Abstract
Cardiovascular diseases cause more mortality and morbidity worldwide than any other diseases. Although many intracellular signaling pathways influence cardiac physiology and pathology, the mitogen-activated protein kinase (MAPK) family has garnered significant attention because of its vast implications in signaling and cross-talk with other signaling networks. The extensively studied MAPKs ERK1/2, p38, JNK, and ERK5, demonstrate unique intracellular signaling mechanisms, responding to a myriad of mitogens and stressors and influencing the signaling of cardiac development, metabolism, performance, and pathogenesis. Definitive relationships between MAPK signaling and cardiac dysfunction remain elusive, despite 30 years of extensive clinical studies and basic research of various animal/cell models, severities of stress, and types of stimuli. Still, several studies have proven the importance of MAPK cross-talk with mitochondria, powerhouses of the cell that provide over 80% of ATP for normal cardiomyocyte function and play a crucial role in cell death. Although many questions remain unanswered, there exists enough evidence to consider the possibility of targeting MAPK-mitochondria interactions in the prevention and treatment of heart disease. The goal of this review is to integrate previous studies into a discussion of MAPKs and MAPK-mitochondria signaling in cardiac diseases, such as myocardial infarction (ischemia), hypertrophy and heart failure. A comprehensive understanding of relevant molecular mechanisms, as well as challenges for studies in this area, will facilitate the development of new pharmacological agents and genetic manipulations for therapy of cardiovascular diseases.
Keywords: heart, MAPK, cardiac diseases, mitochondria, cell signaling
1. Introduction
Cardiovascular diseases cause more mortality and morbidity worldwide than any other class of diseases, accounting for 31.9% of the 2.47 million deaths in the United States in 2010 (Go et al., 2014). The pathogenesis of cardiac decompensation, or heart failure (HF), can be chronic or acute in nature and is of major clinical concern. Coronary artery disease, myocardial infarction (MI), hypertension, cardiomyopathy, myocarditis, arrhythmia, hyperlipidemia, and diabetes, among other diseases, can promote the onset or progression of HF by increasing blood pressure or blood volume or reducing contractility. Indeed, myocardial ischemia is the most common cause of HF. Insufficient blood supply due to partial or complete occlusion of coronary arteries deprives the myocardium of the oxygen and substrates needed for cardiac metabolism, leading to MI and localized necrosis. The remaining cardiomyocytes must compensate for the lack of function from necrotic myocytes, so the ventricles adaptively remodel immediately after MI to maintain cardiac output (Sutton and Sharpe, 2000, Dorn, 2009). Remodeling is a complex process involving multiple signaling pathways associated with ionic regulation, reactive oxygen species (ROS) generation, substrate utilization and energy synthesis in response to cellular remodeling. Initially, cardiomyocytes can overcome the increase in workload by undergoing hypertrophic remodeling; cardiomyocytes increase heart contractility by increasing protein synthesis and adding sarcomeres. However, if the heart is too stressed, hypertrophy can become deleterious due to functional decompensation, and cause heart HF, characterized by a decreased ejection fraction, progressive chamber dilation, pro-inflammatory cytokines release, apoptosis, and fibrogenesis (Frey and Olson, 2003, Ertl and Frantz, 2005).
The heart is regulated by various intracellular signaling pathways. In particular, mitogen-activated protein kinase (MAPK) signaling has been widely implicated in cardiac pathology for several reasons. First, in vitro and in vivo stimulation of MAPK signaling promotes or suppresses cardiac pathology. Second, cardiac diseases are associated with changes in the expression and activity of MAPKs in the heart. Third, pharmacological or genetic inhibition of MAPKs affects cardiac diseases. Four classic MAPKs, including extracellular signal-regulated kinases 1/2 (ERK1/2), p38, c-Jun N-terminal kinases (JNK), and ERK5, distinctly mediate heart development, metabolism, function, and pathology. Notably, ERK1/2 and ERK5 are activated by hypertrophic stimuli, whereas JNK and p38 responded mostly to stressors, such as oxidative stress, hyperosmosis and radiation (Sugden and Clerk, 1998). MAPKs are significantly integrated in intracellular signaling and the regulation of gene expression; they target an array of cytosolic and nuclear proteins, including proteins from other signaling pathways and transcription factors (Yang et al., 2003). In addition, MAPKs directly and indirectly target mitochondria, which synthesize 80% of the ATP needed for cardiomyocyte function. Furthermore, mitochondria are the nexus of various stressors, and they initiate cell death through apoptosis, necrosis and autophagy. Previous studies revealed that MAPKs directly interact with the outer mitochondrial membrane and even translocate into mitochondria (Kharbanda et al., 2000, Baines et al., 2002, Ballard-Croft et al., 2005). Other studies demonstrated indirect effects between MAPKs and mitochondria; MAPKs affected mitochondria-mediated cell survival and cell death through their effects on ROS and calcium signaling (Bogoyevitch et al., 2000, Zhao et al., 2001, Yue et al., 2002, Kaiser et al., 2004, Kong et al., 2005, Wall et al., 2006).
Although the precise mechanisms underlying MAPK-mitochondria signaling in cardiac diseases have not yet been established, a significant amount of evidence confirms that MAPKs profoundly influence cellular signaling underlying cardiac compensation and decompensation, in part, through interactions with the mitochondria. Since MI is the most common cause of HF, pharmacological and conditional interventions must be developed to prevent MI or otherwise delay its progression. This review integrates lessons from previous studies into a comprehensive discussion of the implications of MAPK signaling in the physiological and pathological heart. An understanding of the molecular mechanisms underlying canonical MAPK signaling and MAPK-mitochondria signaling in the heart will promote the development of new therapeutic approaches for the treatment of cardiac diseases.
2. The MAPK family in the healthy heart
To elucidate the potential therapeutic implications of targeting MAPK signaling, understanding the MAPK family in the context of a healthy heart, including genealogy, three-tiered activation cascades, the unique physiological functions of subfamilies and isoforms, and signaling regulation is important. Currently, studies on the role of MAPKs in the heart are mainly based on the following approaches: (i) analysis of the activity of MAPKs in the myocardium under physiological and pathological conditions; (ii) elucidating the effects of pharmacological inhibition/activation of MAPKs on cardiac diseases; (iii) assess the effects of gene targeted modulation of MAPKs expression on the healthy or diseased heart (Ravingerova et al., 2003). Four classical subfamilies represent the majority of the MAPK family in humans: ERK1/2, (also known as MAPK 3/1), p38 (also known as MAPKs 11-14), JNK (also known as MAPKs 8-10), and ERK5 (also known as Big MAPK 1 or MAPK 7). Atypical MAPKs, in contrast to their classical counterparts, are evolutionary primitive and apparently less implicated in cardiac physiology (Feijoo et al., 2005). MAPK enzymes are so conserved among eukaryotes that the genealogy of classical human MAPKs was traced back to evolutionary divergences in primitive eukaryotes. Indeed, studies of the yeast species Saccharomyces cerevisiae have directed and supplemented studies of MAPKs in mammalians including humans. ERK1/2 and ERK5 are considered pheromone response pathway-type MAPKs, or Fus3/Kss1-type MAPKs, because they are commonly activated by peptide mitogens. p38 and JNK are considered high-osmolarity growth pathway-type MAPKs, or Hog1-type MAPKs, because they respond strongly to cellular stress and inflammatory cytokines; they are appropriately dubbed stress-activated protein kinases (SAPKs) (Gustin et al., 1998, Doczi et al., 2012). Nonetheless, human MAPKs respond to an array of stimuli. ERK1/2 and ERK5 can also respond to stressors, like ROS, G-protein-coupled receptor (GPCR) agonists, and cytokines (McKay and Morrison, 2007, Raman et al., 2007). p38 and JNK can also respond to growth factors and GPCRs (Ono and Han, 2000, Raman et al., 2007).
Although some overlap exists with regard to molecular structure, substrate specificity, and signaling functions, MAPKs and their isoforms can uniquely influence cardiac physiology based on cell type and stimulus characteristics (Gerits et al., 2007). This is, in part, due to the co-evolution of regulatory mechanisms, which impart specificity and preserve physiological signaling. Facilitated by these regulatory mechanisms, the MAPK family of serine/threonine-specific protein kinases targets a remarkable assortment of transcription factors, protein kinases, and other proteins, both in the cytosol and the nucleus, to mediate cellular adaption, growth, cellular survival, apoptosis, proliferation, differentiation, metabolism, and motility (Davis, 2000, Pearson et al., 2001, Ramos, 2008, Rincon and Davis, 2009, Wang and Tournier, 2006). MAPK signaling is like a potent molecular switch; after it prompts an appropriate cellular response, it must be deactivated by regulatory mechanisms (Ferrell, 1996). This compensatory downregulation may be illustrated in the context of physical exercise, a normal yet acute stress. Transient hypoxia and mechanical overload stimulate the MAPK signaling during exercise. Within minutes, MAPKs rectify cardiac output by increasing contractility via cardiomyocyte hypertrophy without inflammation or fibrosis (Hunter et al., 1995). If a stressor is only temporary, as in the case of normal exercise, regulatory mechanisms, such as MAPK phosphatases (MKPs) and tyrosine phosphatases, properly downregulate MAPK signaling.
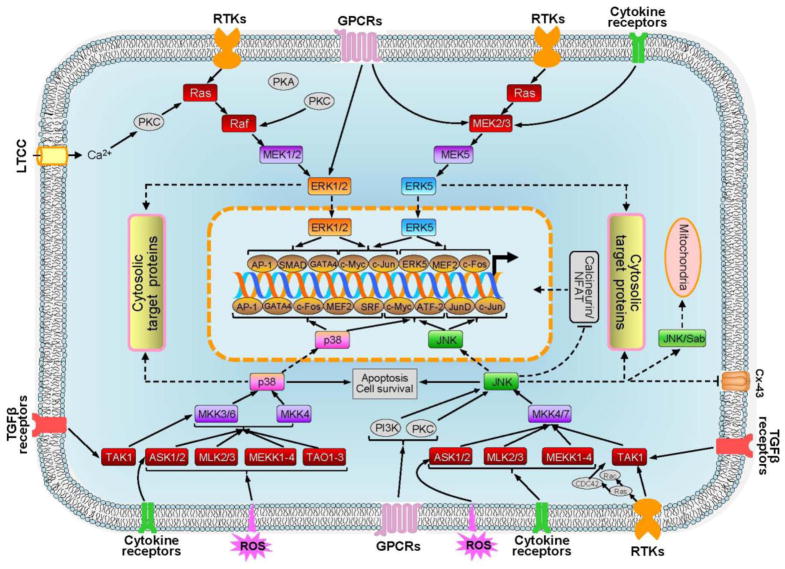
Each MAPK subfamily is activated by a unique cascade; cascade intermediates exhibit exceeding substrate specificity for their associated MAPKs (Feijoo et al., 2005, Doczi et al., 2012). Yet, even before a stimulus reaches a MAPK kinase kinase (MAP3K), the first tier of a MAPK cascade, it can be influenced by an array of upstream activities (Pearson et al., 2001). Indeed, intracellular macromolecular complexes, which include docking and scaffold proteins, alter the timing and location of intracellular signaling (Whiteside and Goodbourn, 1993). Highly conserved three-tiered serial phosphorylation cascades begin when MAP3Ks phosphorylate and activate second tier MAPK kinases (MAP2Ks). MAP2Ks dually phosphorylate the activation loop of their corresponding MAPKs on a characteristic threonine-X-tyrosine motif, where X is a variable amino acid residue (glutamic acid in ERK1/2, glycine in p38, proline in JNK, aspartic acid in ERK5). This precise, highly conserved mechanism stimulates changes in MAPK global conformation and facilitates access to substrates (Pearson et al., 2001, Doczi et al., 2012). Since, removal of either phosphate from the activation loop of a cascade kinase essentially abolishes signaling, MKPs and tyrosine phosphatases, which hydrolyze two or one activating phosphate, respectively, can significantly downregulate MAPK signaling (Ferrell, 1996). Many intracellular signaling pathways, including mitochondrial signaling pathways and other MAPK signaling pathways, manipulate upstream and downstream proteins of MAPK signaling to influence dynamic myocardial growth or adaptation (Junttila et al., 2008).
