Abstract
The aim was to examine the impact of TLR5 ligation in rheumatoid arthritis (RA) and experimental arthritis pathology. Studies were conducted to investigate the role of TLR5 ligation on RA and mouse myeloid cell chemotaxis or osteoclast formation; in addition, to uncover the significance of TNF-α function in TLR5 mediated pathogenesis. Next, the in vivo mechanism of action was determined in collagen induced arthritis (CIA) and local joint TLR5 ligation models. Last, to evaluate the importance of TLR5 function in RA, anti-TLR5 antibody therapy was employed in CIA mice. We show that TLR5 agonist, flagellin, can promote monocyte infiltration and osteoclast maturation directly through myeloid TLR5 ligation and indirectly via TNF-α production from RA and mouse cells. These two identified TLR5 functions are potentiated by TNF-α, as inhibition of both pathways can more strongly impair RA synovial fluid driven monocyte migration and osteoclast differentiation compared to each factor alone. In preclinical studies, flagellin post onset treatment in CIA and local TLR5 ligation in vivo, provoke homing and osteoclastic development of myeloid cells, which are associated with the TNF-α cascade. Reversely, CIA joint inflammation and bone erosion is alleviated when TLR5 function is blockade. We found that TLR5 and TNF-α pathways are interconnected, as TNF-α is produced by TLR5 ligation in RA myeloid cells, and anti-TNF-α therapy can markedly suppress TLR5 expression in RA monocytes. Our novel findings demonstrate that a direct as well as an indirect mechanism is involved in TLR5 driven RA inflammation and bone destruction.
Keywords: TLR5, TNF-α, monocyte migration, osteoclast differentiation, collagen induced arthritis
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune disorder in which the numbers of monocyte derived macrophages are greater than in normal joints and are well correlated with radiological damage, joint pain and inflammation (1, 2). Yet, the mechanism that derives RA myeloid cell trafficking and further facilitates their maturation to osteoclasts is incompletely understood.
Osteoclasts are multinucleated bone resorbing cells differentiated from the myeloid lineage found in RA peripheral blood (PB) and synovial tissue (ST) (3–5). In RA, dominance of proinflammatory factors such as TNF-α, IL-1β, IL-6 and IL-17 can promote osteoclast maturation through enhancing myeloid receptor activator for nuclear factor κB (RANK) expression as well as RANKL production from RA ST fibroblasts and T cells (4, 6–9). Interestingly, ligation of TNF-α to myeloid TNFR1 and TNFR2 can directly facilitate osteoclast differentiation through a mechanism that is independent of RANK/RANKL cascade (10, 11). Confirming this notion, others have shown that RA synovial fluid (SF) macrophages can transform into mature osteoclasts in presence of M-CSF in combination with RANKL or TNF-α/IL-1β suggesting that proinflammatory factors such as TNF-α/IL-1β can replace the function of RANKL (12). While TNF-α and IL-17 are known to be responsible for joint myeloid cell retention directly through myeloid cell ligation and indirectly via induction of monocyte chemokines (13–15).
TLRs belong to the family of pattern recognition receptors and TLR2 and TLR4 are abundantly expressed in RA PB monocytes, RA SF and ST macrophages (16–19). Identification of TLR2 and TLR4 endogenous ligands in RA synovium has triggered an interest in discovering the role of these receptors in the RA pathogenesis (20). Hence, the impact of TLR2 and TLR4 ligation has been extensively studied on bone degradation, primarily in mouse bone marrow cells (21–23) with a few studies performed in normal human myeloid cells (24). Despite these comprehensive in vitro investigations, the obtained results are controversial and the effect of TLR4 ligation on osteoclast differentiation is greatly dependent on the treatment time point, cell type used and the concentration of reagents employed (21, 24, 25). However, the in vivo studies consistently support the significance of TLR4 activation in experimental arthritis bone loss (26–28). Unlike TLR2 and TLR4, the role of TLR5 in RA and murine models of RA is undefined.
In our recent paper, we uncovered for the first time that the TLR5 expression is markedly accentuated in RA compared to normal (NL) ST and PB myeloid cells (29). We also found that ligation of myeloid TLR5 to potential endogenous ligands in the RA joint can modulate SF TNF-α transcription (29). Notably expression of myeloid TLR5 closely correlates with RA disease activity and TNF-α levels (29), suggesting that ligation of TLR5 in RA myeloid cells contributes to disease progression. Therefore, the significance of the TLR5 cascade was investigated in myeloid cell function employing RA PB myeloid cells and mouse PB and bone marrow cells as well as in acute and chronic experimental arthritis models.
In this study we demonstrate that the TLR5 agonist, flagellin, can dose dependently promote monocyte migration and osteoclast maturation through its direct effect on myeloid cell function and indirectly via TNF-α production from RA and mouse myeloid cells or CIA ankle joints. Conversely, anti-TLR5 antibody therapy attenuates CIA joint myeloid cell homing and bone erosion. Consistent with our findings in RA, flagellin treatment can strongly transform mouse bone marrow progenitor cells into mature osteoclasts through a TNF-α dependent and IFN-β independent mechanism. In conclusion, a strong positive feedback regulation exists between TLR5 and TNF-α pathways in attracting the circulating monocytes and further remodeling the newly recruited cells into mature osteoclasts; therefore disruption of TLR5 ligation can dysregulate both functions in preclinical arthritis models.
MATERIALS AND METHODS
Monocyte chemotaxis
Experiments were performed to determine the effect of flagellin on monocyte chemotaxis. Mononuclear cells were isolated by Histopaque (GE Healthcare Bio-Sciences, Pittsburgh, PA) gradient centrifugation and monocytes were isolated from NL or RA PB using negative selection kit (StemCell Technology, Vancouver, Canada) according to the manufacturer’s instruction (30, 31). Chemotaxis was performed in a Boyden chamber (Neuroprobe; Gaithersburg, MD) using NL monocytes for 2h with varying concentrations (0.001 to 100 ng/ml) of flagellin (Ultrapure; endotoxin level <50 EU/mg) (InvivoGen, San Diego, CA), fMLP (f; 10 nM) was used as positive control and PBS was utilized as negative control (14, 15). Cell culture media, FBS, culture plates and all reagents utilized were tested for endotoxin contamination.
To demonstrate that RA SF mediated monocyte trafficking involves TLR5 ligation, cells were incubated with anti-TLR5 (10 µg/ml; InvivoGen) or IgG antibodies (Abs) for 1h prior to performing monocyte chemotaxis in response to 8 different RA SFs (20% dilution). To show that TLR5 and TNF-α pathways are interconnected in facilitating monocyte migration, RA SFs (20%) were incubated with IgG or anti-TNF-α (10 µg/ml; R&D Systems) and monocytes were either immunoneutralized by anti-TLR5 or IgG Abs (10 µg/ml) prior to performing monocyte chemotaxis.
To examine the signaling pathways associated with flagellin induced monocyte chemotaxis, monocytes were treated with DMSO or 1 and 5 µM of inhibitors to PI3K (LY294002), ERK (PD98059), p38 (SB203580), JNK (SP600125) and NF-κB (Bay 11-7085) (EMD Millipore; Billerica, MA) for 1h. Subsequently, monocyte chemotaxis was performed in response to 100 ng/ml of flagellin.
To document that flagellin and TNF-α synergistically contribute to monocyte chemotaxis, monocyte migration was examined in response to various concentrations of flagellin (0.1 and 10 ng/ml) or TNF-α (0.1 and 5 ng/ml) alone or in combination.
Flagellin signaling in monocytes
NL monocytes were untreated or treated with flagellin (100 ng/ml) for 15–65 min. Cell lysates were examined by Western blot analysis (14, 15). Blots were probed with phospho (p)-AKT1, pERK, p-p38, pJNK, p-paxillin and pFAK (Cell Signaling; 1:1000 dilution) and IκB Abs (Santa Cruz; 1:3000 dilution) or probed with AKT, ERK, p38 or actin Abs (Cell Signaling or Sigma; 1:3000 dilution).
RA patient population
These studies were approved by the University of Illinois at Chicago Institutional Ethics Review Board and all donors gave informed written consent. RA patients were diagnosed according to the 1987 revised criteria from the ACR (32). PB was obtained from 68 patients, 64 women and 4 men (mean age 48.6 ± 14.6 years). At the time of evaluation, patients were either on DMARDs (n=34, 3 male and 31 female, mean age 51.6 ± 16.2), or treatment with anti-TNF therapy (n=34, 1 male, 33 female, mean age 45.7 ± 12.4). Treatment with DMARDs (n=34) consisted of DMARDs alone (methotrexate, leflunomide, sulfasalazine, azathioprine, hydroxychloroquine or minocycline) (n=27) of which 2 were on hydroxychloroquine only, or treatment with DMARD plus prednisone (n=7). Patients treated with anti-TNF therapy (n= 34) were either on anti-TNF therapy alone (n=7), anti-TNF plus prednisone (n=1), anti-TNF therapy plus DMARD (n=21), or anti-TNF with DMARD and prednisone (n=5).
Quantification of TRAP+ cells in in vitro and in vivo experiments
To generate mature osteoclasts, NL or RA peripheral blood mononuclear cells (PBMC)s or monocytes were cultured in 0% RPMI media and allowed to attach for 1h. Thereafter cells were cultured in 10% α-MEM and were either untreated (negative control) or treated with 20 ng/ml of human M-CSF and RANKL (positive control) (ProSpec, Brunswick, NJ) for 14–21 days with the reagents and the culture media replenished every 3 days. The ability of test reagents to differentiate human precursor cells to fully mature osteoclasts was examined in the suboptimal culture conditions which consisted of 10 ng/ml of human M-CSF and RANKL. To quantify osteoclast formation, Tartrate Resistant Acid Phosphatase (TRAP) staining was performed using Acid Phosphatase Leukocyte Kit (Sigma-Aldrich) in cells or mice ankles according to manufacturer’s instruction. In vitro experiments were performed in triplicates and the total number of osteoclasts was determined by counting TRAP+ multinuclear (> 4 nuclei) cells in each well.
