags. PLoS Genetics. 2010;6:e1000862. doi: 10.1371/journal.pgen.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huycke TR, Eames BF, Kimmel CB. Hedgehog-dependent proliferation drives modular growth during morphogenesis of a dermal bone. Development. 2012;139:2371–2380. doi: 10.1242/dev.079806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamniczky HA, Harper EE, Garner R, Cresko WA, Wainwright PC, Hallgrímsson B, Kimmel CB. Association between integration structure and functional evolution in the opercular four-bar apparatus of the threespine stickleback, Gasterosteus aculeatus (Pisces, Gasterosteidae) . Biol. J. Linn. Soc. 2014;111:375–390. [Google Scholar]
- Jones FC, Grabherr MG, Chan YF, Russell P, Mauceli E, Johnson J, Swofford R, Pirun M, Zody MC, White S, Birney E, Searle S, Schmutz J, Grimwood J, Dickson MC, Myers RM, Miller CT, Summers BR, Knecht AK, Brady SD, Zhang H, Pollen AA, Howes T, Amemiya C Broad Institute Genome Sequencing Platform & Whole Genome Assembly Team. Lander ES, Di Palma F, Lindblad-Toh K, Kingsley DM. The genomic basis of adaptive evolution in threespine sticklebacks. Nature. 2012;484:55–61. doi: 10.1038/nature10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, DeLaurier A, Ullmann B, Dowd J, McFadden M. Modes of developmental outgrowth and shaping of a craniofacial bone in zebrafish. PLoS One. 2010;5:e9475. doi: 10.1371/journal.pone.0009475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Cresko WA, Phillips PA, Ullmann B, Currey M, von Hippel F, Kristjánsson BK, Gelmond O, McGuigan K. Independent axes of genetic variation and parallel evolutionary divergence of opercle bone shape in threespine stickleback. Evolution. 2012a;66:419–434. doi: 10.1111/j.1558-5646.2011.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Hohenlohe PA, Ullmann B, Currey M, Cresko WA. Developmental dissociation in morphological evolution of the stickleback opercle. Evol. Dev. 2012b;14:326–337. doi: 10.1111/j.1525-142X.2012.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg CP. Heterochrony and allometry: the analysis of evolutionary change in ontogeny. Biol. Rev. 1998;73:79–123. doi: 10.1017/s000632319800512x. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP. Morphological integration and developmental modularity. Annual Review of Ecology and Systematics. 2008;39:115–132. [Google Scholar]
- Klingenberg CP. Morphometric integration and modularity in configurations of landmarks: tools for evaluating a priori hypotheses. Evol. Dev. 2009;11:405–421. doi: 10.1111/j.1525-142X.2009.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michikami I, Fukushi T, Honma S, Yoshioka S, Itoh S, Muragaki Y, Kurisu K, Ooshima T, Wakisaka S, Abe M. Trps1 is necessary for normal temporomandibular joint development. Cell Tissue Res. 2012;348:131–140. doi: 10.1007/s00441-012-1372-1. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Gunz P, Weber GW, Bookstein FL. Regional dissociated heterochrony in multivariate analysis. Ann. Anat. 2004;186:463–470. doi: 10.1016/S0940-9602(04)80085-2. [DOI] [PubMed] [Google Scholar]
- Naiche LA, Holder N, Lewandoski M. FGF4 and FGF8 comprise the wavefront activity that controls somitogenesis. Proc. Nat. Acad. Sci. USA. 2011;108:4018–4023. doi: 10.1073/pnas.1007417108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham J. On the dissociability of the fundamental process in ontogenesis. Biol. Rev. 1933;8:180–223. [Google Scholar]
- Nichols JT, Pan L, Moens CB, Kimmel CB. barx1 represses joints and promotes cartilage in the craniofacial skeleton. Development. 2013;140:2765–2775. doi: 10.1242/dev.090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson ME. The developmental renaissance in adaptationism. Cell. 2012;27:278–287. doi: 10.1016/j.tree.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Patterson C. Cartilage bones, dermal bones, and membrane bones, or the exoskeleton versus the endoskeleton. In: Andrews S, Miles R, Walker A, editors. Problems in Vertebrate Evolution. London: Academic Press; 1977. pp. 77–121. [Google Scholar]
- Pourquié O. Vertebrate segmentation: from cyclic gene networks to scoliosis. Cell. 2011;145:650–663. doi: 10.1016/j.cell.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff EC, Raff RA. Dissociability, modularity, evolvability. Evol. Dev. 2000;2:235–237. doi: 10.1046/j.1525-142x.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- Raff RA. Genes, Development, and the Evolution of Animal Form. Chicago: University of Chicago Press; 1996. The Shape of Life; p. 520. [Google Scholar]
- Raff RA, Wray GA. Heterochrony: Developmental mechanisms and evolutionary results. J. Evol. Biol. 1989;2:409–434. [Google Scholar]
- Rasskin-Gutman . Modularity: jumping forms within morphospace. In: Callebaut W, Rasskin-Gutman D, editors. Modularity: Understanding the Development and Evolution of Natural Complex Systems. Cambridge Mass.: MIT Press; 2005. pp. 207–219. [Google Scholar]
- Roff DA, Fairbairn DJ. A test of the hypothesis that correlational selection generates genetic correlations. Evolution. 2002;66:2953–2960. doi: 10.1111/j.1558-5646.2012.01656.x. [DOI] [PubMed] [Google Scholar]
- Schluter D. Adaptive radiation along genetic lines of least resistance. Evolution. 1996;50:1766–1774. doi: 10.1111/j.1558-5646.1996.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Shubin N, Tabin C, Carroll S. Fossils, genes and the evolution of animal limbs. Science. 1997;388:639–648. doi: 10.1038/41710. [DOI] [PubMed] [Google Scholar]
- Sinervo B, Svensson E. Correlational selection and the evolution of genomic architecture. Heredity. 2002;89:329–338. doi: 10.1038/sj.hdy.6800148. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Flowering Plants: Evolution Above the Species Level. Cambridge, Mass: Belknap Press; 1974. p. 480. [Google Scholar]
- Talbot JC, Johnson SL, Kimmel CB. hand2 and Dlx genes specify dorsal, intermediate and ventral domains within zebrafish pharyngeal arches. Development. 2010;137:2507–2517. doi: 10.1242/dev.049700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GP. Homologues, natural kinds and the evolution of modularity. Am. Zool. 1996;36:36–43. [Google Scholar]
- Wagner G, Altenberg L. Complex adaptations and the evolution of evolvability. Evolution. 1996;50:967–976. doi: 10.1111/j.1558-5646.1996.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Wilkins AS. The Evolution of Developmental Pathways. Sunderland MA: Sinaur Associates; 2002. p. 603. [Google Scholar]
- Willems B, Büttner A, Huysseune A, Renn J, Witten PE, Winkler C. Conditional ablation of osteoblasts in medaka. Dev. Biol. 2012;364:128–137. doi: 10.1016/j.ydbio.2012.01.023. [DOI] [PubMed] [Google Scholar]
- Young NM, Hallgrímsson B. Serial homology and the evolution of mammalian limb covariation structure. Evolution. 2005;59:2691–2704. [PubMed] [Google Scholar]