2.1. ERK1/2
The ERK1/2 activation cascade, also known as the Ras-ERK pathway, has been investigated extensively. Calcium channels, RTKs, and GPCRs can internalize a stimulus. In a well-studied example, growth factor receptor-bound protein 2, a docking protein, binds to activated RTKs and, then, complexes with and activates the guanine nucleotide exchange factor, son of sevenless (SOS). Activated SOS promotes removal of GDP from Ras, which then binds GTP (McKay and Morrison, 2007). Ras is a GTPase with an extensive reach in intracellular signaling. Among its prominent signaling mechanisms, Ras phosphorylates and activates Raf proto-oncogene serine/threonine-protein kinase (Raf-1 or c-RAF), a MAP3K. In turn, Raf activates MAPK/ERK kinase 1/2 (MEK1/2, also known as dual specificity MAPK kinase 1), a MAP2K, which activates ERK. Interestingly, ERK1/2 also is autophosphorylated via a GPCR-dependent mechanism (Lorenz et al., 2009). Within the cascade, scaffold proteins, like β-arrestin, kinase suppressor of Ras (KSR), MAPK organizer 1 (MORG1), and MEK partner 1 (MP1), interact with ERK1/2 to facilitate and modulate signaling (Dhanasekaran et al., 2007). MKPs, protein serine/threonine phosphatases and protein tyrosine phosphatases downregulate ERK1/2 signaling (Junttila et al., 2008, Owens and Keyse, 2007). Once activated, ERK1/2 can activate the proto-oncogene c-Myc and SMAD1-4, transcriptional activators implicated in heart development (MacLellan and Schneider, 2000). It also activates c-Jun and activating protein 1 (AP-1); the AP-1 transcription factor complex, which is involved in cellular proliferation and survival, can be formed by the transcription factors Fos, jun, and activating transcription factor (ATF), each of which is targeted by MAPKs (Hai and Curran, 1991, Pearson et al., 2001). Furthermore, during neonatal heart development, ERK activates transcription factor GATA4, which inhibits apoptosis and is important in the formation of the septum arteriosum (Davidson and Morange, 2000, Liang et al., 2001, Eriksson and Leppa, 2002, Naito et al., 2003). Ubiquitous expression of ERK1/2 also implicates it in signaling that influences cell death, migration, immune function, insulin signaling, cardiac hypertrophy, and cell structure including cytoskeletal properties and cell adhesion (Pearson et al., 2001, Ramos, 2008). MEK1-restricted transgenic (TG) mice demonstrated concentric hypertrophy, a phenotype similar to that observed in a weight lifter. In fact, in response to an increase in pressure from resistance training, MAPK signaling promotes parallel sarcomere addition and thickening of the left ventricular wall. This physiological phenomenon, referred to as “athlete’s heart,” improves the ability of the heart to maintain cardiac output during exercise-induced acute stress (Mihl et al., 2008). Finally, with regard to isoforms, ERK2 appears to be more important than its counterpart, ERK1, because only ERK2 null mice are nonviable. ERK2 seems to compensate for a lack of ERK1, so it is thought to be capable of fulfilling most ERK1 functions (Gerits et al., 2007).
2.2. p38
The p38 cascade exhibits more variability than the ERK1/2 cascade; unlike ERK1/2, the p38 cascade can begin with MEK kinase 1-4 (MEKK1-4), mixed lineage kinase 2/3 (MLK2/3, also known as MAPK kinase kinase 10/11), apoptosis signal-regulating kinase 1/2 (ASK1/2, also known as MAPK kinase kinase 5/6), transforming growth factor-β-activated kinase 1 (TAK1, also known as MAPK kinase kinase 7), and one amino acid protein kinases 1-3 (TAO1-3, also known as serine/threonine-protein kinases) (Raman et al., 2007). With the exception of TAO1-3, all p38 MAP3Ks activate the MAP2Ks MEK3/6 and MEK4. Finally, MEK3/6 and MEK4 activate p38 (Gerits et al., 2007). TAK1 also mediates noncanonical p38 autophosphorylation via TAB-1 (transforming growth factor-β-activated protein 1-binding protein 1), a MEK3/6-independent mechanism (Ge et al., 2002). Osmosensing scaffold for MEKK1 (OSM), JNK-interacting protein 2 (JIP2), and JNK-associated leucine-zipper protein can alter p38 signaling (Dhanasekaran et al., 2007). p38 phosphatases include MKPs 1, 2, 5, and 7, and protein serine/threonine phosphatases (PP2C) (Junttila et al., 2008, Owens and Keyse, 2007).
p38 signaling most prominently influences immune responses by affecting proinflammatory cytokine production in the cytosol and regulating immune cell proliferation, differentiation, and function. It activates c-Myc, c-Fos, GATA4, AP-1, and ATF-2 (Liang and Molkentin, 2003, Petrich et al., 2004, Rose et al., 2010). ATF-2, in particular, is activated by SAPKs, binds to cAMP response element (CRE), and, at the transcriptional level, functions as a histone acetyltransferase, activating transcription of genes important in cardiac development and cell survival (Hai and Curran, 1991, Sano et al., 1999). Moreover, p38 activates myocyte-specific enhancer factor 2A/C (MEF2A/C) and serum response factor (SRF), both of which influence cardiac differentiation and development (Sano et al., 1999). Activated p38 may also remain in the cytosol to inhibit nuclear factor of activated T-cells (NFAT), a family of calcium-regulated transcription factors, that influences immune response and cardiac development (Zarubin and Han, 2005, Macian, 2005). One study implied that p38 might be physiologically anti-hypertrophic in response to swimming (Taniike et al., 2008), but the majority of studies implies that p38 activation is associated mostly with pathological hypertrophy (Nishida et al., 2004, Watanabe et al., 2007). p38 may be negatively inotropic by modifying cardiac sarcomeric proteins and decreasing phosphorylation of α-tropomyosin (Zechner et al., 1997, Liao et al., 2001, Chen et al., 2003, Vahebi et al., 2007). The p38 MAPK subfamily has four isoforms: p38α and p38β are ubiquitously expressed, p38γ is expressed primarily in the heart and skeletal muscle, and p38δ is found in the small intestine, kidney, pancreas, and testis (Ono and Han, 2000). Knock-out of p38α in mice, but not other isoforms, caused embryonic lethal defects in erythropoiesis (Tamura et al., 2000). The p38α isoform appears to promote apoptosis via ROS production, and calcium overload (Dhingra et al., 2007). On the other hand, p38β seems to inhibit apoptosis by activating heat shock protein 27 (Hsp27), which prevents proteolysis of myofilament proteins (Li et al., 2008).
2.3. JNK
The JNK cascade mostly overlaps with the p38 cascade. It begins with the same MAP3Ks as p38, with the exception of TAO1-3 (Raman et al., 2007). After the first tier, these two signaling cascades diverge. All of the mentioned MAP3Ks can activate MEK4/7, which activates JNK. JNK can also be activated noncanonically via Wnt signaling, which involves Rac and RhoA intermediates (Zhang et al., 2009). JNK scaffold proteins include JIP1, JIP2, JNK/stress-activated protein kinase-associated protein 1 (JSAP1), JNK-associated leucine-zipper protein, and SH3 (POSH) (Dhanasekaran et al., 2007), and JNK phosphatases are MKPs 1, 2, 5, and 7 (Owens and Keyse, 2007)...
JNK, like p38, is strongly implicated in cardiac response to stress. Like other MAPKs, JNK signaling influences cell survival, apoptosis, proliferation, and differentiation, as observed from its phosphorylation of c-Myc, c-Jun, and ATF2. JNK1 and JNK2 each has four isoforms and are ubiquitously expressed. JNK3 has two isoforms and is found in the heart, brain, and testis (Davis, 2000). Confounding results reported enhanced myocyte survival as a result of both JNK activation and inhibition (Aoki et al., 2002, Dougherty et al., 2002, Engelbrecht et al., 2004). In vivo studies suggest that JNK impedes physiological hypertrophic growth by activating transcription factor jun-D, which mediates anti-hypertrophic and anti-apoptotic effects as part of the AP-1 transcription factor complex (Hilfiker-Kleiner et al., 2005). Lastly, JNK1 and JNK2 are connected to T cell development and cytokine release (Gerits et al., 2007) inhibition of NFAT translocation into the nucleus (Liang et al., 2003), which blocks its regulation of thymocyte development and T cell differentiation (Macian, 2005). JNK signaling also influences heart development, metabolism, insulin signaling, cell mobility, and actin reorganization (Davis, 2000, Pearson et al., 2001, Raman et al., 2007).
2.4. ERK5
As mentioned, ERK5 is more similar to ERK1/2 than p38 and JNK. While p38 and JNK can each be activated by several MAP3Ks, ERK5, like ERK1/2, has only been shown to be activated by one MAP3K, which is MEKK2/3. MEKK2/3 activates MEK5, which activates ERK5. ERK5 scaffold proteins include Lck-associated adaptor (LAD), Grb-2-associated binder 1 (Gab1), and muscle specific A-kinase anchoring protein (Wang and Tournier, 2006). ERK5 phosphatases include MKPs 1 and 3 and the phosphotyrosine specific phosphatases PTP-SL (Buschbeck et al., 2002). ERK5 is unusual because a large COOH-terminal extension makes it more than twice the size of other MAPKs. It promotes postnatal, eccentric hypertrophic heart growth, cell survival, differentiation, growth, and proliferation by activating immediate-early response genes fos, myc, jun, and MEF2 (MacLellan and Schneider, 2000, Wang and Tournier, 2006). Ventricular volume overload promotes eccentric hypertrophy, which is characterized by serial sarcomere addition, cardiomyocyte elongation, and dilation and thinning of the left ventricle (Mihl et al., 2008). This phenotype is often observed in athletes who engage in endurance training, such as marathon runners. Unlike other MAPKs, ERK5 functions directly as a transcriptional activator, binding directly to DNA (Kasler et al., 2000, Akaike et al., 2004). Besides, numerous studies have implicated ERK5 in the inhibition of apoptosis and maintenance of vascular integrity by discovering its pro-survival role in endothelial cells and smooth muscle cells, both of which are found significantly in blood vessels (Hayashi and Lee, 2004). In fact, ERK5 is essential in the formation of a vascular system; global ERK5 deletion caused defects in vascular formation that resulted in an embryonic lethal phenotype (Regan et al., 2002, Hayashi and Lee, 2004, Hayashi et al., 2004). ERK5a is the most ubiquitously expressed isoform of the only known erk5 gene. Other isoforms, ERK5b, ERK5c, and ERK-T appear to downregulate ERK5a (Yan et al., 2001).
Thus, despite extensive studies, the precise mechanisms of the MAPKs network in the regulation of cell differentiation, cell growth and death are not yet fully understood. Elucidating the cross-talk between the MAPKs family and other signaling pathways in the regulation of cell metabolism require further studies that can be helpful for understanding the contribution of MAPKs to myocardial damages induced by various exogenous stressors.
3. MAPKs in chronic cardiac stress (hypertrophy and heart failure)
3.1. ERK1/2
As mentioned previously, growth factors stimulate ERK1/2 signaling through RTKs and GPCRs to promote cardiac hypertrophy. Stimulation of GPCRs, such as α- and β-adrenergic, angiotensin II (AngII) and endothelin 1 (ET-1) receptors induce hypertrophy through activation of Ras-ERK cascade through Gq/G11 (reviewed in Liang and Molkentin, 2003, Petrich et al., 2004, Rose et al., 2010). Interestingly, β-adrenergic ligands can activate ERK1/2 in a G protein-independent, β-arrestin-dependent manner through RTKs. The β-blockers alprenolol and carvedilol induced β1-adrenergic receptor-mediated transactivation of epidermal growth factor receptor by arrestin, and ERK activation (Kim et al., 2008). Stimulation of both receptor complexes can mediate hypertrophic signaling through the Ras-ERK pathway. Upregulation of individual signaling molecules from the Ras-ERK pathway was associated with cardiac hypertrophy. Increased Ras expression (Kai et al., 1998) and Ras-ERK pathway activity (Eisenberg and Eisenberg, 2006) positively correlated with severity of cardiac hypertrophy in patients with hypertrophic cardiomyopathy. In animal models, TG mice with cardiac-specific expression of constitutively active (CA)-Ras (Hunter et al., 1995, Zheng et al., 2004) or CA-MEK1 (Bueno et al., 2000) developed cardiac hypertrophy, including changes in gene expression and myofibers. Targeted overexpression of RAS transgenic mice was associated with extracellular matrix remodeling, cardiac dysfunction, hypertrophic cardiomyopathy and HF (Hunter et al., 1995, Mitchell et al., 2006). Gene expression of the key regulators for energy metabolism, such as PPARα, GLUT4, and fatty acids oxidation enzymes were remarkably suppressed in these hearts.