To examine the impact of TLR5 ligation on osteoclastogenesis, NL and RA PBMCs were exposed to varying concentrations of flagellin (0.001 to 100 ng/ml) in suboptimal culture conditions (10 ng/ml of human M-CSF and RANKL) prior to TRAP staining.
To determine whether T cell function plays a critical role in flagellin mediated osteoclastogenesis, negatively selected RA myeloid cells were cultured in suboptimal conditions in the presence or absence of flagellin (10 ng/ml) prior to TRAP staining.
To show that flagellin mediated osteoclast maturation is due to TLR5 ligation and TNF-α induction, RA PBMCs cultured in suboptimal conditions were incubated with 10 µg/ml IgG, anti-TNF-α or anti-TLR5 prior to flagellin (10 ng/ml) treatment and followed by TRAP staining.
To demonstrate whether TLR5 and TNF-α pathways synergize in promoting osteoclast differentiation, RA PBMCs cultured in suboptimal conditions were either untreated or treated with TNF-α (1 ng/ml), flagellin (1 ng/ml) alone or in combination prior to TRAP staining.
To determine that in RA joint, TLR5 and TNF-α mediated osteoclastogenesis are interconnected, RA PBMCs cultured in suboptimal conditions were immunoneutralized by anti-TLR5 or IgG control (10 µg/ml) and cells were then incubated with 2% RA SF plus IgG or 2% RA SF plus anti-TNF-α (10 µg/ml) prior to TRAP staining.
To generate mature osteoclasts from mouse PB, mononuclear cells were isolated by Histopaque (GE Healthcare Bio-Sciences) gradient centrifugation and mouse monocytes were isolated by negative selection kit (cat# 19701A; StemCell Technology) according to the manufacturer’s instruction. Thereafter cells were cultured in 10% α-MEM and were treated with 20 ng/ml of mouse M-CSF and RANKL (suboptimal condition) (R&D Systems) with or without flagellin (10 ng/ml) for 14–21 days. The reagents and culture media were replenished every 3 days. Mouse PB monocytes cultured in 10% α-MEM alone were considered as negative control and cells cultured in presence of 40 ng/ml M-CSF and 40 ng/ml RANKL served as the positive control.
To examine the role of TLR5 ligation in mouse bone marrow derived osteoclasts, bone marrow cells were isolated from C57/BL6 mice femur and tibia. Non-adherent mouse bone marrow cells (5×105 cells/96 wells) from 1h culture were incubated for 4 days in 10% α-MEM supplemented with 10 ng/ml mouse M-CSF plus 25 ng/ml mouse RANKL (suboptimal condition). Following 4 day culture, cells were untreated or stimulated with flagellin (10 ng/ml) plus IgG (10 µg/ml), flagellin (10 ng/ml) plus anti-TLR5 (10 µg/ml; InvivoGen) or flagellin (10 ng/ml) plus anti-TNF-α (10 µg/ml) for 3 additional days prior to TRAP staining. Non-adherent mouse bone marrow cells cultured in 10% α-MEM alone were considered as negative control and cells cultured in the presence of mouse 10 ng/ml M-CSF and 100 ng/ml RANKL were regarded as positive control.
To determine that TLR5 ligation contributes to bone erosion in acute and chronic models of arthritis, ankles ectopically treated with flagellin or vehicle as well as ankle joints harvested from CIA post onset treatment of flagellin or PBS were TRAP stained. To document that blockade of TLR5 ligation can impair osteoclast differentiation; TRAP staining was performed on CIA ankle joints harvested from IgG or anti-TLR5 antibody therapy. Number of osteoclasts stained in mouse ankles was quantified by counting the number of TRAP+ cells in each section (33).
FACS analysis
To determine the percentage of TLR5+ T cells in NL and RA PBMC, cells were blocked with 50% human serum in 0.5% BSA. Subsequently cells were stained with PE conjugated anti-TLR5 (Imgenex, San Diego, CA) and FITC labeled anti-CD3 (BD Pharmingen, Franklin Lakes, NJ) or IgG Abs (BD Pharmingen).
Real-time RT-PCR
Total cellular RNA was extracted using TRIzol (Life Technologies, Carlsbad, CA) and relative gene expression was determined by real-time RT-PCR using the Ct method (29–31). To determine the mechanism by which TLR5 ligation promotes osteoclast formation, RA PBMCs were cultured for 7 days at suboptimal conditions (10 ng/ml M-CSF and RANKL). Thereafter cells cultured in α-MEM in the absence of M-CSF and RANKL were untreated or stimulated with flagellin (100 ng/ml) for 6h and the expression of RANK, RANKL, TNF-α and IFN-β was quantified. TNF-α and IFN-β real time RT-PCR was performed on mouse osteoclasts precursor cells from day 4 cultured in 10 ng/ml mouse M-CSF plus 25ng/ml mouse RANKL treated with or without flagellin (100 ng/ml) for 6, 24, 48 and 72h. Additionally, transcription of calcitonin receptor (CTR), Cathepsin K (CTSK), RANKL and IFN-β was determined in CIA ankles treated with flagellin (20 µg) or PBS. TLR5 expression was quantified in RA monocytes treated with DMARDs or anti-TNF-α with or without DMARDs.
Cytokine Quantification
Mouse TNF-α protein concentration was determined by ELISA (R&D Systems) according to the manufacturer’s instructions in CIA mouse ankles treated with PBS or flagellin (20 µg) as well as in day 4 mouse bone marrow precursor cells untreated (PBS) or treated with flagellin (100 ng/ml) plus IgG (10 µg/ml) versus flagellin (100 ng/ml) plus anti-TLR5 (10 µg/ml) for 24 h. Joint IL-6 and CCL2 protein levels were determined in CIA mice treated with IgG or anti-TLR5 antibody.
Study protocol for animal models
Eight week old DBA/1 mice were immunized with collagen type II (Chondrex, Redmond, WA) on days 0 and 21 (34, 35). Flagellin (20 µg, n=10) (InvivoGen) or PBS (n=10) was injected i.p. on day 33 post CIA induction, mice were sacrificed on day 57 and experiments were repeated twice. Ankle circumference was determined by Caliper using the following formula: circumference = 2Bx(sqrt(a2 + b2/2)), where a and b represent the diameters (34–36). In a different experiment, C57BL/6 mice were i.a. injected on day 0 with 20 µg of flagellin or PBS and mice were sacrificed on day 10 and ankle circumference was determined by Caliper (34–36). To validate that the loss of TLR5 function can critically impact joint inflammation and bone erosion, following CIA induction (34, 35), mice were treated i.p. with IgG or monoclonal rat anti-mouse TLR5 antibody (100 µg/injection) (Invivogen) on days 23, 27, 30, 34, 37, 41, 44 and 48. Animals were sacrificed on day 49 post induction.
Immunohistochemistry
Mouse ankles were decalcified, formalin fixed, paraffin embedded and sectioned. Briefly, slides were deparaffinized in xylene and antigens were unmasked by incubating slides in Proteinase K digestion buffer (Dako, Carpinteria, CA). Nonspecific binding of avidin and biotin was blocked using an avidin/biotin blocking kit (Dako). CIA ankles were stained with F480 (1:100 dilution; Serotec), iNOS (1:200 dilution; Santa Cruz) or control IgG Abs (Beckman Coulter). Joint myeloid cell or M1 macrophage staining were scored on a 0–5 scale by two blinded observers.
Statistical Analysis
One way ANOVA was employed for comparison among multiple groups followed by post hoc two-tailed Student’s t-test. The data were also analyzed using two-tailed Student’s t-test for paired or unpaired comparisons between two groups. Values of p < 0.05 were considered significant.
RESULTS
Activation of PI3K/AKT1, JNK and NF-κB pathways contribute to TLR5 induced monocyte chemotaxis
Since TLR5+ monocyte derived macrophages are elevated in RA compared to NL ST, we asked whether circulating monocytes can migrate into the RA joint where TLR5 endogenous ligands are expressed (29). We found that flagellin is chemotactic for monocytes at concentrations ranging from 1 to 100 ng/ml (Fig. 1A). Further blockade of myeloid TLR5 suppressed RA SF mediated monocyte chemotaxis suggesting that the potential TLR5 endogenous ligands can contribute to joint monocyte homing (Fig. 1B). We next demonstrate that monocytes stimulated with flagellin phosphorylate AKT1, ERK, p38, JNK, paxillin and degrade IκB pathways; in contrast FAK was not activated by TLR5 ligation (Fig. 1C). Interestingly, while inhibition of ERK and p38 was ineffective, suppression of PI3K, JNK and NF-κB pathways markedly reduced flagellin mediated chemotaxis starting at 1 µM (Fig. 1D). These results suggest that ligation of TLR5 by SF endogenous ligands can modulate monocyte homing through activation of PI3K/AKT1, JNK and NF-κB pathways.
Figure 1. TLR5 ligation promotes monocyte migration through activation of AKT1/PI3K, JNK and NF-κB pathways.
A. Flagellin monocyte chemotaxis was performed in a Boyden chemotaxis chamber with varying concentration (0.001–100 ng/ml), n=3. B. Monocytes were incubated with anti-TLR5 antibody (10 µg/ml) or control IgG for 1h, thereafter chemotaxis was performed in response to 20% RA SF, n=8. C. Monocytes were stimulated with 100 ng/ml flagellin for 0–65 min, and the cell lysates were probed for pAKT1, pERK, p-p38, pJNK, pFAK, p-paxillin and degradation of IκB, n=3. D. Cells were pre-incubated with DMSO (D) or 1 and 5 µM inhibitors to PI3K (LY294002), ERK (PD98059), p38 (SB203580), JNK (SP600125) and NF-κB (Bay 11-7085) for 1h. Subsequently, monocyte chemotaxis was performed in response to 100 ng/ml of flagellin for 2h, n=3. For all experiments PBS and FMLP (f) served as negative and positive controls. Values demonstrate mean ± SE.* represents p <0.05.