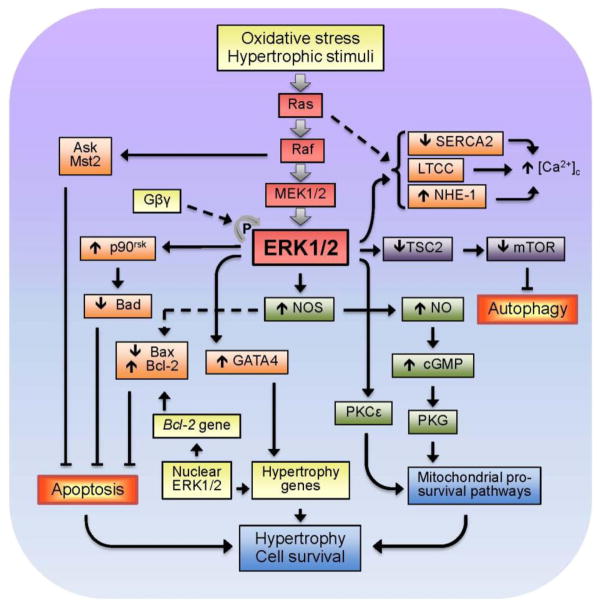
Potential downstream targets of ERK1/2 activation in response to growth stimuli and oxidative stress are shown in Fig. 2. Numerous studies reported that the Ras-ERK pathway regulates Ca2+ through modulation of the activity of ion channels/exchangers and pumps. Cardiac dysfunction in Ras TG mice can occur due to changes in regulation of Ca2+ homeostasis in the cytoplasm. Ras upregulation in intact hearts (Zheng et al., 2004) and cultured cardiomyocytes (Ho et al., 1998) resulted in reduced expression of SERCA2 associated with low Ca2+ uptake by the sarcoplasmic reticulum. In addition, alterations in Ca2+ homeostasis can result from reduced L-type Ca2+-channel (LTCC) activity and defective excitation-calcium release coupling with Ras activation in cardiomyocytes (Ho et al., 2001). Yet, recent studies revealed ET-1-induced stimulation of the LTCC via activation of ERK1/2 (Yu et al., 2013). In addition, ERK1/2 can phosphorylate and modulate the activity of Na+/H+ exchanger 1 (NHE-1), which is indirectly involved in Ca2+ regulation in the heart (Moor and Fliegel, 1999). Inhibition of ERK1/2 also significantly reduced the K+ channels (IK and IK1) activity in hypertrophied adult cardiomyocytes (Teos et al., 2008).
Figure 2.
Major downstream targets of ERK1/2 MAPK in response to oxidative stress and growth stimuli in the heart.
Although most of studies show that the Ras-ERK cascade plays an important role in the signaling pathway leading to cardiac hypertrophy, ERK-independent mechanisms can also play a significant role in the pathogenesis of hypertrophy. In favor of the latter, genetic ablation of cardiac ERK1/2 promoted stress-induced apoptosis and HF without effect on hypertrophy in mice, suggesting that ERK1/2 signaling is not a requirement in the mediation of cardiac hypertrophy, though it does play a protective role in response to pathologic stimuli (Purcell et al., 2007). Activation of Raf-1 was not sufficient to induce cytoskeletal changes similar to those seen in hypertrophy (Thorburn et al., 1994). Notably, dominant negative (DN) mutants and pharmacological inhibitors of Raf-ERK1/2 signaling attenuated hypertrophy and increased cell death in isolated cardiomyocytes and intact hearts (Purcell et al., 2007, Lorenz et al., 2009, Cheng et al., 2011). A recent study suggested a scenario in which selective blocking of ERK-mediated hypertrophy occurs without an increase in apoptotic cardiomyocyte death. Autophosphorylation of ERK on Thr188 (ERK2Thr188) due to direct protein-protein interaction between ERK and Gβγ subunits was observed in mice upon stimulation of Gq-coupled receptors or after aortic banding and in failing human hearts (Lorenz et al., 2009). Notably, ERK autophosphorylation, which requires the activation and assembly of the entire Ras-ERK cascade and dimerization of ERK is a critical event in the induction of ERK-mediated cardiac hypertrophy in response to various stimuli (Lorenz et al., 2009). ERK2T188A, which is DN for ERKThr188 signaling, attenuated cardiomyocyte hypertrophic responses to phenylephrine (PE) and to chronic pressure overload in isolated cells and intact hearts without any effect on anti-apoptotic ERK1/2 signaling and physiological cardiac function (Ruppert et al., 2013). Interestingly, despite inhibition of pathological hypertrophy, ERK2T188A did not affect physiological cardiac growth associated with age or exercise, therefore, suggesting that interference with ERKThr188 phosphorylation may be a selective therapeutic strategy in pathological ERK1/2-mediated cardiac hypertrophy.
In addition to hypertrophy, activation of the ERK cascade promotes resistance to apoptosis (Bueno et al., 2000, Yamaguchi et al., 2004), although the anti-apoptotic effects of individual components such as Raf likely do not associate with hypertrophy and can occur through a MEK-ERK- independent mechanism (Chen et al., 2001). Conversely, inhibition of Ras-ERK signaling attenuates hypertrophic response of the heart and cardiomyocytes. Anti-remodeling and anti-hypertrophic effects of mechanical unloading caused by a left ventricular assist device in patients with HF were associated with reduced cardiac ERK1/2 activity in the myocardium (Flesch et al., 2001). Likewise, hearts with DN-Raf demonstrated reduced hypertrophy in response to pressure overload (Harris et al., 2004), and DN-MEK1 inhibited ET-1- and PE-induced hypertrophy in cardiomyocytes (Ueyama et al., 2000).
Thus, activation of ERK due to stimulation of both RTKs and GPCRs promotes hypertrophy indicating a key role it plays in the pathogenesis of cardiac hypertrophy and HF. Development of hypertrophy and its progression from compensated to pathological (decompensated) state and HF is a complex process which along with Ras-ERK includes other signaling pathways and depends on exposure time, severity and nature of hypertrophic stimuli. Discrepancies between different studies are also due to variability of animal/cell models and specificity of cell metabolism in neonatal and adult cardiomyocytes used in these studies.
3.2. p38
p38, a stress-activated MAPK, is stimulated in response to various extracellular stresses including inflammatory cytokines, oxidative stress, radiation, growth factors, hyperosmolarity and others. Substantial variabilities within studies on the role of p38 in cardiac hypertrophy, ventricular remodeling and HF are due to the use of a) neonatal and adult cardiomyocytes which are metabolically different, b) various animal models or hypertrophic agonists, c) pan-p38 inhibitors or genetic upregulation/downregulation of total p38 that affect both p38α and p38β, and d) measurements of p38 activity at different time points after stimulation.
Similar to other MAPKs, p38 is rapidly activated within a few minutes of exposure to stretch, increased aortic pressure, or volume overload, although this activation is not consistent (Hoshijima and Chien, 2002). Analysis of tissue samples from the hearts with post-MI ventricular remodeling demonstrated substantial variability in p38 activity although it was higher in patients with end-stage HF compared to healthy hearts (Ng et al., 2003, Denise Martin et al., 2012). In most cases, a double-peak, transitional activation of MAPKs, including p38, suggests that MAPKs may play discrete roles throughout progression of hypertrophy, from early activation to the late hypertrophic phase (Chien, 1999, Molkentin and Dorn, 2001). Studies on isolated cardiomyocytes, predominantly, neonatal cardiomyocytes, demonstrated that stimulation of p38 promoted hypertrophy (Nemoto et al., 1998, Wang et al., 1998a, Liang and Molkentin, 2003), while pharmacological inhibition or genetic ablation of p38 prevented cell growth in response to hypertrophic stimuli (Liang and Molkentin, 2003, Nemoto et al., 1998), suggesting that p38 plays a causative role in the development of cardiac hypertrophy. Furthermore, overexpression of the upstream activators for the p38, MKK3 and MKK6 elicited pro-hypertrophic responses, including an increase in cell size, enhanced sarcomeric organization, and elevated atrial natriuretic factor expression in neonatal cardiomyocytes (Wang et al., 1998a). However, in vivo studies provided contradictory data on the role of p38 in the development of cardiac hypertrophy. Targeted activation of p38 in intact hearts by transgenic expression of MKK3 and MKK6 resulted in interstitial fibrosis and expression of fetal marker genes characteristic of HF, but no significant cardiac hypertrophy (Liao et al., 2001). Likewise, mice lacking PKCε exhibited enhanced activation of p38 associated with increased collagen deposition and diastolic dysfunction but preserved pressure overload-induced myocardial hypertrophy (Klein et al., 2005). Conversely, TG mice with cardiac-specific expression of DN-p38 developed cardiac hypertrophy but were resistant to cardiac fibrosis in response to pressure overload (Zhang et al., 2003). Inhibition of p38 protected post-infarction remodeling and HF in mice (Liu et al, 2005) and rats (See et al, 2004). Studies of cardiac-specific p38 knock-out mice demonstrated that p38 plays a significant role in the regulation of survival mechanisms in response to pressure overload through modulation of apoptosis and fibrosis, while cardiac hypertrophic growth is unaffected despite a dramatic down-regulation of the kinase (Nishida et al., 2004). These studies provide strong evidence that p38 activation is not the only causative factor in cardiac hypertrophy.
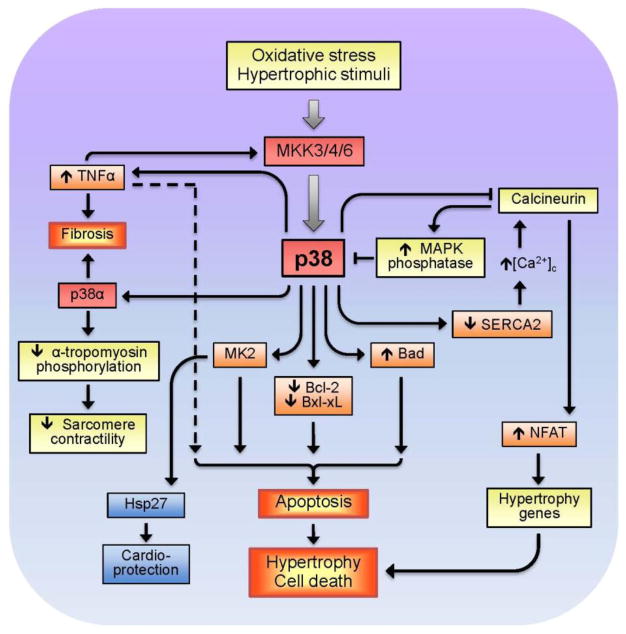
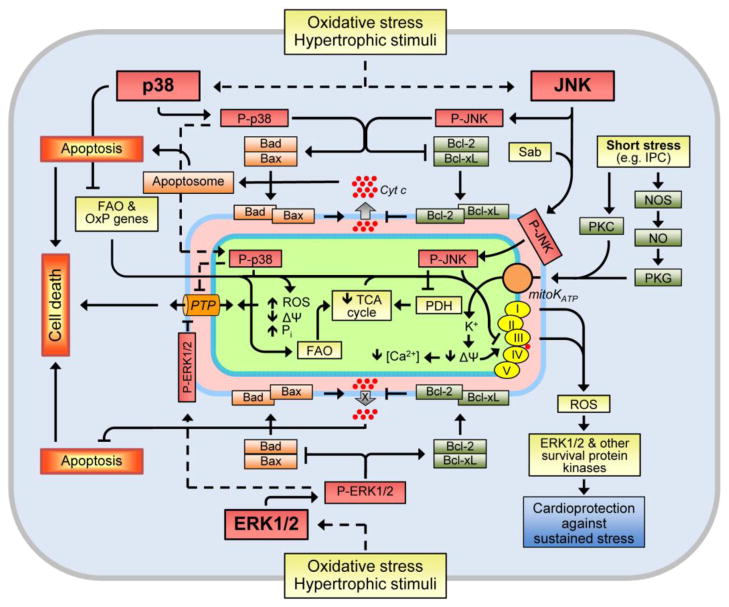
Notably, stimulation of different isoforms of p38 can exert distinct, even, opposite effects. Upregulation of p38α enhanced apoptosis, whereas p38β overexpression promoted hypertrophy in cultured isolated cardiomyocytes (Wang et al., 1998b). In vivo studies on intact hearts provided results different from those seen during in vitro studies, although they confirm diverse downstream targets and functional roles of p38α and p38β in the regulation of hypertrophy-associated signaling pathways. Direct injections of adenoviruses expressing p38α or p38β into the left ventricular wall of adult rats demonstrated that p38α stimulates fibrosis-related factors whereas p38β attenuated the ET-1-induced expression of the B-type natriuretic peptide. These findings indicate that p38α participates in the regulation of fibrotic remodeling process, and p38β stimulates the agonist-induced activation of the B-type natriuretic peptide and, thereby, elicits inhibitory effects on growth factors (Koivisto et al., 2011). DN-p38α transgenic mice exhibited cardiac hypertrophy despite the reduced p38α activity (Braz et al., 2003, Zhang et al., 2003). Interestingly, a negative feedback mechanism exists between p38 and calcineurin in which upregulation of the latter enhances the activity of MAPK phosphatase-1, negatively regulating the hypertrophic response in cardiomyocytes by downregulating p38 (Lim et al., 2001). Cardiac-specific DN-p38α, and MKK3 and MKK6 TG mice exhibited enhanced cardiac hypertrophy in response to pressure overload or infusion of hypertrophic agonists, and this was associated with augmented activity and nuclear translocation of NFAT (Braz et al., 2003). These observations indicate that reduced p38 signaling promotes cardiomyocyte growth through a mechanism involving enhanced calcineurin-NFAT signaling (Molkentin, 2004, Yang et al., 2002). It should be noted that p38 exerts a negative inotropic effect on isolated cardiomyocytes by decreasing myofilament response to Ca2+ (Liao et al., 2002), and p38α activation directly suppresses sarcomeric function in the heart associated with decreased phosphorylation of α-tropomyosin (Vahebi et al., 2007). In contrast to downregulation of the calcineurin-NFAT pathway, activation of the MKK6-p38 MAPK signaling in neonatal cardiomyocytes prolonged the contractile Ca2+ transient by downregulating SERCA2 and increasing diastolic [Ca2+]i and NFAT activity (Andrews et al., 2003). Downstream targets of p38 activation during cardiac and oxidative stress are summarized in Fig. 3.
Figure 3.
p38-induced stimulation of death signaling pathways in response to oxidative stress and hypertrophic stimuli.
Although in vivo studies revealed no consistent activation of p38 in cardiac hypertrophy and ventricular remodeling following MI, inhibition of p38 mostly exerted anti-remodeling effects and improved cardiac function (reviewed in Marber et al., 2011). DN-p38 mice had reduced infarct size which was associated with improved ventricular systolic function after MI (Ren et al., 2005). In addition to the anti-remodeling action, inhibition of p38 activity decreased tumor necrosis factor alpha (TNFα) expression and reduced inflammation-induced fibrosis in post-MI myocardium (Yin et al., 2008). There exists a feed-back mechanism, in which TNFα activated p38 in the intact heart and isolated cardiomyocytes through MKK3 (Bellahcene et al., 2006). Activation of p38 promoted apoptosis via the regulation of apoptotic protein activity. In response to TNFα, p38 induced phosphorylation (inactivation) and downregulation of the anti-apoptotic protein Bcl-xL, eventually leading to apoptosis in endothelial cells (Grethe et al., 2004). In addition, p38 stimulated cardiomyocyte apoptosis through Bcl-xL deamidation (Ren et al., 2005), attenuated phosphorylation of the pro-apoptotic protein Bad, and stimulated TNFα-induced apoptosis in endothelial cells (Grethe and Porn-Ares, 2006). Likewise, p38 inhibition up-regulated Bcl-2, whereas its activation down-regulated Bcl-2 in p38 transgenic mice hearts and neonatal cardiomyocytes, thereby, indicating that p38 functions as a pro-apoptotic signaling effector (Kaiser et al., 2004).
A diversity of basic science and preclinical studies obscures understanding of the precise role of p38 MAPK in cardiac diseases. Still, collectively, both in vitro and in vivo studies of various cells, animals, and human heart models show that p38, concurrently with other signaling pathways, plays a significant role in the pathogenesis of hypertrophy and its progression to HF. Acute activation of p38 signaling in early phases of hypertrophy may serve as an adaptive response to extracellular stresses while chronic activation of the kinase apparently exerts detrimental effects, including adverse cardiac remodeling and HF.
3.3. JNK
Studies using both animal models and cultured cells provide strong evidence that JNK is involved in pathogenesis of hypertrophy and HF. This conclusion comes from studies that demonstrated that i) cardiac hypertrophy and HF change JNK activity and ii) upregulation or inhibition of JNK using gain- and loss-of-function approaches influences cardiac hypertrophy and HF. However many questions on the cause-and-effect relationship between JNK activation and cardiac dysfunction induced by hypertrophy and HF still remain unanswered. Similar to p38, activation of JNK is transient, cyclic, and it varies depending on timing, models, severity of stress, and types of stimuli. Early studies demonstrated that hypertrophic agents, such as α1-adrenergic receptor agonists (Ramirez et al., 1997), AngII (Kudoh et al., 1997), and ET-1 (Bogoyevitch et al., 1995) cause transient activation of JNK in cultured cardiomyocytes isolated from neonatal rats.
In vivo studies demonstrated that pressure-overload hypertrophy induced by transverse aortic constriction in rats resulted in rapid activation of JNK and its target transcription factors c-Jun and ATF-2 (Fischer et al., 2001, Nadruz et al., 2004). However, there was no difference between control hearts and hearts with pressure- or volume-overload hypertrophy induced by aortic banding for 24h (Miyamoto et al., 2004). Mechanical stress induced by hemodynamic overload plays an important role in the development of cardiac hypertrophy and ventricular remodeling associated with early activation of the hypertrophic genetic program. Mechanical stress induced by cyclic stretch in neonatal cardiomyocytes activated the JNK/c-Jun pathway (Nadruz et al., 2005). Specific activation of the MKK7/JNK pathway by CA-MKK7 induced hypertrophy in cultured cardiomyocytes (Wang et al., 1998b). Resistin promoted cardiac hypertrophy via activation of the JNK/insulin receptor substrate pathway in neonatal cardiomyocytes and adult rat hearts in vivo (Kang et al., 2011). Conversely, targeted inhibition of JNK using DN-MKK4, an upstream kinase of JNK, prevented JNK activation and ET-1-induced hypertrophy in cardiomyocytes (Choukroun et al., 1998) and pressure-overload in intact hearts (Choukroun et al., 1999). Importantly, studies using cultured cardiomyocytes, particularly from neonatal hearts, revealed pro-hypertrophic action of JNK activation, however, the majority of in vivo studies demonstrated no positive correlation between JNK activation and hypertrophy.
Transgenic animals with targeted expression of MKK7 developed congestive HF with extracellular matrix remodeling in the absence of ventricular hypertrophy, although the hearts exhibited increased levels of the hypertrophy marker genes α-skeletal actin and atrial natriuretic factor (Petrich et al., 2004). Inhibition of MEKK1-JNK signaling attenuated cardiac hypertrophy induced by heart-restricted overexpression of Gαq in mice (Miyamoto et al., 2004) whereas it had no effect on pressure overload hypertrophy (Sadoshima et al., 2002). Genetic ablation of three JNK isoforms (JNK1, JNK2 and JNK3) individually in the heart did not promote greater cardiac hypertrophy compared to wild type mice, although hearts with JNK1 deletion exhibited increased fibrosis in response to pressure overload (Tachibana et al., 2006). On the other hand, downregulation of the ASK1-JNK pathway was associated with reduced fibrosis and myocardial remodeling during AngII-induced cardiac hypertrophy (Izumiya et al., 2003). These studies support implications of JNK signaling in specific aspects of myocardial remodeling associated with cardiac pathology, while they exclude a causative role of JNK in hypertrophy. Furthermore, TG mice expressing DN-JNK1/2 showed enhanced cardiac hypertrophy following transverse aortic constriction and spontaneous cardiac hypertrophy with aging, suggesting an anti-hypertrophic role, rather than pro-hypertrophic role, for this signaling pathway in the heart (Liang et al., 2003). Mice lacking JunD, a downstream target of JNK, exhibited enhanced pressure overload-induced hypertrophy, increased mortality, and enhanced cardiomyocyte apoptosis and fibrosis compared to wild type animals (Hilfiker-Kleiner et al., 2005, Ricci et al., 2005), suggesting that JunD limits cardiomyocyte hypertrophy. Moreover, JunD KO mice developed low adaptive pressure overload cardiac hypertrophy, while cardiac-specific overexpression of JunD resulted in spontaneous ventricular dilation and decreased contractility (Ricci et al., 2005). These data indicate that JunD, promotes both adaptive-protective and maladaptive hypertrophy in the heart depending on its expression levels.
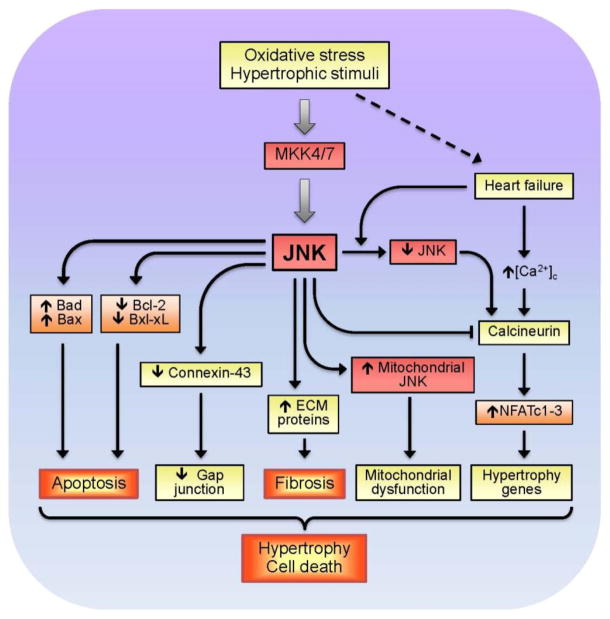
JNK-induced activation of downstream targets in response to growth stimuli and oxidative stress are given in Fig. 4. The anti-hypertrophic action of JNK is mediated, at least partially, through inhibition of calcineurin-NFAT signaling, which plays a critical role in regulating cardiac hypertrophic growth (Molkentin et al., 1998). JNK was originally shown to phosphorylate NFATc2 (Porter et al., 2000) and NFATc3 (Chow et al., 1997) but not NFATc4 (Yang et al., 2002). In cultured cardiomyocytes, DN-JNK1/2 significantly enhanced activity of NFATc1, NFATc2 and NFATc3, indicating that JNK signaling can inhibit calcineurin-mediated translocation of NFAT isoforms to the nucleus (Liang et al., 2003), (Ricci et al., 2005). Conversely, activation of NFAT during HF may be due to downregulation of JNK, as well as Ca2+ overload. Activation of JNK can result in cardiac dysfunction through modulation of gap junctions. TG hearts with chronic JNK activation exhibited impaired intercellular communication associated with significant downregulation of connexin-43 expression and loss of gap junctions in myocardium (Petrich et al., 2002). The extent of reduction in Cx43 mRNA expression (40% of normal) in JNK-activated cardiomyocytes shown in these studies was similar to that found in end-stage human HF (Dupont et al., 2001). Another mechanism underlying JNK-mediated detrimental, cardiac remodeling is JNK association with extracellular matrix proteins, such as matrix metalloproteinase-2. Upregulation of matrix metalloproteinase-2 was associated with its activation in both cultured cardiomyocytes (Shimizu et al., 1998) and intact hearts (Krishnamurthy et al., 2007). Finally, as will be discussed below (see 6.3), adverse effects of JNK are mediated, at least partially, through mitochondria.
Figure 4.
Major downstream targets of JNK MAPK in response to oxidative stress and growth stimuli in the heart.
Thus, JNK plays play an important role in cardiac hypertrophy, remodeling and HF. Contradictory data obtained in vitro and in vivo studies show that the activation of JNK contributes differently to cardiac hypertrophy depending on the duration and severity of stimuli. During early-stage hypertrophy, JNK apparently involves in compensatory mechanisms in response to extracellular stimuli, however, sustained JNK activation concurrently with other signaling pathways promotes pathological hypertrophy. However, it still remains unclear why JNK activation has a dual nature, when Dr. Jekyll becomes Mr. Hyde, and vice versa.
3.4. ERK5
The ERK5 kinase cascade begins with activation of MEKK2/3, which activates MEK5. MEK5, then, activates ERK5 (Pearson et al., 2001). As discussed earlier, ERK5 is highly specific, and its overexpression in cultured cells does not activate other MAPKs (English et al., 1995). Many studies demonstrated that MEK5-ERK5 signaling is activated by growth stimuli via RTKs and GPCRs (Kato et al., 1997, Kamakura et al., 1999, Garcia-Hoz et al., 2012), as well as by oxidative and osmotic stresses (Abe et al., 1996). Epidermal growth factor receptors have been shown to mediate hypertrophic signaling through an MEK5-ERK5-MEF2A pathway in H9c2 cardiomyocytes (Lee et al., 2011). ERK5 signaling may be regulated differently from ERK1/2 in cardiac cells (Takeishi et al., 1999), although MEK1 inhibitors were able to inhibit ERK5, suggesting that the growth stimuli previously attributed to ERK1/2 may also be mediated via ERK5 (Kamakura et al., 1999). Gain- and loss-of-function studies demonstrated that ERK5 regulated many transcription factors responsible for postnatal cardiac growth and hypertrophy, suggesting an essential role for ERK5 signaling during cardiac development and pathogenesis (MacLellan and Schneider, 2000). Similar to other MAPKs, in vivo and in vitro studies demonstrated that various hypertrophic stimuli and growth factors rapidly and transiently enhanced ERK5 activity in cultured cardiomyocytes (Ikeda et al., 2005, Nicol et al., 2001) and intact hearts (Takeishi et al., 2001, Kacimi and Gerdes, 2003). Recent studies in dogs demonstrated that volume overload-induced eccentric hypertrophy increased the localization of p-ERK5 in caveolae and selectively activated ERK5 signaling (Liu et al., 2013). However, the progression of hypertrophy to HF reduced ERK5 activity to normal levels (Kacimi and Gerdes, 2003), or even lower levels (Takeishi et al., 2002).