TLR5 interconnets with TNF-α in mediating monocyte chemotaxis
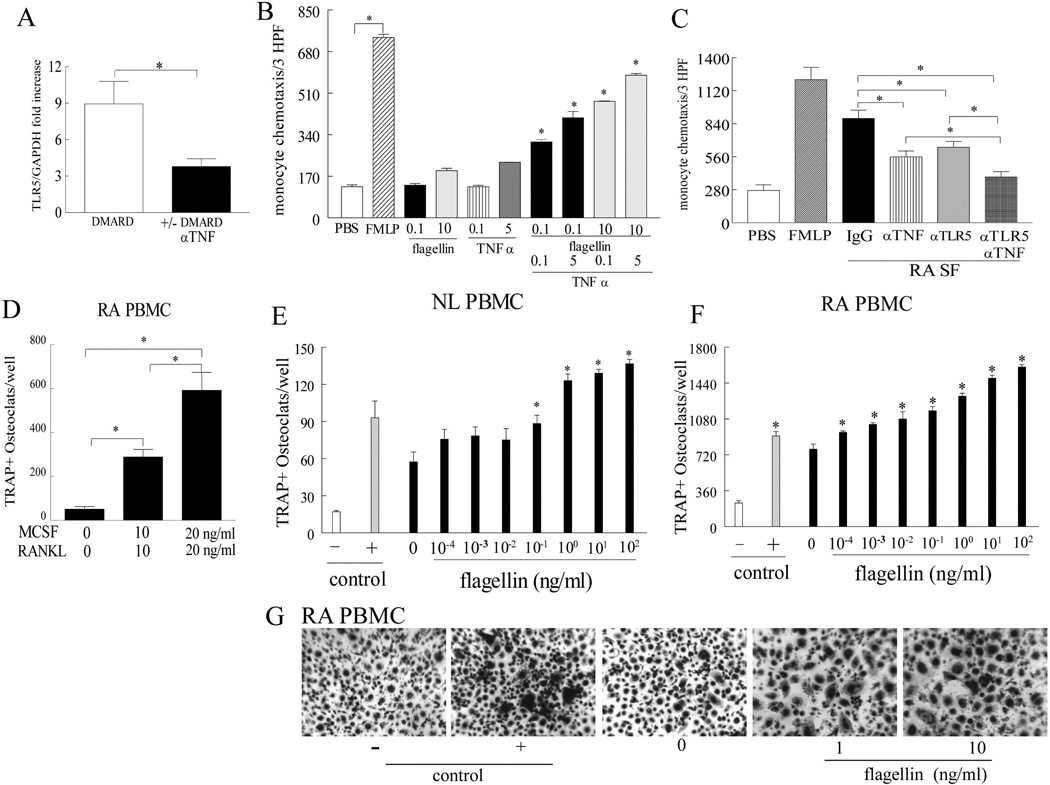
Ligation of TLR5 strongly induces production of TNF-α in RA monocytes and macrophages (29), thus we asked whether TLR5 expression can be affected by anti-TNF-α therapy. Interestingly, myeloid TLR5 expression was 2.5 fold higher in RA patients treated with DMARDs compared to those treated with anti-TNF-α agents (Fig. 2A) suggesting that these two pathways are cross regulated. Consistently, we demonstrate that TNF-α and flagellin can synergistically induce myeloid cell migration (Fig. 2B) and hence blockade of both cascades can more potently suppress RA SF mediated monocyte chemotaxis compared to each pathway alone (Fig. 2C). These results indicate that in the RA joint, expression of TNF-α triggered by myeloid TLR5 ligation can further enhance TLR5 driven myeloid cell infiltration.
Figure 2. TLR5 mediated monocyte trafficking is interconnected to TNF-α pathway and ligation of myeloid TLR5 is a strong promoter of RA osteoclast differentiation.
A. Expression of TLR5 was quantified in RA monocytes treated with DMARDs (n=34) or with anti-TNF-α in the presence or absence of DMARDs (n=34). B. Monocyte migration was examined in response to various concentrations of flagellin (0.1 and 10 ng/ml) or TNF-α (0.1 and 5 ng/ml) alone or in combination. The combined doses were compared to the same doses treated alone, n=3. C. 20% RA SFs were incubated with 10 µg/ml IgG or anti-TNF-α and monocytes were either immunoneutralized by 10 µg/ml anti-TLR5 or IgG control prior to performing chemotaxis, n=7. D. RA PBMCs were differentiated to fully mature osteoclasts in the presence 20 ng/ml of M-CSF and RANKL, while suboptimal conditions consisted of 10 ng/ml M-CSF and RANKL, n=4. NL (n=3) (E) and RA PBMCs (n=4) (F) were exposed to varying concentrations of flagellin (0.001 to 100 ng/ml) in the presence of 10 ng/ml human M-CSF and RANKL (suboptimal condition) prior to TRAP staining and significance is compared to no flagellin treatment (0) in the suboptimal condition in E and F. G. Shows the representative TRAP staining (original magnification x 200) of F. Negative (-) and positive (+) controls consisted of untreated cells or cells treated with 20 ng/ml M-CSF and RANKL. Values demonstrate mean ± SE.* represents p <0.05.
Myeloid TLR5 ligation activates RA osteoclast formation
Since TLR5 ligation contributes to RA joint myeloid cell chemotaxis, we asked whether TLR5 activation could transform the recruited myeloid cells into mature osteoclasts. Experiments performed established that PBMCs could be differentiated into fully mature osteoclasts in the presence 20 ng/ml of M-CSF and RANKL, while the suboptimal conditions consisted of 10 ng/ml M-CSF and RANKL in RA PBMCs (Fig. 2D). We next demonstrated that TLR5 ligation in RA and NL PBMCs could dose dependently contribute to osteoclast formation when cultured in suboptimal conditions (Figs. 2E–G), suggesting that flagellin can promote transcription of essential osteoclastogenic factors.
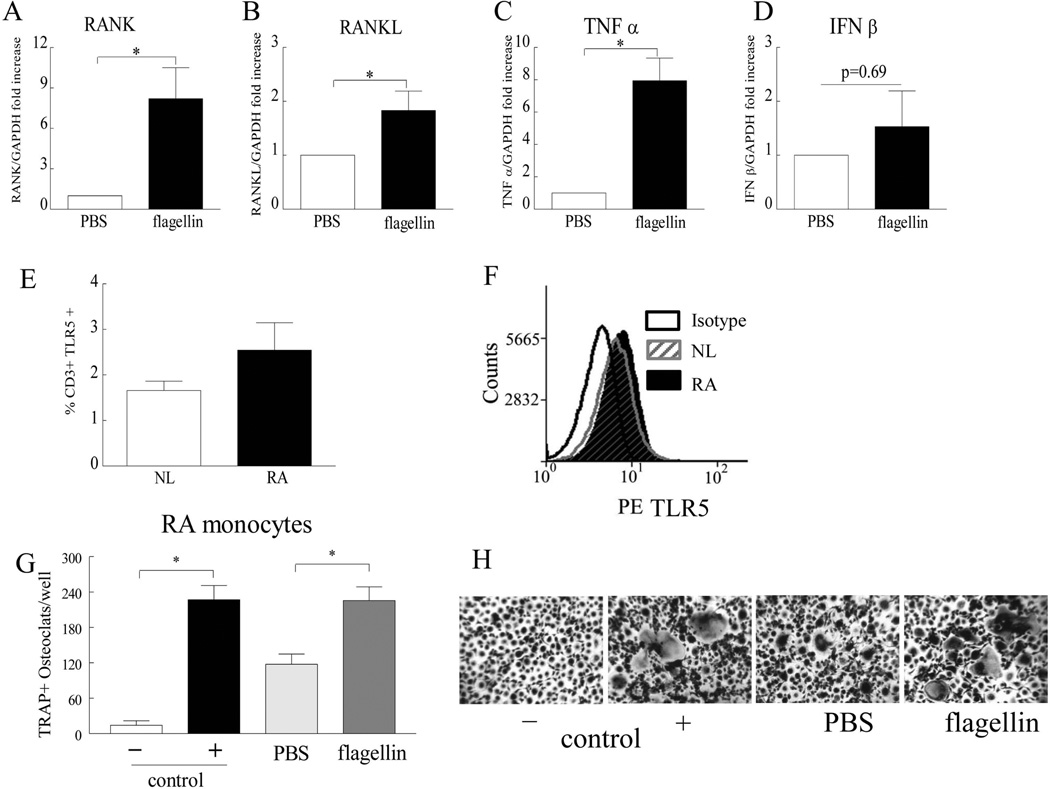
We found that in RA PBMCs, flagellin treatment upregulates RANK, RANKL and TNF-α expression levels by 2–8 folds (Figs. 3A–C). In contrast to our findings, others have shown that TLR5 ligation inhibits mouse bone marrow cell differentiation to mature osteoclast through IFN-β induction (37); therefore transcription of IFN-β was also assessed in our culture system. Interestingly, we document that there was an insignificant higher trend of IFN-β expression in RA cells treated with flagellin compared with the PBS treatment (Fig. 3D).
Figure 3. Ligation of TLR5 in RA PBMCs drives the transcription of pro-osteoclastogenic factors and TLR5 ligation promotes RA myeloid cells to form mature osteoclasts in suboptimal culture conditions.