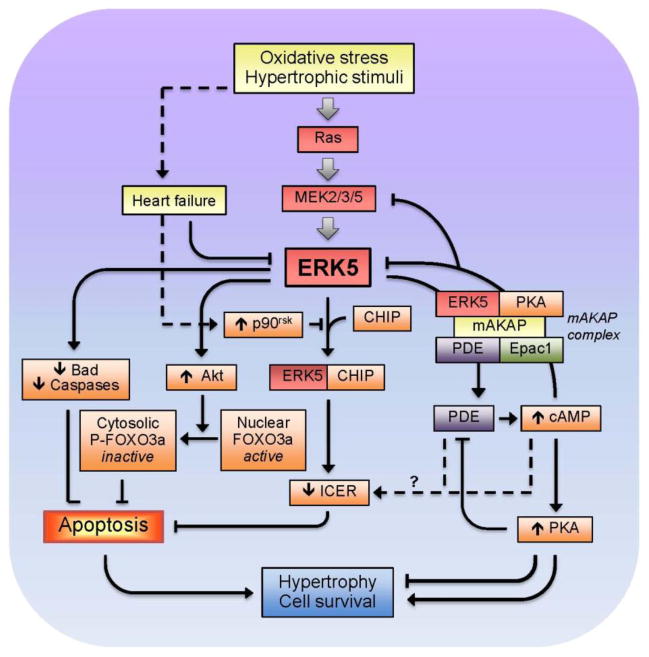
ERK5 has been shown to play an essential role in the regulation of the cardiovascular network, including vascular metabolism, heart contractility and cell growth (Deng et al., 2007, Roberts et al., 2010, Wang et al., 2005). Targeted deletion of ERK5 in adult mice was associated with altered vascular integrity and endothelial failure (Hayashi et al., 2004). Deletion of the erk5 gene in mice caused defects in the development of the heart and its blood vessels, which were severe enough to result in embryonic lethality (Regan et al., 2002). Likewise, inhibition of ERK5 activity by the overexpression of DN-ERK5 stimulated apoptosis in microvascular endothelial cells of the lung (Pi et al., 2004). In contrast, CA-MEK5 overexpression inhibited growth factor deprivation-induced apoptosis in these cells. The anti-apoptotic effects were associated with the ability of ERK5 to phosphorylate Bad, independent of Akt, PKA, or p90RSK kinase activity (Pi et al., 2004). The MEK5-ERK5 pathway can also modulate cardiac metabolism and hypertrophy by regulating cAMP, an important second messenger in physiological and pathological hearts. In cardiomyocytes, the muscle-specific A-kinase anchoring protein has been shown to maintain the cAMP-responsive signaling complex, which includes PKA, phosphodiesterase 4D3 (PDE4D3) and Epac1. The muscle-specific A-kinase anchoring protein-ERK5 complex suppresses PDE4D3 and facilitates cytokine-induced cardiomyocyte hypertrophy (Dodge-Kafka et al., 2005). Cardiac specific expression of CA-MEK5α reduced pressure overload-induced apoptosis and cardiac dysfunction by inhibiting a PDE3A/inducible cAMP early repressor (ICER) feedback loop (Yan et al., 2007). In addition, ERK5 interacts with the C terminus of Hsc70-interacting protein (CHIP); the ERK5-CHIP complex plays an obligatory role in the inhibition of ICER expression, cardiac apoptosis, and pressure overload-induced dysfunction (Woo et al., 2010). Collectively, these data suggest that ERK5-induced regulation of cAMP-dependent feedback loops prevents myocardial remodeling and HF (Fig. 5).
Figure 5.
Proposed role of ERK5 MAPK during oxidative stress and cardiac hypertrophy.
The MEK5-ERK5 pathway also regulates myofibril contractility by influencing sarcomeric assembly and organization. Activation of MEK5 signaling in cultured cardiomyocytes resulted in serial sarcomere assembly, a process also induced by the interleukin-6 family cytokines leukemia inhibitory factor (LIF) and cardiotrophin-1 (CT-1) (Nicol et al., 2001). Expression of DN-MEK5 specifically abrogated elongation of cardiomyocytes without blocking parallel assembly of sarcomeres and reduced expression of a subset of fetal genes induced by LIF (Nicol et al., 2001, Nakaoka et al., 2003). Furthermore, activated MEK5 induced rapidly decompensating eccentric cardiac hypertrophy in TG mice, indicating a key role for MEK5 in the regulation of in vivo serial sarcomere assembly (Nicol et al., 2001). Likewise, eccentric cardiac hypertrophy induced by long-term, intermittent hypoxia in rats was associated with activation of the MEK5-ERK5 pathway (Chen et al., 2007). It is known that PE or ET-1 induces hypertrophy increasing cell size in all dimensions whereas LIF or CT-1 increases cell length by adding sarcomere units in a serial rather than parallel fashion in cultured myocytes (Wollert et al., 1996). Targeted inactivation of gp130 in ventricular myocytes resulted in rapid chamber dilation and myocyte apoptosis upon pressure overload (Hirota et al., 1999). These studies suggested that gp130 signaling may have a specific role in eccentric hypertrophy, which is mostly associated with volume overload. Accordingly, cell hypertrophy induced by CT-1, an activator of several signaling pathways via gp130, was suppressed by overexpression of DN-MEK5 (Takahashi et al., 2005), indicating that the MEK5-ERK5 pathway is a major pathway responsible for the hypertrophic responses to CT-1. Furthermore, activation of gp130 in cardiomyocytes mediated hypertrophic signaling through the scaffolding/docking protein Gab1-tyrosine phosphatase SHP2 complex, indicating that Gab1-SHP2 interaction plays a crucial role in gp130-dependent longitudinal elongation of cardiomyocytes through activation of ERK5 (Nakaoka et al., 2003).
It should be noted that, like other MAPKs, isoforms of MEK5, MEK5α and MEK5β, differently contribute to cardiac hypertrophy. As mentioned previously, transgenic mice with CA-MEK5β developed eccentric cardiac hypertrophy (Nicol et al., 2001) whereas mice with MEK5α overexpression demonstrated no significant difference in response to hypertrophic stimuli (Cameron et al., 2004). This difference may be due to distinct tissue-wide distribution and cellular localization of MEK5α and MEK5β. Interestingly, MEK5α, but not MEK5β, activates BMK1 (Kobayashi et al., 1997).
In conclusion, MEK5-ERK5 signaling possesses specific features, such as regulation of vascular metabolism, and assembly of sarcomeres, which significantly distinguish these kinases from other MAPKs. However, many questions related to the role of the MEK5-ERK5 axis in the pathogenesis of cardiac hypertrophy and HF still remain unclear. Lack of specific inhibitors hampers an understanding of the contribution of MEK5/ERK5 to cell metabolism.
4. MAPKs in acute cardiac stress (myocardial infarction and ischemia/reperfusion)
4.1. ERK1/2
Both in vivo and in vitro studies have yielded conflicting results on the effect of acute cardiac ischemia (infarction) on ERK1/2 activity. Global 10-min or 20-min ischemia did not activate ERK1/2 in the Langendorff-perfused rat heart (Bogoyevitch et al., 1996). Likewise, IR failed to activate cytosolic ERK1/2, however, it markedly increased phosphorylation of nuclear ERK1/2 in rabbit hearts (Ping et al., 1999). The authors suggested that the IR-induced activation of ERK1/2 occurred in the cytoplasm and was followed by translocation to the nucleus. As mentioned earlier, activation of the Ras-ERK pathway exerts anti-apoptotic effects. Genetic inhibition of cardiac ERK1/2 (Purcell et al., 2007) and Raf (Yamaguchi et al., 2004) promoted stress-induced apoptosis and HF, which suggests that the activation of ERK signaling can provide cardioprotection against oxidative stress. Notably, Raf may exert anti-apoptotic effects by downregulating the apoptotic proteins Ask1 and Mst2, independent of MEK-ERK activity (Chen and Sytkowski, 2005). TG mice hearts with activated MEK1-ERK signaling were protected from apoptosis and were resistant to ischemia-reperfusion injury (Bueno et al., 2000, Lips et al., 2004). Anti-apoptotic effects of ERK1/2 may be explained by its activation of multiple downstream effectors that diminish apoptotic pathways and stimulate pro-survival mechanisms. For example, ERK1/2 can phosphorylate p90RSK, which in turn induces phosphorylation and inactivation of multiple pro-apoptotic proteins, including the Bcl-2 family member Bad, eventually resulting in cellular protection (Bonni et al., 1999) (Fig. 2). Activation of ERK1/2 increased expression of iNOS and eNOS, and enhanced the Bcl-2/Bax ratio associated with cardioprotection against IR (Das et al., 2008). NOS-derived NO may trigger pro-survival mechanisms through the activation of guanylate cyclase leading to cGMP production, PKG activation, and opening of mitochondrial ATP-sensitive potassium (mitoKATP) channels. ERK1/2 can interact with PKCε in the mitochondria to facilitate cardioprotection (Baines et al., 2002) (see 5.1.). ERK1/2 can phosphorylate GATA4, a transcription factor that promotes the expression of anti-apoptotic Bcl-2 proteins (Liang et al., 2001, Kobayashi et al., 2006). Thus, there is growing evidence that activation of the Ras-ERK pathway exerts cardioprotective effects against MI and IR through a number of downstream targets. Cardioprotective effects of ERK signaling involve a complex interplay of various regulatory mechanisms which are still being unraveled.
4.2. p38
Studies of both isolated cell cultures and intact hearts reported early transient activation of p38 in response to oxidative stress induced by ischemia or reperfusion (Bogoyevitch et al., 1996, Ma et al., 1999, Abe et al., 2000, Ping and Murphy, 2000). Activation of p38 does not always correlate with its detrimental or beneficial actions due to cyclicity of p38 activation and variability of oxidative stress. Using different p38 inhibitors, a large number of studies demonstrated that activation of p38 promotes cardiac dysfunction (Nagarkatti and Sha’afi, 1998, Ma et al., 1999, Mackay and Mochly-Rosen, 1999, Barancik et al., 2000, Clark et al., 2007, Martin et al., 2001), whereas others revealed cardioprotective effects of p38 activation (Weinbrenner et al., 1997, Maulik et al., 1998, Mocanu et al., 2000, Bell et al., 2008). Interestingly, cardioprotective effects of p38 activation are mostly induced by ischemic preconditioning (IPC); brief repeated periods of IR prior to sustained ischemia protected hearts against oxidative stress, in part, by rapidly activating p38 (Weinbrenner et al., 1997, Maulik et al., 1998, Mocanu et al., 2000, Steenbergen, 2002, Bell et al., 2008).
The use of non-specific inhibitors along with variability in models, timing, and severity of stresses complicates understanding downstream targets of p38 signaling and its contribution to cardiac IR injury. Genetic studies discovered differential contributions of p38 isoforms, predominantly p38α and p38β, to cardiac dysfunction during ischemia. The use of the chemical-genetic approach and p38 inhibitors revealed that p38α is the dominant-active p38 isoform (Kumphune et al., 2010). It is activated by autophosphorylation and contributes to infarction, which is prevented by the direct binding of SB203580, a p38 inhibitor. Neonatal rat cardiomyocytes infected with adenoviruses encoding p38α or p38β showed isoform-selective activation during sustained, simulated ischemia; p38α remained activated but p38β did not. Moreover, cells expressing DN-p38α were resistant to lethal simulated ischemia, which suggests that inhibition of p38α reduces ischemic injury in this model (Saurin et al., 2000). Likewise, both cultured cardiomyocytes and intact hearts with inactive p38α were resistant against IR injury (Kaiser et al., 2004). Selective inhibition of p38α improved cardiac function and reduced myocardial apoptosis in a rat model of myocardial injury (Li et al, 2006). Ischemic injury was increased in hearts with DN-p38β but not DN-p38α (Cross et al., 2009), which demonstrates that loss of p38β promotes cardiac dysfunction during ischemia. It is important to note that many previous studies used pharmacological inhibitors of p38 that were not isoform-specific. Concurrent inhibition of p38α or p38β complicates the interpretation of results because activation of these isoforms in cardiac cells has different consequences. Furthermore, ATP-competitive inhibitors including the p38 type I inhibitor, SB203580, are not always specific, since they compete for the ATP binding site of all kinases (Clerk and Sugden, 1998, Hall-Jackson et al., 1999, Lali et al., 2000). For p38, this issue was partially solved by developing type II inhibitors that bind to the lower edge of the ATP-binding pocket in the C-terminal lobe. Type II inhibitors of p38 (e.g. BIRB796) selectively interact with and stabilize an inactive, ATP-binding transit pocket, thus, preventing its further activation (Pargellis et al., 2002, Kuma et al., 2005, Denise Martin et al., 2012).