Employing real-time RT-PCR, RANK (A), RANKL (B), TNF-α (C) and IFN-β (D) mRNA levels were quantified in RA osteoclast precursor cells that were cultured in suboptimal condition (10 ng/ml M-CSF and RANKL) for 7 days prior to being treated with PBS or 10 ng/ml flagellin for 6h in the absence of M-CSF and RANKL, n=7. Results are shown as fold increase above the PBS group and are normalized to GAPDH. E. NL and RA PBMC were immunostained with FITC labeled anti-CD3 antibody and PE-conjugated anti-TLR5 in order to determine the percentage of CD3+ and TLR5 positive cells, n=3 and F is a representative flow cytometry histogram of E. G. Negatively selected RA monocytes were cultured in the suboptimal condition and were either untreated (PBS) or treated with 10 ng/ml flagellin prior to TRAP staining. H. Shows the representative TRAP staining (original magnification x 200) of G. Negative and positive control consisted of untreated cells or cells treated with 20 ng/ml M-CSF and RANKL. Values demonstrate mean ± SE.* represents p <0.05.
Since RANKL is produced from RA T cells and fibroblasts, we asked whether TLR5 is expressed on T cells and if these cells are critical for flagellin mediated osteoclastogenesis. Although the cell surface TLR5 levels were not significantly higher, there was however a greater expression trend in RA compared to NL CD3+ T cells (Figs. 3E–F). We show that ligation of TLR5 could differentiate osteoclast precursor cells into fully mature osteoclasts in the absence of T cells when monocytes were cultured in a suboptimal condition (Figs. 3G–H). Taken together these results suggest that monocytes are the effector cells in TLR5 mediated osteoclastogenesis and flagellin can facilitate osteoclast formation by increasing RANK expression and allowing the cells to be more responsive to RANKL binding, resulting in less RANKL being required for this process. In addition it is possible that the pro-osteoclastogenic factors counterbalance the inhibitory effect of IFN-β in part because flagellin induced TNF-α transcription can potentiate RANK/RANKL cascade while suppressing the IFN-β transcription (38).
TLR5 links with TNF-α in enhancing joint osteoclastogenesis
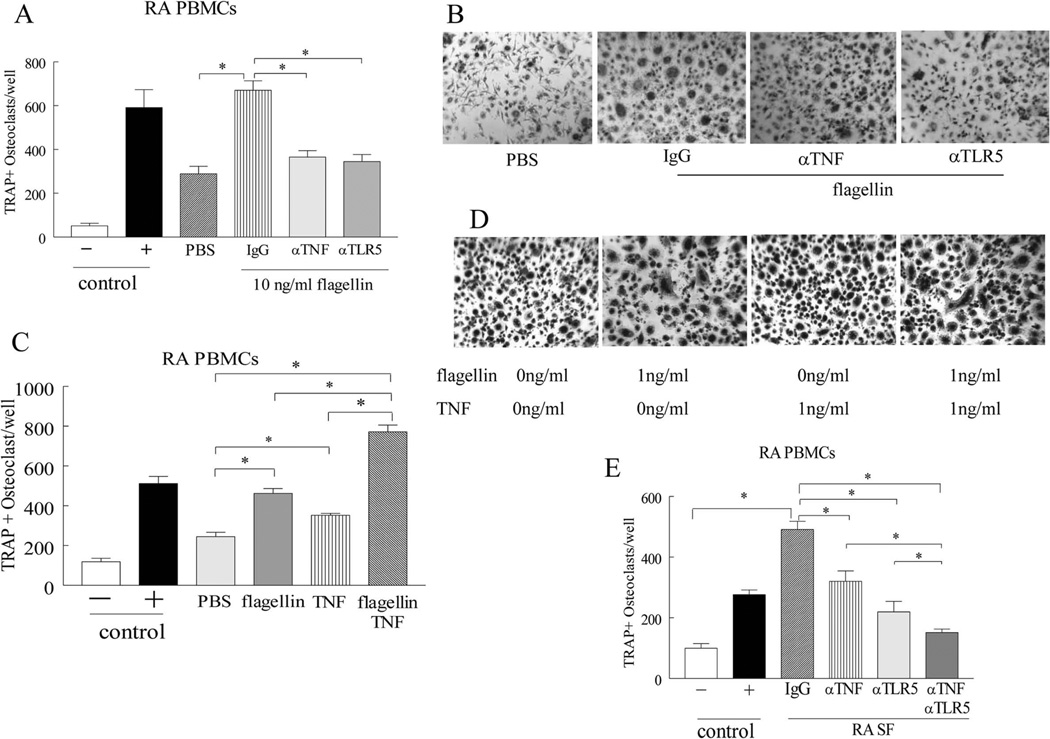
Next, experiments were performed to document whether TNF-α is capable of potentiating TLR5 mediated osteoclast formation. We show that TLR5 mediated osteoclastogenesis is in part due to TNF-α produced from RA myeloid cells (Figs. 4A–B). However since osteoclast differentiation driven by TLR5 and TNF-α is dose dependent, supplementing the flagellin treated cells with exogenous TNF-α further increased the number of TRAP+ osteoclast by 2 fold (Figs. 4C–D). Confirming this notion, we show that in RA SF, TLR5 endogenous ligand(s) connect with TNF-α in promoting osteoclastogenesis, as inhibition of both pathways can suppress RA SF mediated osteoclast formation more potently compared to each pathway alone (Fig. 4E). Our results indicate that TLR5 and TNF-α pathways are linked in fostering RA myeloid cell recruitment and osteoclast differentiation.
Figure 4. In RA joint, TLR5 and TNF-α mediated osteoclastogenesis is interconnected.
A. RA PBMCs cultured in suboptimal conditions (10 ng/ml M-CSF and RANKL) were untreated or pretreated with 10 µg/ml of IgG, anti-TNF-α and anti-TLR5 antibodies prior to being stimulated with 10 ng/ml flagellin, followed by TRAP staining. B. Is a representative image of TRAP+ cells (original magnification x 200) from A, n=3. C. RA PBMC were cultured in suboptimal condition and were either untreated or treated with 1ng/ml flagellin, 1ng/ml TNF-α or both prior to TRAP staining. D. Is a representative image of TRAP staining (original magnification x 200) from C, n=3. E. RA PBMCs cultured in suboptimal condition were immunoneutralized by 10 µg/ml IgG control or anti-TLR5 antibodies and cells were then incubated with 2% RA SF plus IgG or 2% RA SF plus anti-TNF-α (10 µg/ml) subsequent to TRAP staining, n=4. Negative and positive control consisted of untreated cells or cells treated with 20 ng/ml M-CSF and RANKL. Values demonstrate mean ± SEM, * represents p<0.05.
Flagellin fosters osteoclastogenesis in mouse PB and bone marrow cells via TNF-α which is distinct from IFN-β pathway
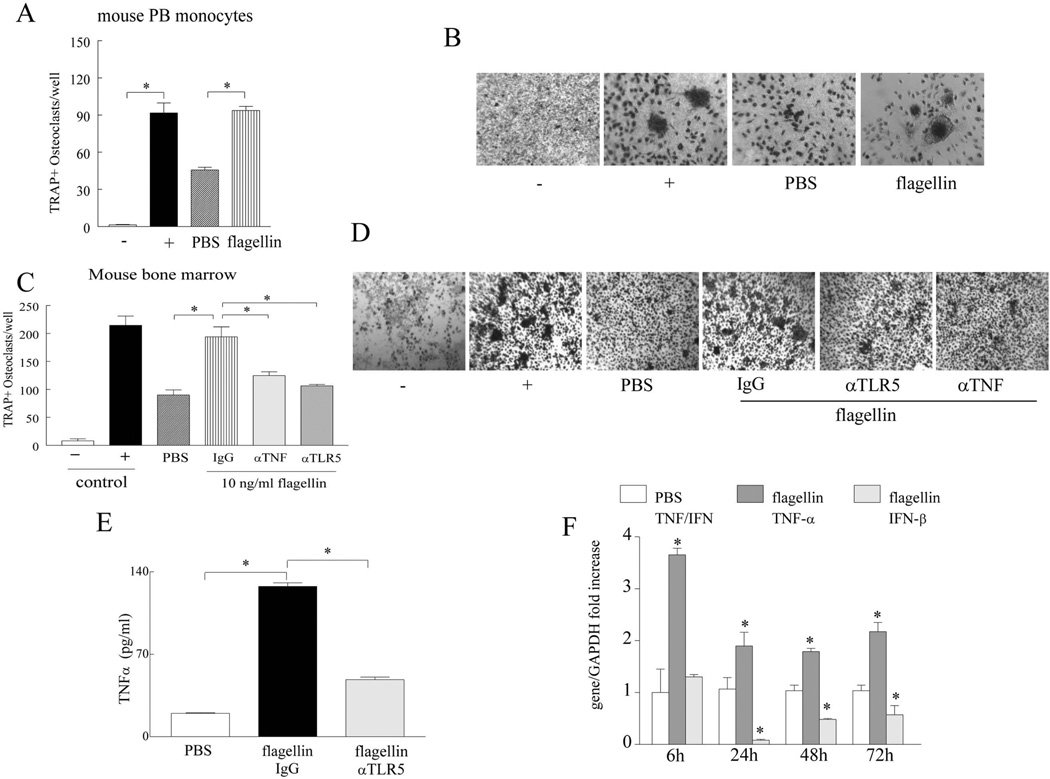
In contrast to previous findings (37), we document that ligation of TLR5 plays a critical role in osteoclastogenesis as determined in vitro in RA PB myeloid cells and in vivo in acute and chronic models of experimental arthritis. Therefore experiments were performed for the first time in mouse PB myeloid cells as well as in bone marrow cells to address the data discrepancy. We show that flagellin activation transforms negatively selected mouse PB monocytes cultured in suboptimal conditions into multi-nuclei mature osteoclasts (Figs. 5A–B). Because of limited access to mouse PB monocytes (blood from 20 mice was utilized in order to obtain adequate mouse PB myeloid cells for 4 wells/conditions), mouse bone marrow cells were employed to determine the mechanism by which flagellin ligation to TLR5 promotes osteoclastogenesis. Consistent with our results in RA cells, we demonstrate that blockade of TLR5 or TNF-α in mouse bone marrow osteoclast precursor cells significantly reduces flagellin induced osteoclast maturation in part by inhibiting TNF-α production (Figs. 5C–E). In mouse bone marrow osteoclast precursor cells, flagellin treatment elevates TNF-α transcription by 4 fold at 6h and these levels remain constantly 2 fold higher from 24 to 72h of stimulation. While IFN-β mRNA levels are not significantly accentuated at 6h stimulation and are further reduced following 24 to 72h of flagellin activation (Fig. 5F). These results suggest that similar to RA myeloid cells, ligation of murine TLR5 cells by flagellin can strongly promote osteoclast formation in part through production of TNF-α and dysregulation of IFN-β cascade.