Many gain- and loss-of-function studies revealed that p38 participates in induction of pro-apoptotic signals in cardiac IR (Fig. 3). p38 activation during cardiac IR was associated with TNFα-induced apoptosis, ROS generation, Ca2+ overload and mitochondrial dysfunction, and inhibition of p38 exerted cardioprotective effects and abrogated metabolic alterations (Dhingra et al., 2007, Schwertz et al., 2007, Sucher et al., 2009b). In addition, p38 participates in the regulation of glycogen synthesis and glycolysis in ischemic hearts (Jaswal et al., 2007).
Hearts of the MAPK activated protein kinase (MAPKAP) kinase 2 (MK2) knock-out mice were resistant to myocardial IR injury as evidenced by enhanced post-ischemic recovery of ventricular performance, reduced myocardial infarct size and apoptosis confirming that MK2, a downstream target of p38, is also involved in transmitting the death signal to the ischemic myocardium (Shiroto et al, 2005). Activation of p38 in response to cellular stresses was associated with increased cellular resistance through enhanced actin cytoskeleton reorganization via the p38-MK2-Hsp27 pathway (Guay et al, 1997, Huot et al, 1997). Activation of the p38α-MK2 pathway stimulated phosphorylation of αB-crystallin, a heat shock protein family member, and protected cardiomyocytes against stress-induced apoptosis (Hoover et al, 2000) or myocardial infarction (Shu et al, 2005). Likewise, cardioprotective effects of MKK6 over-expression against IR and myocardial infarction was associated with increased αB-crystallin levels in the TG mice further supporting the protective role for this pathway (Degousee et al., 2003, Martindale et al, 2005).
Activation of p38 mediated cardioprotective effects of insulin against IR in rat hearts through phosphorylation of Hsp27 (Li et al., 2008). However, p38-induced phosphorylation may not be required for the cardioprotective effects of Hsp27 since overexpression of wild-type Hsp27 or a non-phosphorylatable Hsp27 mutant protein was equally capable of protecting the mouse heart from global IR. This indicates that the protection may be caused by different mechanisms or loci of action (Hollander et al., 2004). In this study, nonphosphorylatable Hsp27 mutants even reduced oxidative stress with greater efficacy than wild-type Hsp27 TG mice. Notably, inhibition of p38 with SB203580 stimulated cell necrosis but blocked contractility during reperfusion and offered cardioprotection against IR indicating a dual role of p38 activation (Sumida et al, 2005). Diminished contractility was observed in hearts of MKK3 or MK2 knock-out mice in response to TNFα (Bellahcene et al, 2006). Differential role of p38 activation was also supported by a study in which p38-mediated F-actin reorganization may stimulate apoptotic cell death and, at the same time, conversely protect against osmotic stress-induced necrosis in neonatal cardiomyocytes (Okada et al, 2005).
In conclusion, existing in vivo and in vitro studies demonstrate that oxidative stress induced by acute MI or IR causes activation of p38 in isolated cultured cardiomyocytes and hearts. Short activation of p38 in the heart, for instance, in IPC, may be cardioprotective whereas chronic activation within sustained ischemia (infarction) and reperfusion may have detrimental effects on heart function. Activation of p38 is cyclic and may have different consequences depending on models, and timing and severity of oxidative stress. Although inhibition of p38α on various animal and cell models leads to cardioprotection confirming the role of p38α in cardiac dysfunction, greater basic and preclinical research is still needed to identify appropriate targets of p38 for clinical conditions.
4.3. JNK
Similar to p38, JNK plays a dual role in IR, mediating both protective and detrimental effects depending on the timing and severity of oxidative stress. Interestingly, both genetic inhibition and activation of JNK1/2 protected the heart from IR-induced apoptosis in vivo (Kaiser et al., 2005). These studies suggest that cellular protection induced by sustained inhibition or activation of JNK is likely mediated through different mechanisms. Many studies using various in vitro and in vivo models revealed robust activation of JNK upon reperfusion following ischemia (Laderoute and Webster, 1997, Yin et al., 1997, Fryer et al., 2001). Conversely, pharmacological inhibition of JNK reduced IR-induced infarct size and apoptosis in hearts (Ferrandi et al., 2004, Milano et al., 2007). Inhibition of JNK in H9c2 cardiomyocytes blocked stress-induced apoptosis (Gabai et al., 2000), and DN-JNK mutants antagonized H2O2-induced apoptosis in adult cardiomyocytes (Kwon et al., 2003). Mutated JNK attenuated β-adrenergic-stimulated apoptosis in cardiomyocytes through a mitochondria-dependent mechanism (Aoki et al., 2002, Remondino et al., 2003). Interestingly, inhibition of JNK1 protected cardiomyocytes from ischemia-induced apoptosis, whereas JNK2 inhibition had no effect (Hreniuk et al., 2001). On the other hand, deletion of MEKK1, a direct upstream activator of MKK4 and MKK7, induced more cardiac apoptosis in mouse hearts following pressure overload stimulation (Sadoshima et al., 2002). This is consistent with the observation that JNK inhibitory mutants increased the rates of apoptosis in cultured neonatal cardiomyocytes subjected to hypoxia and reoxygenation by almost 2-fold compared with control cultures (Dougherty et al., 2002). Likewise, cultured cardiomyocytes with DN-JNK1 or DN-MKK4 exhibited enhanced nitric oxide-induced apoptosis (Andreka et al., 2001). In conclusion, confounding experimental results suggest that JNK may simultaneously and distinctly modulate both pro-and anti-apoptotic signaling pathways in the heart. The effects of JNK on apoptosis are mediated, at least in part, by stimulation of caspase-dependent (Aoki et al., 2002) and caspase-independent pathways (Song et al., 2008, Zhang et al., 2009) in the mitochondria (see 6.3). Thus, in response to oxidative stress in cardiomyocytes, complex JNK signaling may be simultaneously protective and detrimental. Ultimate effects apparently depend on crosstalk between JNK and other signaling pathways.
4.4. ERK5
Few studies discuss the role of ERK5 in cardiac IR. Initial studies in perfused guinea pig hearts revealed maximal activation of BMK1 within 30 min of ischemia (Takeishi et al., 1999). Reperfusion of the hearts reduced ERK5 activity to normal values, but, interestingly, activated ERK1/2. These results suggest that ERK5 and ERK1/2 mediate different signaling during reperfusion. Furthermore, maximal ERK5 activation by ischemia was significantly enhanced by IPC (Takeishi et al., 2001). Cardiac-specific CA-MEK5α TG mice exhibited a 3–4-fold increase in ERK5 activation and were highly resistant to IR, as evidenced by their greater cardiac recovery after IR compared to wild-type mice (Cameron et al., 2004). However, cardiac function did not improve after MI induced by permanent ligation in CA-MEK5α TG mice (Shishido et al., 2008). Cardioprotection from MI was enhanced in hyperglycemic CA-MEK5α TG mice. These data suggest that ERK5 activation does not have a significant cardioprotective role in the permanent ligation-induced MI model per se, although it has a significant role in diabetes after MI, at least during the acute (1-week) phase of post-ligation (Shishido et al., 2008). We have previously discussed the role of ERK5 to prevent pressure overload-induced apoptosis and cardiac dysfunction via interaction with ICER and CHIP (see 3.4)(Yan et al., 2007, Woo et al., 2010). ICER and CHIP influence apoptosis by regulating cAMP levels. Interestingly, diabetic mice after MI demonstrated significantly high levels of ICER, which was blunted in CA-MEK5α TG mice. This suggests that ERK5 exerts cardioprotective effects against MI in diabetic mice via downregulation of ICER (Shishido et al., 2008). ERK5 can protect diabetic hearts against MI by receiving posttranslational SUMOylation. SUMOylation of ERK5 was found to inhibit its transcriptional activity in cardiomyocytes (Woo et al., 2008). Diabetes complicated by MI, but not MI alone, increased ERK5-SUMOylation. Furthermore, CA-MEK5α TG mice inhibited ERK5-SUMOylation, thereby preventing cardiac dysfunction and apoptosis (Shishido et al., 2008). This study demonstrates that ERK5 activity can be downregulated by diabetes-dependent SUMOylation leading to post-MI cardiac dysfunction. Furthermore, the ERK5-CHIP complex is targeted by p90RSK in diabetic hearts, and p90RSK prevents ERK5-mediated CHIP activation and promotes apoptosis and cardiac dysfunction in post-MI diabetic mice. Activation of p90RSK disrupts ERK5-CHIP interaction by phosphorylation of ERK5 at S496 and binding of p90RSK to ERK5, causing dislocation of CHIP from ERK5 (Le et al., 2012). In conclusion, many questions with regard to the cause-and-effect relationship between ERK5 activation and attenuation of cardiac dysfunction and cell death remain unanswered. ERK5 participates in MI- and IR-induced cardiac response to oxidative stress. However, the precise mechanisms underlying ERK5 regulation of IR and permanent (non-reperfused) MI signaling are still unknown.
5. Mitochondrial localization of MAPKs
Mitochondria are subcellular organelles that serve as the targets and end-effectors for a myriad of cellular metabolic pathways, including cell signaling, redox control, ion homeostasis, lipid metabolism, cell growth, and cell death. Indeed, they provide approximately 90% of the ATP required for cell metabolism in the heart and represent the major source of cellular physiological and pathological ROS. In response to various extra- and intracellular stimuli, the dynamic mitochondria exhibit bidirectional motility and undergo extensive shape changes via fission-fusion (Hom and Sheu, 2009). Furthermore, mitochondria have their own genome which involves over 1,000 genes. Mitochondrial DNA encodes for 13 proteins of the electron transport chain (ETC) and oxidative phosphorylation, and the RNA genes for their translation in mitochondria. Mitochondrial DNA is not protected by histones and, therefore, has a very high (~1000 times) mutation rate compared to nuclear DNA (Wallace, 2010).
Adult cardiomyocytes contain about 5000 mitochondria, which occupy 30–35% of the cell volume. Moreover, cardiomyocytes contain two functionally distinct mitochondrial subpopulations, which differ with regard to oxygen consumption rates, Ca2+ sensitivity and ROS production; subsarcolemmal mitochondria (SSM) reside beneath the plasma membrane, and interfibrillar mitochondria (IFM) are located between myofibrils (Lesnefsky and Hoppel, 2003, Marzetti et al., 2013). Structurally, mitochondria consist of two membranes, the outer mitochondrial membrane (OMM) and the inner mitochondrial membrane (IMM), and the intermembrane space (IMS) between them. The OMM is permeable to molecules with a molecular mass less than 6 kDa, which pass through the membrane using voltage-dependent anion channel pores. The IMS contains over 100 proteins, including apoptotic proteins such as cytochrome c, apoptosis-inducing factor, the second mitochondrial activator of caspases (Smac)/direct IAP-binding protein with low pI (DIABLO), mammalian serine protease Omi/high-temperature requirement protein A2 (Omi/HtrA2), and endonuclease G. The release of such proteins from mitochondria into the cytoplasm stimulates both caspase-dependent and caspase-independent apoptosis. In contrast, the IMM is impermeable and uses distinct channels, exchangers and pumps to transport ions and other compounds to and from the matrix. A major part of ~500 proteins localized in the matrix are encoded by nuclear genome and transported into the matrix through the transporter outer membrane and transporter inner membrane (TOM/TIM) protein translocation machinery (Muro et al., 2003).
Mitochondrial regulation of MAPK cascades, in particular, is especially intriguing because mitochondria-MAPK interactions are, perhaps, potent regulators in the pathogenesis of cardiac diseases. Several studies revealed direct interactions between MAPKs and mitochondria. Major lines of evidence are derived from studies using isolated mitochondria and/or morphological observations. However, the claim of translocation of MAPKs to the mitochondrial matrix was questioned by studies that reported interactions between MAPKs and OMM, as well as MAPK translocation to the IMS, but did not observe translocation to the matrix. Growing evidence suggests that not only MAPKs, but also their interacting kinases interact with mitochondria (Aoki et al., 2002, Ferreira et al., 2014). In the following sections, mitochondrial localization of MAPKs, as well as the role of MAPKs in the modulation of mitochondrial metabolism and, vice versa, the impact of mitochondria on MAPK signaling, will be discussed.
5.1. ERK1/2
In addition to the cytoplasm and the nucleus, ERK1/2 is also found in mitochondria of the heart (Baines et al., 2002), brain (Alonso et al., 2004, Rumora et al., 2007), renal cells (Nowak et al., 2006, Zhuang et al., 2008), human alveolar macrophages (Monick et al., 2008) and different cell lines, such as B65 cells (Kulich et al., 2007) and SHSY5Y cells (Dagda et al., 2008). Active (phosphorylated) ERK1/2 was found mostly at the OMM and in the IMS of brain mitochondria. ERK1/2 translocation to brain mitochondria followed a brain developmental pattern in rats (Alonso et al., 2004). In the same study, MEK1/2 was detected in brain mitochondria. Severe oxidative stress induced by H2O2 or antimycin decreased ERK1/2 activity significantly in mitochondria. Initial studies using double label confocal microscopy and immuno-electron microscopy reported localization of phospho-ERK1/2 at high labeling densities in mitochondria and autophagosomes of brain tissue in subjects with Parkinson’s disease and Lewy body dementia (Zhu et al., 2003). Phospho-ERK1/2 immunoreactivity in these studies was often associated with mitochondrial proteins, mitochondrial superoxide dismutase and mitochondrial antigens. Transmission electron microscopy of immunolabeled LP07 cells detected ERK1/2 in mitochondria. In these cells, increased ERK1/2 activity in mitochondria and nuclei was observed within 1h of oxidative stress, after which the activity returned to basal levels in mitochondria but remained elevated in nuclei (Galli et al., 2008). Cardiac mitochondria of exercised rats exhibited elevated levels of RAF, an upstream mediator of ERK1/2 (Ferreira et al., 2014). Cardiac-targeted PKCε TG mice demonstrated that transgenic activation of PKCε greatly increased mitochondrial PKCε and its interaction with mitochondrial ERK1/2. Interestingly, both active and inactive PKCε bound to ERK1/2, however, increased phosphorylation of mitochondrial ERK1/2 was observed only in mice expressing active PKCε (Baines et al., 2002). Pre-ischemic increases in phospho-ERK1/2 induced by the adenosine A1/A2a receptor agonist AMP-579 were blunted by the ERK1/2 inhibitor U-0126, though only in cardiac mitochondrial and membrane fractions (Reid et al., 2005).
5.2. p38
Few studies demonstrated direct interactions between p38 and mitochondria in the heart in response to oxidative stress (Ballard-Croft et al., 2005, Sharma et al., 2010). In one study, p38 bound to and stimulated carnitine palmitoyltransferase-1 in mitochondria isolated from rat hearts, thus suggesting a novel regulatory mechanism of mitochondrial fatty acid metabolism (Sharma et al., 2010). Ischemia only induced activation of p38 in the mitochondria, whereas reperfusion increased p38 activity in cytosolic, mitochondrial and membrane fractions. Treatment with the adenosine receptor agonist AMP-579 before ischemia significantly increased p38 activity in the nuclear/myofilament fraction, whereas no activation occurred during ischemia or reperfusion (Ballard-Croft et al., 2005).
5.3. JNK
Previous in vitro and in vivo studies demonstrated the existence of mitochondrial JNK signaling in different cell types (Kharbanda et al., 2000, Ito et al., 2001, Hanawa et al., 2008, Zhou et al., 2008, Zhou et al., 2009, Zhao and Herdegen, 2009), including adult cardiomyocytes (Aoki et al., 2002). Active recombinant JNK1 was found in isolated mitochondria, but its presence was proteinase-sensitive, implying that JNK interacted with mitochondria and did not cross OMM (Zhou et al., 2008). Activation of JNK by upstream MAP2Ks stimulated translocation of a small population of JNKs to mitochondria (Weston and Davis, 2007), and inhibition of JNK activation by N-acetylcysteine, an antioxidant that prevents JNK activation during stress, blocked JNK translocation to the mitochondria in HeLa cells. Active JNK can bind to mitochondria via the mitochondria-related JNK interacting protein, Sab (Wiltshire et al., 2002, Wiltshire et al., 2004). Two kinase interaction motifs (KIMs), KIM1 and KIM2, have been shown to facilitate the interaction between JNK and Sab, although, only KIM1 was necessary for JNK binding and JNK-mediated Sab phosphorylation (Wiltshire et al., 2002). Interestingly, Sab is associated with the mitochondria and co-localizes with activated JNK in response to oxidative stress (Wiltshire et al., 2004). Furthermore, inhibition not only of JNK, but also of Sab reduced infarct size in rat hearts after heart IR (Chambers et al., 2013). Inhibition of Sab also prevented 6-hydroxydopamine (6-OHDA)-induced oxidative stress, mitochondrial dysfunction, and neurotoxicity in vitro and in vivo (Chambers et al., 2013). Apparently, inhibition of JNK-Sab interactions prevents cell death by inhibiting the detrimental mitochondrial ROS amplification loop. Currently, a peptide form of the JNK-Sab inhibitor is used as a specific inhibitor (Chambers et al., 2011). Interestingly, only JNK2 translocated to mitochondria in response to 6-OHDA-induced stress in PC12 cells (Eminel et al., 2004), indicating that JNK1 and JNK2 may play different roles in cardiomyocytes. In addition, upstream kinases of JNK also can interact with mitochondria. Oxidative stress induced translocation of activated JNK and its upstream kinase SEK1 (MKK4) to mitochondria in adult rat cardiomyocytes. The subcellular distribution of total JNK1 and SEK1 (MKK4) did not change significantly upon oxidative stress, however, mitochondrial JNK was phosphorylated, whereas cytosolic JNK remained non-phosphorylated in response to oxidative stress (Aoki et al., 2002). Notably, inhibition of mitochondrial JNK signaling also blocked activation of MKK4, an upstream activator of JNK (Win et al., 2011).
6. MAPKs regulate mitochondrial metabolism
Many studies report that the pro-survival and pro-death effects of MAPKs converge on mitochondria. MAPKs can modulate mitochondrial metabolism directly, through the interaction of individual MAPK members with mitochondria, or indirectly, by activation/inhibition of MAPK-dependent downstream signaling molecules that modulate metabolism and function of mitochondria. The roles of mitochondria in the mediation of MAPK signaling are summarized in Table 1 and shown in Fig. 6. An analysis of existing studies on MAPKs revealed little information with regard to the role of ERK5 in the regulation of mitochondrial metabolism and function. Therefore, bidirectional interactions between ERK5 and mitochondria will not be discussed in following sections.
Table 1.
The role of mitochondria in MAPK signaling
| Model | Role of mitochondria | Reference |
|---|---|---|
| ERK | ||
| Neonatal rat cardiomyocytes | ERK signaling required normal mitochondrial function during H2O2-induced oxidative stress | (Bogoyevitch et al., 2000) |
| TG mice | All MAPKs colocalized in cardiac mitochondria, but PKCε overexpression increased mitochondrial translocation of ERK and Bad | (Baines et al., 2002) |
| THP-1 cells | MitKATP channel openers activated ERK by oxidant-dependent mechanism | (Samavati et al., 2002) |
| Primary pig aorta endothelial cells | Mitochondria-generated ROS stimulated hypoxia-induced activation of ERK1/2 | (Schafer et al., 2003) |
| Neonatal mouse astrocytes | MEK1/ERK pathway may modulate F1F0-ATPase activity | (Yung et al., 2004) |
| SK-N-SH cells | Inhibition of ERK leads to disruption of mitochondrial membrane potential, and resulting in enhanced cell death | (Lee et al., 2004) |
| Rat heart IR | Adenosine receptor preconditioning-activated ERK was found in all subcellular fractions. Loss of activated ERK was observed in mitochondrial and membrane fractions upon blocking of preconditioning | (Reid et al., 2005) |
| Neonatal rat cardiomyocytes | Inhibition of mitochondrial PTP resulted in decreased activation of ERK from phenylephrine-induced hypertrophy | (Javadov et al. 2006) |
| Adult rat cardiomyocytes | Protective effects of PKG-Iα were associated with mitoKATP channel and enhanced phosphorylation of ERK | (Das et al., 2006) |
| SH-SY5Y cells | Mitochondrial ERK2 regulates stress-induced mitophagy and autophagy | (Dagda et al., 2008) |
| Alveolar macrophages | ERK maintained mitochondrial integrity and inhibition of ERK led to cell death through both apoptosis and necrosis | (Monick et al., 2008) |
| Adult rat cardiomyocytes | Mitochondrial ROS production and subsequent ERK phosphorylation are necessary for temperature preconditioning of isolated ventricular myocytes | (Bhagatte et al., 2012) |
| Rat heart mitochondria | Raf levels in mitochondria were elevated by sustained exercise | (Ferreia et al., 2014) |
| p38 | ||
| Mouse heart IR | Inhibition of p38/mitKATP channel abolished adenosine receptor-induced preconditioning | (Zhao et al., 2001) |
| Embryonic chick cardiomyocytes | Mitochondrial ROS stimulated activation of p38 during hypoxia | (Kulisz et al., 2002) |
| Rat heart IR | Ischemic preconditioning induced by opening of the mitKATP channel was the upstream of p38/ROS | (Yue et al., 2002) |
| Mouse heart IR | DN-p38α and MKK6 showed reduced IR-induced injury and enhanced Bcl-2 associated with activation of mitochondrial death signaling | (Kaiser et al., 2004) |
| Rat heart IR | AMP-579 blocked the activation of mitochondrial p38 MAPK during ischemia and reperfusion. | (Ballard-Croft et al., 2005) |
| Mouse embryonic fibroblasts | Activation of p38α and HIF-1 was dependent on the production of mitochondrial ROS. p38 signaling was essential for HIF-1 activation | (Emerling et al., 2005) |
| Rat heart | AngII-induced activation of p38 was prevented by 5-hydroxydecanoate, an inhibitor of mitoKATP channels | (Kimura et al., 2005b) |
| Embryonic chick heart | Inhibition of p38 blocked mitochondrial apoptosis pathway | (Kong et al., 2005) |
| Neonatal rat cardiomyocytes | The AMPK-p38 pathway activated Bax translocation to mitochondria, and inhibition of p38 blocked the process from simulated ischemia | (Capano and Crompton, 2006) |
| Neonatal rat cardiomyocytes | Inhibition of PTP resulted in decreased activation of p38 in phenylephrine-induced hypertrophy dependent on mitochondrial ROS | (Javadov et al., 2006) |
| Neonatal rat cardiomyocytes | Hypoxia/reoxygenation-induced mitochondrial ROS activated p38α | (Kim et al., 2006) |
| TG mice | MKK6 overexpression reduced ETC proteins in heart mitochondria | (Wall et al., 2006) |
| Mouse embryonic cardiomyocytes | Inhibiting p38 reduced mitochondrial ROS, Ca2+ overload, and cell death | (Sucher et al, 2009) |
| Rat heart mitochondria | p38 bound to carnitine palmitoyltransferase-1B and activated its activity in isolated mitochondria | (Sharma et al., 2010) |
| Neonatal rat cardiomyocytes | p38 was a part of ROS-induced formation of a positive-feedback loop during development | (Matsuyama and Kawahara, 2011) |
| JNK | ||
| U-937, Ionizing radiation | Translocation of SAPK to mitochondria was functionally important for apoptosis | (Kharbanda et al., 2000) |
| Adult rat cardiomyocytes | JNK activated mitochondrial apoptosis machinery induced by hydrogen peroxide | (Aoki et al., 2002) |
| Adult rat cardiomyocytes | DN-JNK reduced mitochondria-mediated cell death | (Remondino et al., 2003) |
| Neonatal rat cardiomyocytes | Hypoxia/reoxygenation-induced JNK activation was initiated by mitochondria. Blocking calcium flux and ETC prevented activation of JNK | (Dougherty et al., 2004) |
| PC12 cells | JNK2, not JNK1 was responsible for cytochrome c release. DN-JNK2, but not DN-JNK1 prevented cell death | (Eminel et al., 2004) |
| Rat heart | AngII-induced activation of JNK was prevented by the inhibitor of mitoKATP channels, 5-hydroxydecanoate | (Kimura et al., 2005b) |
| Adult rat cardiomyocytes | Protective effects of PKG-Iα were associated with mitKATP channel and enhanced phosphorylation of JNK | (Das et al., 2006) |
| HeLa cells | Activation of JNK, but not p38 or NAD(P)H oxidase induced mitochondrial ROS generation | (Chambers and LoGrasso, 2011) |
| H9C2 cells, Rat heart IR | Inhibition of mitochondrial JNK signaling reduced ROS, mitochondrial dysfunction, and attenuated MI following IR | (Chambers et al., 2013) |
Figure 6.
Bidirectional interactions between MAPKs and mitochondria.
6.1. ERK1/2
ERK1/2 MAPK participates in regulating processes like cell differentiation, proliferation, growth, adaptation, survival, and death. The role of the Ras-Raf-MEK-ERK1/2 pathway in the regulation of metabolism and function of mitochondria still remains unclear. Individual members of this pathway can mediate pro- and anti-apoptotic signals depending on their intracellular localization and types of stimuli (Majewski M et al., 1999, Alavi et al., 2003,Chu et al., 2004 Hetman et al., 2004). Activation of ERK1/2 can improve mitochondrial function, and inhibition of the Ras-ERK1/2 pathway is associated with cell death. Both MEK1 and ERK1/2 inhibitors diminished F1F0-ATPase activity and induced necrosis in glucose-deprived astrocytes (Yung et al., 2004). In response to toxic stress, inhibition of ERK1/2 with PD98059 induced collapse of ΔΨmit and promoted cytochrome c release from mitochondria into the cytosol that was associated with enhanced neuronal cell death (Lee et al., 2004). Blocking of CB2 receptors exerted cardioprotective effects against IR and increased ERK1/2 phosphorylation associated with reduced cytochrome c release, high ΔΨmit and low PTP opening (Li et al., 2014). In alveolar macrophages, ERK1/2 has been shown to regulate mitochondrial integrity and ATP production. DN-MEK cells treated with U0126 exhibited ATP depletion in a time-dependent manner. Also, ERK inhibition resulted in a rapid loss of ΔΨmit and induced cell death (Monick et al., 2008). PKCα and ERK1/2 mediated cisplatin-induced mitochondrial dysfunction and apoptosis through cytochrome c release from mitochondria in renal cells.