Figure 5. Flagellin strongly drives differentiation of murine PB monocytes and bone marrow cells to mature osteoclasts through TNF-α activation.
A. Mouse PB monocytes were negatively selected and cultured in 10% α-MEM, 20 ng/ml of mouse M-CSF and RANKL. Mouse PB monocytes were either untreated (PBS) or treated with flagellin (10 ng/ml) for 14–21 days prior to TRAP staining. Mouse PB monocytes cultured in 10% α-MEM alone were considered as negative control and cells cultured in presence of 40 ng/ml M-CSF and RANKL served as the positive control. B. Is a representative image of TRAP+ cells (original magnification x 400) from A, n=3. C. Mouse bone marrow cells cultured for 4 days in 10% α-MEM, 10 ng/ml of mouse M-CSF plus 25 ng/ml mouse RANKL were untreated or stimulated with flagellin (10 ng/ml) plus IgG (10 µg/ml), flagellin (10 ng/ml) plus anti-TLR5 (10 µg/ml) or flagellin (10 ng/ml) plus anti-TNF-α (10 µg/ml) for 3 additional days before TRAP staining, n=3. Mouse bone marrow cells cultured in 10% α-MEM alone served as negative control and cells supplemented with mouse 10 ng/ml M-CSF and 100 ng/ml RANKL served as positive control. D. Is a representative image of TRAP+ cells (original magnification x 200) from C. E. Mouse TNF-α protein concentration was determined by ELISA in day 4 mouse bone marrow precursor cells cultured in 10% α-MEM, 10 ng/ml of mouse M-CSF plus 25 ng/ml mouse RANKL either untreated (PBS) or treated with flagellin (100 ng/ml) plus IgG (10 µg/ml) versus flagellin (100 ng/ml) plus anti-TLR5 (10 µg/ml) for 24 h, n=5. F. TNF-α and IFN-β real time RT-PCR was performed on mouse bone marrow cells from day 4 cultured in 10% α-MEM, 10 ng/ml of mouse M-CSF plus 25 ng/ml mouse RANKL and treated with or without flagellin (100 ng/ml) for 6, 24, 48 and 72h, n=3. Values demonstrate mean ± SEM, * represents p<0.05.
Post onset treatment of CIA with flagellin contributes to elevated joint myeloid cell infiltration and osteoclastogenesis
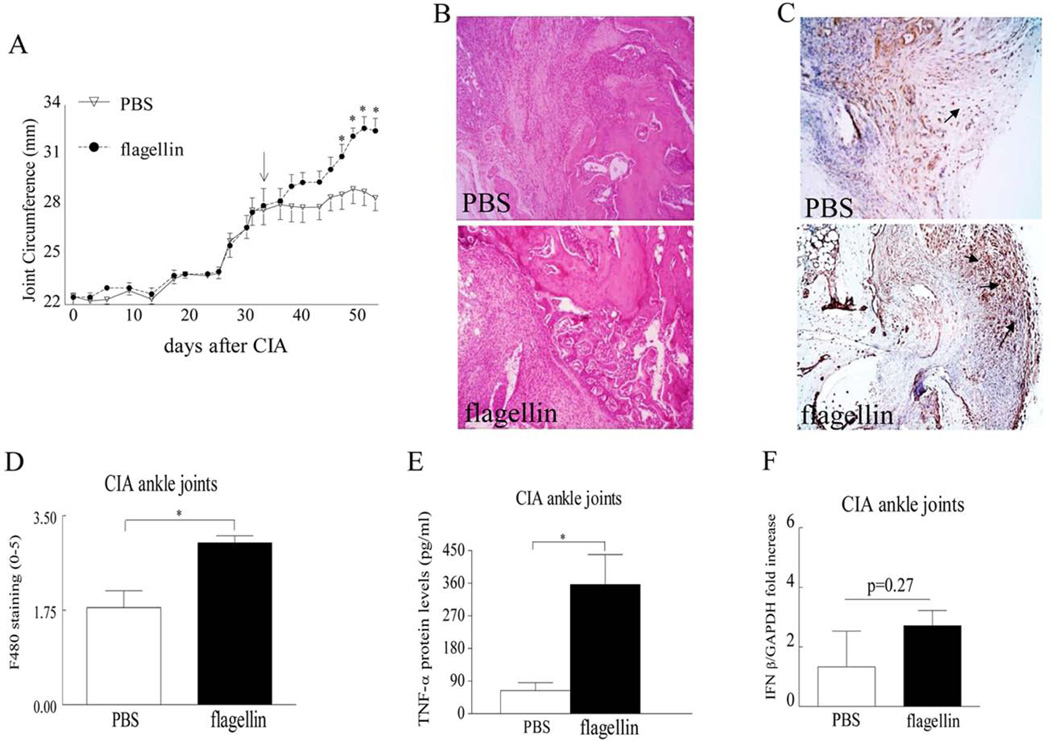
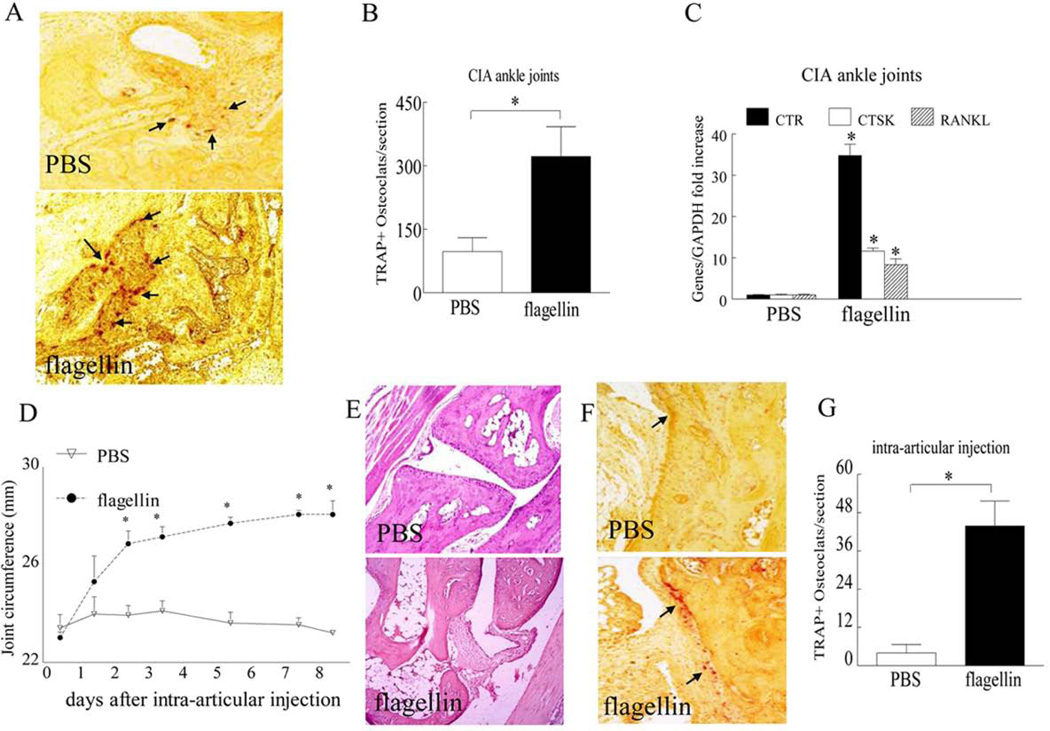
We next asked whether acute and/or chronic arthritis driven by TLR5 ligation are due to homing and differentiation of myeloid cells into mature osteoclasts. We document that when CIA mice were therapeutically treated with TLR5 agonist, joint swelling was markedly greater in mice that received flagellin treatment compared to PBS control (Figs. 6A–B). Similar to our in vitro studies, we found that post onset treatment with flagellin could strongly facilitate myeloid cell recruitment into the CIA ankle joints (Figs. 6C–D). Further, joint TNF-α production levels were 5.5 fold higher, while transcription of IFN-β was unchanged in CIA mice that received post onset treatment of flagellin compared to the control group (Figs. 6E–F). We also show that the number of TRAP+ cells (Figs. 7A–B) and the concentration of bone erosion markers calcitonin receptor, Cathepsin K and RANKL (Fig. 7C) were lower in the control ankles compared to the flagellin treated CIA joints. These results indicate that the elevated joint myeloid cell migration and their differentiation to osteoclasts may be due to TLR5 ligation as well as TNF-α production or it is possible that both mechanism of action contribute to the detected observations.
Figure 6. Flagellin post onset treatment in CIA contributes to joint inflammation, elevated joint myeloid cell trafficking and TNF-α production.
A. Changes in joint circumference was determined in CIA mice that were treated i.p. with PBS or flagellin (20 µg) on day 33, n= 10. B. Ankles were harvested on day 57 from CIA mice treated with PBS or flagellin and were H&E stained (original magnification x 200), n=7. C. STs from CIA mice treated with PBS or flagellin were harvested on day 57 and were immunostained with anti-F480 antibody (original magnification x 200), arrows demonstrate F480+ cells. D. Macrophage staining was quantified on a 0–5 scale, n=7. E. TNF-α protein levels (pg/ml) were quantified by ELISA in ankle homogenates from CIA mice treated with PBS or flagellin, n=7. F. Transcription of IFN-β was determined by real-time RT-PCR in CIA ankles which had received post onset treatment of flagellin or control and the data are shown as fold increase above PBS group and are normalized to GAPDH, n=5.Values are mean ± SE. * indicates p<0.05.
Figure 7. TLR5 ligation facilitates osteoclast formation in vivo in RA animal models.
A. Ankles harvested from CIA mice treated with PBS or flagellin were TRAP stained (TRAP+ cells are shown by arrows) and (B) the number of TRAP+ cells were counted per section, n=7. C. Transcription of calcitonin receptor (CTR), Cathepsin K (CTSK) and RANKL was determined by real-time RT-PCR in CIA ankles which had received post onset treatment of flagellin or control and the data are shown as fold increase above PBS group and are normalized to GAPDH, n=5. D. Changes in joint circumference in mice i.a. injected with PBS or 20µg of flagellin; n=5. Ankles harvested from local injection of PBS or flagellin on day 10 were H&E (E) and TRAP (F) stained (TRAP+ cells are shown by arrows) and (G) number of TRAP+ cells were counted per section, n=5. Values are mean ± SE. * indicates p<0.05.
Since CIA ankle swelling and bone erosion were exacerbated by flagellin post onset treatment, we next asked whether local injection of flagellin alone could drive joint inflammation and osteoclastogenesis. We demonstrate that ectopic TLR5 ligation elevates ankle circumference from day 0 to 2, subsequently joint inflammation plateaus until day 8, however swelling remains consistently higher than PBS group (Figs. 7D–E). Corroborating with the CIA data, i.a. injection with flagellin resulted in 10 fold greater mature osteoclasts compared to the control group (Figs. 7F–G). Hence consistent with our in vitro studies, data generated in acute and chronic animal models suggest that flagellin ligation to joint TLR5 contributes directly as well as indirectly to myeloid cell infiltration and osteoclast maturation.
Blockade of TLR5 function impairs CIA joint monocyte infiltration and osteoclast formation
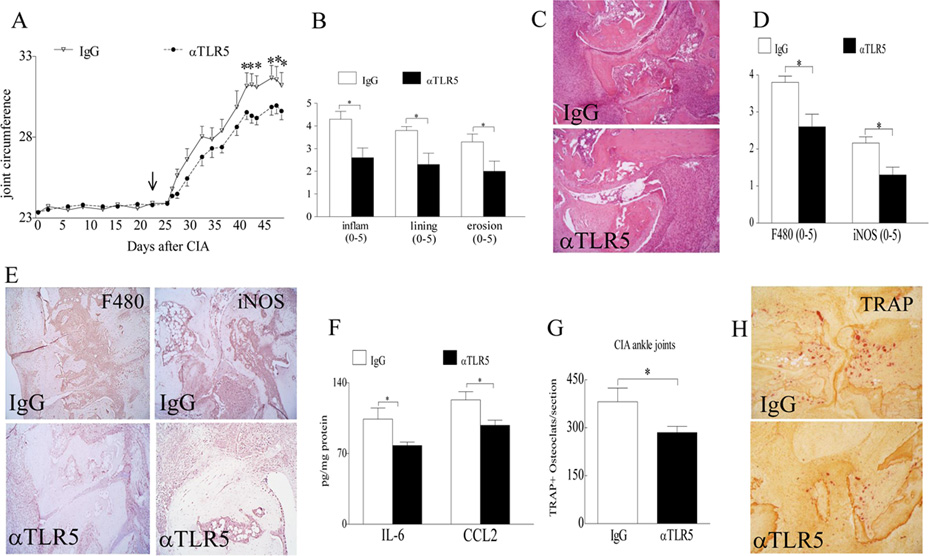
To document the critical role of TLR5 in RA pathogenesis, CIA mice were systemically treated with IgG or anti-TLR5 antibody. Results from these experiments demonstrate that CIA mice treated with anti-TLR5 antibody have markedly reduced joint swelling starting on day 44 until day 48 compared to the IgG control group (Figs. 8A–C). When the underlying mechanism of function was examined, we found that blockade of TLR5 inhibited F480CD80+iNOS+ M1 macrophage differentiation in CIA ankle joints (Figs. 8D–E) which resulted in significantly lower production of joint IL-6 and CCL2 protein levels compared to the IgG control group (Fig. 8F). Additionally, we show that joint myeloid cell migration and their remodeling to mature osteoclasts were compromised in the anti-TLR5 antibody group compared to the control group (Figs. 8G–H). Since blockade of TLR5 function ameliorates joint myeloid cell trafficking and their transformation into M1 macrophages and/or fully mature osteoclasts, these results suggest that TLR5 can be utilized as a target for RA therapy.
Figure 8. Anti-TLR5 antibody treatment alleviates CIA joint swelling and bone resorption.
A. Changes in joint circumference were recorded for CIA mice that were treated i.p. with IgG or anti-TLR5 Ab (100 µg/mouse) on days 23, 27, 30, 34, 37, 41, 44 and 48 and mice were sacrificed on day 49 post induction, n=6 mice (12 ankles). B. Effect of anti-TLR5 Ab treatment on inflammation, lining thickness, and bone erosion was scored on a 0–5 scale, n=6. C. Is the representative ankle H&E staining (original magnification × 200) of Fig. B. D. Synovial tissues from CIA mice treated with IgG or anti-TLR5 antibody were harvested on day 49 and immunostained with anti-F480 (1:100 dilution) or iNOS Abs (1:200 dilution) (original magnification × 200). Joint myeloid cells and iNOS+ M1 macrophages staining were quantified on a 0–5 scale, n=6. E. Is the representative F480 and iNOS immunostaining (original magnification × 200) of Fig. D. F. Changes in IL-6 and CCL2 protein levels in ankle homogenates from CIA mice treated with IgG control or anti-TLR5 antibody were determined by ELISA, n=6. G. Number of TRAP+ cells were counted per section in CIA mice treated with IgG or anti-TLR5 Ab, n=6. H. Is the representative ankle TRAP staining (original magnification × 200) of Fig. G. Values are mean ± SE. *p < 0.05.
DISCUSSION
In the current study, we demonstrate for the first time that myeloid TLR5 ligation to endogenous ligands expressed in the RA joint strongly promotes monocyte trafficking and osteoclast formation. We document that TLR5 and TNF-α pathways are cross regulated, as ligation of TLR5 in RA and mouse osteoclast precursor cells activates TNF-α production and anti-TNF-α therapy markedly reduces RA myeloid TLR5 expression. Flagellin treatment in mouse PB and bone marrow cells as well as in CIA ankle joints reveal that joint TLR5 ligation contributes to elevated osteoclast maturation through a TNF-α dependent and IFN-β independent mechanism. Finally, amelioration of joint inflammation and bone destruction by anti-TLR5 antibody therapy in CIA, further establishes TLR5 as a novel RA therapeutic target.
Our initial observation that TLR5 expression is elevated in RA myeloid cells and has a close correlation with disease activity and myeloid TNF-α concentration (29) triggered our interest in unraveling how ligation of myeloid TLR5 impacts RA pathogenesis. Since increase in the number of joint myeloid cells can be due to increased chemotaxis, reduced efflux or cell death, we examined the role of TLR5 in monocyte chemotaxis. We demonstrate that RA SF TLR5 endogenous ligand(s) participate in joint monocyte homing through activation of PI3K/AKT, JNK and NF-κB which is distinct from the pathway employed by classical monocyte chemoattractants such as CCL2/MCP-1, CCL5/RANTES, CCL3/MIP-1α, FMLP and IL-17 that utilize p38 cascade (14, 39).
We further demonstrate that TLR5 induced monocyte migration is potentiated by TNF-α as blockade of both pathways can more efficiently reduce RA SF mediated myeloid cell recruitment. TNF-α is shown to be a potent in vitro (13) and in vivo (40) monocyte chemoattractant that facilitates monocyte trans-endothelial migration in part via enhancing endothelial adhesion molecules ICAM-1 and CD44 (41). Consistently, RA responders to anti-TNF-α therapy have significantly reduced numbers of RA ST CD68+ sublining macrophages (42). Others demonstrate that trans-endothelial monocyte trafficking mediated by TLR4 ligation is directly facilitated via induction of PECAM1 on endothelial cells (43) and indirectly through production of CCL2/MCP-1 from myeloid cells (44) and that anti-TNF-α treatment had no effect on this function (45). This suggests that monocyte trafficking driven by TLR4 is differentially modulated compared with TLR5 and consequently this process is unassociated with the TNF pathway.
Our novel data demonstrate that in RA PBMCs, TLR5 ligation can potently facilitate osteoclast differentiation by activating transcription of RANK and TNF-α from myeloid cells and RANKL from T cells. Interestingly, upregulation of RANK on myeloid cells and further production of TNF-α from osteoclast precursors is adequate for promoting TLR5 mediated osteoclast formation and T cell presence is not required when suboptimal doses of RANKL is employed. Previous studies demonstrate that TLR4 ligation can promote osteoclast formation in RANKL pretreated mouse bone marrow cells through a TNF-α related cascade (21). However, when mouse pre-osteoclasts were simultaneously treated with LPS, RANKL and M-CSF, osteoclast maturation was suppressed by TLR4 ligation despite elevated TNF-α production (21, 23). It was shown that the inhibitory effect of TLR4 ligation on osteoclastgenesis was due to downregulation of RANK as well as elevated expression of osteoprotegerin (OPG) or IFN-γ (22, 24). Others have shown that similar to TLR4, ligation of TLR5 inhibits transformation of mouse bone marrow cells to differentiated osteoclasts through IFN-β induction (37). In contract to the previous studies (37), we document that in RA and murine cells as well as in CIA ankles, flagellin treatment was unable to enhance IFN-β transcription, and this may in part be due to the robust TNF-α transcription, as earlier findings demonstrate that TNF-α signaling can counterbalance type I (IFN-β and other isoforms) and type II (IFN-γ) IFN function (38, 46–48).
Much like our findings in RA (29), earlier studies demonstrate that TLR5 is elevated in cancer compared to normal lingual epithelium and may be a novel predictive marker for tongue cancer recurrence (49). Notably, while flagellin can strongly facilitate migration and invasion of the salivary gland adenocarcinoma cell line, LPS and Pam3CSK4 did not have any effect on these functions (50) suggesting that TLR5 shows some unique characteristics that do not overlap with other TLRs both in cancer and RA.
Despite extensive studies, controversial results were obtained when the effect of TLR4 ligation was examined on osteoclastogenesis of mouse bone marrow cells as the outcome was heavily dependent on the cell treatment conditions (21–23). Therefore, in the current study the pathogenic role of TLR5 was assessed in RA and mouse cells as well as in experimental arthritis models. Unlike results generated in mouse bone marrow studies, ligation of TLR2 and TLR4 in RA ST fibroblasts co-cultured with myeloid cells could strongly promote osteoclast formation through induction of IL-1β and RANKL (51). In contrast to TLR2 and TLR4 mediated osteoclastogenesis which is driven by IL-1β (51), our data demonstrate a strong connection between TLR5 and TNF-α in joint bone erosion.
Notably, it was demonstrated by two independent groups that ligation of TNFR1 and TNFR2 by TNF-α could strongly drive osteoclast differentiation and this process was unaffected by blockade of RANKL via OPG (10, 11). Confirming this finding, others show that macrophages from RA SF can differentiate to fully mature osteoclasts in presence of M-CSF with RANKL or TNF-α/IL-1β suggesting that factors such as TNF-α and IL-β can substitute RANKL’s function in the RA joint (12). The same group found that osteoclast formation was more pronounced in RA SF that contained higher TNF-α levels (52). We document that transcription of RA SF TNF-α is modulated by SF TLR5 endogenous ligands (29) and that TLR5 interconnects with TNF-α in enhancing joint osteoclastogenesis. Therefore these observations indicate that SF with most abundant TNF-α may be a result of elevated TLR5 endogenous ligands that contribute to markedly enhanced bone degradation.
Interestingly a number of endogenous ligands have been identified that can activate TLR4 function (including fibrinogen, surfactant protein-A, fibronectin extra domain A, heparan sulfate, soluble hyaluronan, and defensin 2), while other endogenous ligands can trigger both TLR2 and TLR4 pathways [such as heat shock proteins (HSP60, 70 and 96) and HMGB1] (53, 54). Most recently RNA extracted from RA SF was shown to be a TLR7 endogenous ligand that could dose dependently modulate RA SF TNF-α transcription (55). Earlier studies demonstrate that stimulation with HSP70 can further potentiate flagellin mediated NF-κB luciferase activity in TLR5 transfected HEK 293T reporter cells, but since this effect was not detected with HSP70 treatment alone these results suggest that HSP70 may operate as a chaperone protein for TLR5 endogenous ligands (56). There is still a possibility that other HSPs can serve as a potential TLR5 endogenous ligand in the RA joint, therefore experiments are currently being conducted to identify these TLR5 endogenous ligands in RA SFs and STs.
While gain of TLR5 function promotes myeloid cell recruitment and osteoclast formation partly through potentiating the joint TNF-α production, loss of TLR5 function reverses both mechanism of function in part by suppressing the proinflammatory M1 macrophage differentiation process in CIA ankle joints. In contrast, joint inflammation and bone erosion mediated by local injection of TNF-α was unaffected in TLR2 and TLR4 deficient mice suggesting that TNF-α driven joint pathology is independent of TLR2 and TLR4 function (26). On the contrary, severity of IL-1 induced bone erosion and cartilage destruction was markedly suppressed in TLR4−/− mice. Similarly, TLR4 induced calvarial bone resorption was significantly reduced in IL-1R−/− compared to wild type mice (28). These results suggest that while TLR5 mediated myeloid cell trafficking and differentiation to osteoclasts is linked to TNF-α pathway, TLR4 induced monocyte infiltration is unrelated to TNF-α, whereas its bone erosion is dependent on IL-1β.
In summary, we show that SF TLR5 endogenous ligands participate in recruiting circulating myeloid cells into the joint and further facilitating their differentiation into mature osteoclasts and both functions are interconnected to joint TNF-α cascade. Thus, the connection between TLR5 and TNF cascade and the preclinical evidence obtained from TLR5 loss of function, highlight the importance of this receptor in RA disease.
Acknowledgments
This work was supported in part by awards from the National Institutes of Health AR056099 and AR065778, grant from Within Our Reach from The American College of Rheumatology, funding provided by Department of Defense PR093477 and Arthritis Foundation Innovative Research Grant.
Abbreviation
- RA
Rheumatoid Arthritis
- OA
osteoarthritis
- NL
normal
- PB
peripheral blood
- SF
synovial fluid
- DAS28
disease activity score based on 28 defined joints
- RANKL
receptor activator for nuclear factor κB
- DMARD
disease modifying anti-rheumatic drug
- HSP
heat shock protein
- TRAPT
artrate Resistant Acid Phosphatase
- PBMCs
peripheral blood mononuclear cells
- CTR
calcitonin receptor
- CTSK
Cathepsin K
- CIA
collagen induced arthritis
- M-CSF
macrophage colony-stimulating factor.
REFERENCES
- 1.Brentano F, Kyburz D, Schorr O, Gay R, Gay S. The role of Toll-like receptor signalling in the pathogenesis of arthritis. Cell Immunol. 2005;233:90–96. doi: 10.1016/j.cellimm.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Drexler SK, Sacre SM, Foxwell BM. Toll-like receptors: a new target in rheumatoid arthritis? Expert Rev Clin Immunol. 2006;2:585–599. doi: 10.1586/1744666X.2.4.585. [DOI] [PubMed] [Google Scholar]
- 3.Fujikawa Y, Sabokbar A, Neale S, Athanasou NA. Human osteoclast formation and bone resorption by monocytes and synovial macrophages in rheumatoid arthritis. Ann Rheum Dis. 1996;55:816–822. doi: 10.1136/ard.55.11.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schett G. Joint remodelling in inflammatory disease. Ann Rheum Dis. 2007;3(66 Suppl):ii42–ii44. doi: 10.1136/ard.2007.078972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun T, Zwerina J. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Res Ther. 2011;13:235. doi: 10.1186/ar3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis. 2002;2(61 Suppl):i84–i86. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 8.Walsh NC, Gravallese EM. Bone loss in inflammatory arthritis: mechanisms and treatment strategies. Curr Opin Rheumatol. 2004;16:419–427. doi: 10.1097/01.bor.0000127824.42507.68. [DOI] [PubMed] [Google Scholar]
- 9.Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8:656–664. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ. TNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology. 2002;143:1108–1118. doi: 10.1210/endo.143.3.8701. [DOI] [PubMed] [Google Scholar]
- 12.Adamopoulos IE, Sabokbar A, Wordsworth BP, Carr A, Ferguson DJ, Athanasou NA. Synovial fluid macrophages are capable of osteoclast formation and resorption. J Pathol. 2006;208:35–43. doi: 10.1002/path.1891. [DOI] [PubMed] [Google Scholar]
- 13.Ming WJ, Bersani L, Mantovani A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol. 1987;138:1469–1474. [PubMed] [Google Scholar]
- 14.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol. 2009;182:3884–3891. doi: 10.4049/jimmunol.0802246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahrara S, Pickens SR, Mandelin AM, 2nd, Karpus WJ, Huang Q, Kolls JK, Pope RM. IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J Immunol. 2010;184:4479–4487. doi: 10.4049/jimmunol.0901942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwahashi M, Yamamura M, Aita T, Okamoto A, Ueno A, Ogawa N, Akashi S, Miyake K, Godowski PJ, Makino H. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2004;50:1457–1467. doi: 10.1002/art.20219. [DOI] [PubMed] [Google Scholar]
- 17.Radstake TR, Roelofs MF, Jenniskens YM, Oppers-Walgreen B, van Riel PL, Barrera P, Joosten LA, van den Berg WB. Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis Rheum. 2004;50:3856–3865. doi: 10.1002/art.20678. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen LK, Havemose-Poulsen A, Sonder SU, Bendtzen K, Holmstrup P. Blood cell gene expression profiling in subjects with aggressive periodontitis and chronic arthritis. J Periodontol. 2008;79:477–485. doi: 10.1902/jop.2008.070309. [DOI] [PubMed] [Google Scholar]
- 19.Huang Q, Ma Y, Adebayo A, Pope RM. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56:2192–2201. doi: 10.1002/art.22707. [DOI] [PubMed] [Google Scholar]
- 20.Huang QQ, Pope RM. The role of toll-like receptors in rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:357–364. doi: 10.1007/s11926-009-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou W, Bar-Shavit Z. Dual modulation of osteoclast differentiation by lipopolysaccharide. J Bone Miner Res. 2002;17:1211–1218. doi: 10.1359/jbmr.2002.17.7.1211. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi T, Matsuguchi T, Tsuboi N, Mitani A, Tanaka S, Matsuoka M, Yamamoto G, Hishikawa T, Noguchi T, Yoshikai Y. Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoblasts via Toll-like receptors. J Immunol. 2001;166:3574–3579. doi: 10.4049/jimmunol.166.5.3574. [DOI] [PubMed] [Google Scholar]
- 23.Takami M, Kim N, Rho J, Choi Y. Stimulation by toll-like receptors inhibits osteoclast differentiation. J Immunol. 2002;169:1516–1523. doi: 10.4049/jimmunol.169.3.1516. [DOI] [PubMed] [Google Scholar]
- 24.Ji JD, Park-Min KH, Shen Z, Fajardo RJ, Goldring SR, McHugh KP, Ivashkiv LB. Inhibition of RANK expression and osteoclastogenesis by TLRs and IFN-gamma in human osteoclast precursors. J Immunol. 2009;183:7223–7233. doi: 10.4049/jimmunol.0900072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh K, Udagawa N, Kobayashi K, Suda K, Li X, Takami M, Okahashi N, Nishihara T, Takahashi N. Lipopolysaccharide promotes the survival of osteoclasts via Toll-like receptor 4, but cytokine production of osteoclasts in response to lipopolysaccharide is different from that of macrophages. J Immunol. 2003;170:3688–3695. doi: 10.4049/jimmunol.170.7.3688. [DOI] [PubMed] [Google Scholar]
- 26.Abdollahi-Roodsaz S, Joosten LA, Koenders MI, van den Brand BT, van de Loo FA, van den Berg WB. Local interleukin-1-driven joint pathology is dependent on toll-like receptor 4 activation. Am J Pathol. 2009;175:2004–2013. doi: 10.2353/ajpath.2009.090262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdollahi-Roodsaz S, Joosten LA, Helsen MM, Walgreen B, van Lent PL, van den Bersselaar LA, Koenders MI, van den Berg WB. Shift from toll-like receptor 2 (TLR-2) toward TLR-4 dependency in the erosive stage of chronic streptococcal cell wall arthritis coincident with TLR-4-mediated interleukin-17 production. Arthritis Rheum. 2008;58:3753–3764. doi: 10.1002/art.24127. [DOI] [PubMed] [Google Scholar]
- 28.Chiang CY, Kyritsis G, Graves DT, Amar S. Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect Immun. 1999;67:4231–4236. doi: 10.1128/iai.67.8.4231-4236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamberlain ND, Vila OM, Volin MV, Volkov S, Pope RM, Swedler W, Mandelin AM, 2nd, Shahrara S. TLR5, a novel and unidentified inflammatory mediator in rheumatoid arthritis that correlates with disease activity score and joint TNF-alpha levels. J Immunol. 2012;189:475–483. doi: 10.4049/jimmunol.1102977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickens SR, Chamberlain ND, Volin MV, Pope RM, Mandelin AM, 2nd, Shahrara S. Characterization of CCL19 and CCL21 in rheumatoid arthritis. Arthritis Rheum. 2011;63:914–922. doi: 10.1002/art.30232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickens SR, Chamberlain ND, Volin MV, Pope RM, Talarico NE, Mandelin AM, 2nd, Shahrara S. Characterization of IL-7 and IL-7R in the pathogenesis of Rheumatoid Arthritis. Arthritis Rheum. 2011 doi: 10.1002/art.30493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TAJ, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 33.Redlich K, Hayer S, Ricci R, David JP, Tohidast-Akrad M, Kollias G, Steiner G, Smolen JS, Wagner EF, Schett G. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest. 2002;110:1419–1427. doi: 10.1172/JCI15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickens SR, Chamberlain ND, Volin MV, Mandelin AM, 2nd, Agrawal H, Matsui M, Yoshimoto T, Shahrara S. Local expression of interleukin-27 ameliorates collagen-induced arthritis. Arthritis Rheum. 2011;63:2289–2298. doi: 10.1002/art.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Z, Kim SJ, Chamberlain ND, Pickens SR, Volin MV, Volkov S, Arami S, Christman JW, Prabhakar BS, Swedler W, Mehta A, Sweiss N, Shahrara S. The Novel Role of IL-7 Ligation to IL-7 Receptor in Myeloid Cells of Rheumatoid Arthritis and Collagen-Induced Arthritis. J Immunol. 2013 doi: 10.4049/jimmunol.1201675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pickens SR, Chamberlain ND, Volin MV, Gonzalez M, Pope RM, Mandelin AM, 2nd, Kolls JK, Shahrara S. Anti-CXCL5 therapy ameliorates IL-17-induced arthritis by decreasing joint vascularization. Angiogen. 2011 doi: 10.1007/s10456-011-9227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ha H, Lee JH, Kim HN, Kwak HB, Kim HM, Lee SE, Rhee JH, Kim HH, Lee ZH. Stimulation by TLR5 modulates osteoclast differentiation through STAT1/IFN-beta. J Immunol. 2008;180:1382–1389. doi: 10.4049/jimmunol.180.3.1382. [DOI] [PubMed] [Google Scholar]
- 38.Cantaert T, Baeten D, Tak PP, van Baarsen LG. Type I IFN and TNFalpha cross-regulation in immune-mediated inflammatory disease: basic concepts and clinical relevance. Arthritis Res Ther. 2010;12:219. doi: 10.1186/ar3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayala JM, Goyal S, Liverton NJ, Claremon DA, O’Keefe SJ, Hanlon WA. Serum-induced monocyte differentiation and monocyte chemotaxis are regulated by the p38 MAP kinase signal transduction pathway. J Leukoc Biol. 2000;67:869–875. [PubMed] [Google Scholar]
- 40.Ruth JH, Haas CS, Park CC, Amin MA, Martinez RJ, Haines GK, 3rd, Shahrara S, Campbell PL, Koch AE. CXCL16-mediated cell recruitment to rheumatoid arthritis synovial tissue and murine lymph nodes is dependent upon the MAPK pathway. Arthritis Rheum. 2006;54:765–778. doi: 10.1002/art.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kindle L, Rothe L, Kriss M, Osdoby P, Collin-Osdoby P. Human microvascular endothelial cell activation by IL-1 and TNF-alpha stimulates the adhesion and transendothelial migration of circulating human CD14+ monocytes that develop with RANKL into functional osteoclasts. J Bone Miner Res. 2006;21:193–206. doi: 10.1359/JBMR.051027. [DOI] [PubMed] [Google Scholar]
- 42.Wijbrandts CA, Dijkgraaf MG, Kraan MC, Vinkenoog M, Smeets TJ, Dinant H, Vos K, Lems WF, Wolbink GJ, Sijpkens D, Dijkmans BA, Tak PP. The clinical response to infliximab in rheumatoid arthritis is in part dependent on pretreatment tumour necrosis factor alpha expression in the synovium. Ann Rheum Dis. 2008;67:1139–1144. doi: 10.1136/ard.2007.080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen Y, Sultana C, Arditi M, Kim KS, Kalra VK. Endotoxin-induced migration of monocytes and PECAM-1 phosphorylation are abrogated by PAF receptor antagonists. Am J Physiol. 1998;275:E479–E486. doi: 10.1152/ajpendo.1998.275.3.E479. [DOI] [PubMed] [Google Scholar]
- 44.Tajima T, Murata T, Aritake K, Urade Y, Hirai H, Nakamura M, Ozaki H, Hori M. Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2) J Pharmacol Exp Ther. 2008;326:493–501. doi: 10.1124/jpet.108.137992. [DOI] [PubMed] [Google Scholar]
- 45.Doherty DE, Zagarella L, Henson PM, Worthen GS. Lipopolysaccharide stimulates monocyte adherence by effects on both the monocyte and the endothelial cell. J Immunol. 1989;143:3673–3679. [PubMed] [Google Scholar]
- 46.Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc. Natl. Acad. Sci. USA. 2005;102:3372–3377. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mavragani CP, Niewold TB, Moutsopoulos NM, Pillemer SR, Wahl SM, Crow MK. Augmented interferon-alpha pathway activation in patients with Sjogren’s syndrome treated with etanercept. Arthritis Rheum. 2007;56:3995–4004. doi: 10.1002/art.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ralston SH, Ho LP, Helfrich MH, Grabowski PS, Johnston PW, Benjamin N. Nitric oxide: a cytokine-induced regulator of bone resorption. J Bone Miner Res. 1995;10:1040–1049. doi: 10.1002/jbmr.5650100708. [DOI] [PubMed] [Google Scholar]
- 49.Kauppila JH, Mattila AE, Karttunen TJ, Salo T. Toll-like receptor 5 (TLR5) expression is a novel predictive marker for recurrence and survival in squamous cell carcinoma of the tongue. Br J Cancer. 2013;108:638–643. doi: 10.1038/bjc.2012.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim SJ, Chen Z, Chamberlain ND, Volin MV, Swedler W, Volkov S, Sweiss N, Shahrara S. Angiogenesis in Rheumatoid Arthritis Is Fostered Directly by Toll-like Receptor 5 Ligation and Indirectly Through Interleukin-17 Induction. Arthritis Rheum. 2013;65:2024–2036. doi: 10.1002/art.37992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim KW, Cho ML, Lee SH, Oh HJ, Kang CM, Ju JH, Min SY, Cho YG, Park SH, Kim HY. Human rheumatoid synovial fibroblasts promote osteoclastogenic activity by activating RANKL via TLR-2 and TLR-4 activation. Immunol Lett. 2007;110:54–64. doi: 10.1016/j.imlet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Adamopoulos IE, Danks L, Itonaga I, Locklin RM, Sabokbar A, Ferguson DJ, Athanasou NA. Stimulation of osteoclast formation by inflammatory synovial fluid. Virchows Arch. 2006;449:69–77. doi: 10.1007/s00428-006-0200-y. [DOI] [PubMed] [Google Scholar]
- 53.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 54.Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009;85:905–910. doi: 10.1189/jlb.0109005. [DOI] [PubMed] [Google Scholar]
- 55.Chamberlain ND, Kim SJ, Vila OM, Volin MV, Volkov S, Pope RM, Arami S, Mandelin AM, 2nd, Shahrara S. Ligation of TLR7 by rheumatoid arthritis synovial fluid single strand RNA induces transcription of TNFalpha in monocytes. Ann Rheum Dis. 2013;72:418–426. doi: 10.1136/annrheumdis-2011-201203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye Z, Gan YH. Flagellin contamination of recombinant heat shock protein 70 is responsible for its activity on T cells. J. Biol. Chem. 2007;282:4479–4484. doi: 10.1074/jbc.M606802200. [DOI] [PubMed] [Google Scholar]