Mitochondrial dysfunction and depolarization of IMM was associated with increased activation and protein expression of ERK1/2 in mitochondria. Inhibition of PKCα did not prevent cisplatin-induced ERK1/2 activation, indicating that activation of ERK1/2 by cisplatin was independent of the PKCα pathway (Nowak, 2002). Oxidant-induced activation of ERK1/2, but not p38 or JNK, attenuated mitochondrial respiration and ATP production, which was associated with decreased complex I activity and substrate oxidation (Nowak et al., 2006). ERK1/2 and PKCε mediated oxidant-induced mitochondrial dysfunction through independent pathways, and protective effects of ERK1/2 inhibition were independent of Akt activation. Interestingly, the ERK1/2 pathway had no effect on the activity of Krebs cycle dehydrogenases in renal proximal tubular cells (Nowak et al., 2006).
Activated ERK1/2 colocalized with mitochondria and phosphorylated Bcl-2 (Deng et al., 2000) and Bad (Kang et al., 2003) through direct interactions with Bcl-2 family members, thereby confirming the contribution of ERK1/2 to the stimulation of anti-apoptotic signaling. Inhibition of the MEK1/2-ERK1/2 pathway reduced phospho-inactivation of the proapoptotic protein Bad in hypoxic cultures of neurons, suggesting that a cell-survival program involving activation of MEK1/2-ERK1/2 signaling is involved in cell survival via inactivation of Bad (Jin et al., 2002). Active Raf-1, fused with OMM targeting sequences, protected cells from apoptosis, a phenomenon associated with phosphorylation of pro-apoptotic Bad, whereas plasma membrane-targeted Raf-1 had no effect on apoptosis and resulted in phosphorylation of ERK1/2 (Wang et al., 1996). These data suggest that Bcl-2 can target Raf-1 to mitochondria to induce phosphorylation of Bad or possibly other protein substrates involved in apoptosis regulation. Also, this study demonstrates divergent signaling roles of plasma membrane-targeted and mitochondria-targeted Raf proteins in apoptosis.
The adapter protein Grb10 interacted with both Raf-1 and MEK1 and regulated signal transduction between plasma membrane receptors and the apoptosis-inducing complex on OMM by modulating the anti-apoptotic activity of mitochondrial Raf-1 (Nantel et al., 1999). Interestingly, the addition of cytochrome c to the cytosol of cells overexpressing B-Raf in fibroblasts failed to induce caspase activation, which indicates that the B-Raf/MEK/ERK pathway apparently confers protection against apoptosis at the level of cytosolic caspase activation, downstream of the release of cytochrome c from mitochondria (Erhardt et al., 1999). On the other hand, mitochondrial Raf-1 in myeloid cells exerted anti-apoptotic effects independent of ERK1/2 activity (Majewski et al., 1999). In endothelial cells, p21-activated protein kinase-1 (PAK-1)-induced phosphorylation of Raf1 at Ser338 and Ser339, promoted mitochondrial translocation of the protein kinase and protected the cells from the intrinsic pathway of apoptosis, independent of MEK1. However, VEGF induced activation of Raf-1 via Src kinase, leading to phosphorylation of Tyr340 and Tyr341 and MEK1-dependent protection from extrinsic-mediated apoptosis (Alavi et al., 2003). These findings demonstrate that Raf-1 regulates both cell survival and death through the modulation of mitochondrial signaling and death receptors
In addition to initiating survival signaling, the MEK-ERK pathway also stimulates cell death, as demonstrated by several studies. In rat astrocytes, toxic stress induced mitochondrial swelling and vacuolation, though this phenomenon was attenuated by ERK1/2 inhibition, indicating that the ERK signaling cascade is involved in the induction of mitochondrial vacuolation (Isobe et al., 2003). Treatment of B65 cells with 6-OHDA resulted in significant phosphorylation of ERK1/2 within mitochondrial fractions and mitochondrial ROS generation. Antioxidants inhibited ERK1/2 activation and protected the cells (Kulich et al., 2007). Insulin-like growth factor I receptor (IGFIR)-induced paraptosis, nonapoptotic cell death, was abrogated by inhibition of MEK2. Although mitochondria were not investigated in this study, the results implicate MEK activation in the induction of paraptosis (Sperandio et al., 2004). Doxorubicin-induced apoptosis in H9c2 cells and neonatal cardiomyocytes was associated with ΔΨmit collapse and activation of ERK1/2 and p53 with no significant changes in p38 and JNK phosphorylation. Specific inhibitors of ERK1/2 and p53 attenuated the increased phosphorylation of ERK1/2 and p53 and prevented the toxic effects of doxorubicin (Liu et al., 2008). The ERK1/2 inhibitor U0126 effectively abrogated the anti-apoptotic effects of lovastatin, a cholesterol-lowering drug, on the mitochondrial apoptotic pathway during hypoxia in mesenchymal stem cells, confirming that ERK1/2 activation can initiate pro-death signaling (Xu et al., 2008). Since activation of mitochondrial ERK1/2 was sufficient to induce autophagy and mitophagy, ERK1/2 could act as a downstream target for different stressors and promote cell death (Dagda et al., 2008). Notably, detrimental effects of ERK1/2 activation are dependent on subcellular compartmentalization of the MAPK (Reviewed in Chu et al., 2004, Dagda et al., 2009). In various models of toxicity in neuronal cells, a major fraction of activated ERK1/2 was found in the cytoplasm and mitochondria, but only a small amount translocated to the nucleus (Zhu et al., 2002, Dagda et al., 2008). Pro-death effects of ERK1/2 can also be mediated through different downstream targets, independent of direct mitochondrial targeting.
Thus, subcellular distribution/activation of ERK1/2 could decisively disrupt the balance between pro-survival and pro-death signals in the cell. Translocation and activation of ERK1/2 within the individual compartments of the cell, including the cytoplasm, nucleus and mitochondria, as well as the temporal and spatial coincidence with other signaling pathways, can distinctly direct survival and death pathways.
6.2. p38
Ischemia induced Bax translocation from the cytoplasm to mitochondria in neonatal cardiomyocytes, and inhibition of p38 with SB203580 blocked the translocation completely. These data link ischemia-induced p38 activation to mitochondria-mediated cell death, indicating that Bax translocation to mitochondria, a hallmark of apoptosis, occurs in response to activation of p38 (Capano, Crompton, 2006). Likewise, activation of p38 attenuated phosphorylation of Bad, another pro-apoptotic protein, and stimulated its mitochondrial translocation, inducing apoptosis in endothelial cells (Grethe, Porn-Ares, 2006). In addition, the pro-apoptotic effects of p38 may be due to its capacity to downregulate expression of Bcl-2, an anti-apoptotic protein that antagonizes interactions of Bax and Bad with mitochondria. In neonatal cardiomyocytes, genetic inhibition of p38 up-regulated Bcl-2, whereas overexpression of the active p38 mutant reduced Bcl-2 protein levels (Kaiser et al., 2004). These studies are consistent with in vivo observations, in which the hearts of mice expressing DN-p38 and MKK6 were protected from IR injury, and inhibition of p38 signaling resulted in up-regulation of Bcl-2 (Kaiser et al., 2004). MKK6 TG mice exhibited reduced expression of oxidative phosphorylation and fatty acid oxidation proteins, along with reductions in several proteins involved in apoptosis (Wall et al., 2006). p38 inhibition reduced IR-induced apoptosis in perfused rabbit hearts (Ma et al., 1999), and expression of DN-p38α reduced deamidation (inactivation) of the antiapoptotic protein Bcl-xL and prevented apoptosis in the mouse heart (Ren et al., 2005). Conversely, in response to TNFα, p38 phosphorylated Bcl-xL and reduced its expression due to its degradation, inducing apoptosis in endothelial cells. Inhibition of p38 by SB203580 significantly attenuated TNFα-induced apoptosis and prevented Bcl-xL phosphorylation and degradation in proteasomes, suggesting that p38 is essential for apoptosis. Interestingly, a time-dependent increase of active p38 was observed in mitochondria of cells exposed to TNFα (Grethe et al., 2004).
6.3. JNK
In addition to phosphorylating several transcriptional growth factors in the nucleus and cytoskeletal proteins in the cytoplasm, activation of JNK signaling affects many aspects of mitochondrial function related to apoptosis and bioenergetics. The spatiotemporal activation of JNK is differently regulated in distinct intracellular compartments, including the cytoplasm, the nucleus and mitochondria (Bonny et al., 2005). Many studies using a variety of animal and cell models of stress demonstrated that activated JNK can be translocated to mitochondria or otherwise activated in mitochondria (Baines et al., 2002, Chauhan et al., 2003, Brichese et al., 2004, Rumora et al., 2007, Hanawa et al., 2008, Zhou et al., 2008).
Apoptosis in multiple myeloma cells was associated with translocation of activated JNK to mitochondria and the mitochondrial release of Smac into the cytosol. DN-JNK, or blocking of JNK with a specific JNK inhibitor SP600125, abrogated stress-induced release of Smac and induction of apoptosis, indicating that JNK activation is a requirement for the release of Smac during stress-induced apoptosis in multiple myeloma cells (Chauhan et al., 2003). Likewise, DN-JNK2, but not DN-JNK1, protected PC12 cells against 6-OHDA-induced cell death, which is associated with reduced translocation of JNK2 to the mitochondria, release of cytochrome c and cleavage of caspase-3 (Eminel et al., 2004). Mitochondrial JNK inactivated Bcl-2 and Bcl-xL through phosphorylation, thereby promoting apoptosis (Kharbanda et al., 2000, Lei et al., 2002, Brichese et al., 2004 Dhanasekaran and Reddy, 2008). Furthermore, translocation of JNK to mitochondria and subsequent interaction with anti-apoptotic Bcl-xL indicates that the JNK-Bcl-xL complex is a functionally important event in the initiation of apoptosis in response to gamma radiation-induced genotoxic stress of myeloid lymphoma cells (Kharbanda et al., 2000). Likewise, β-adrenergic receptor-stimulated apoptosis in cardiomyocytes was mediated through ROS/JNK-dependent activation of the mitochondrial death pathway (Remondino et al., 2003). Activation of mitochondrial JNK in MI was associated with release of cytochrome c from heart mitochondria (Aoki et al., 2002). The molecular mechanisms underlying JNK translocation to mitochondria and JNK-induced mitochondrial dysfunction remain to be fully determined.
It seems that mitochondrial JNK signaling leads to ROS generation. JNK, but not p38 or NADPH oxidase, was responsible for mitochondrial ROS generation in response to anisomycin-induced stress in HeLa cells (Chambers and LoGrasso, 2011). JNK activation, associated with increased mitochondrial ROS generation, has been found to be involved in myocardial cell death induced by hypoxia-reoxygenation (Cicconi et al., 2003, Sucher et al., 2009a). Mitochondrial ROS, in addition to Ca2+ overload and ATP depletion, is a main factor leading to the mitochondrial permeability transition. Therefore, mechanisms involved in permeability transition pore (PTP) opening may also link JNK activation to mitochondrial dysfunction.
Mitochondrial PTP are non-specific channels that allow ions, water and solutes with a molecular mass of 1.5 kDa to enter the matrix, leading to amplification of ROS production, mitochondrial swelling and cell death. The molecular identity of the mitochondrial PTP remains unknown. Although initial studies implicated VDAC and ANT as essential components of the PTP complex, subsequent genetic loss- and gain-of-function studies have questioned this conclusion (reviewed in Javadov et al., 2009, Bernardi, 2013). The regulatory roles of cyclophilin D, ANT and phosphate carrier, in addition to the peripheral benzodiazepine receptor, creatine kinase, hexokinase, and Bcl-2 proteins, in the promotion of PTP opening have been established. Nonetheless, the precise mechanisms underlying the permeability transition still need to be elucidated. Most recently, F1F0-ATPase (complex V) has shown promise as a potential structural protein of the PTP complex (Giorgio et al., 2013). The role of mitochondrial PTP opening in cardiac diseases, especially in IR, has been well reviewed elsewhere (Weiss et al., 2003, Halestrap et al., 2004, Di Lisa and Bernardi, 2006). The mitochondrial PTP has been proposed to be a promising target in the treatment of cardiac diseases, such as IR and HF (Javadov et al., 2009). Direct interaction of activated JNK with proteins of the PTP complex may stimulate pore formation and promote mitochondria-mediated cell dysfunction in response to oxidative stress. However, the lack of knowledge with regard to the molecular composition of the PTP complex limits examination of the possible interactions between JNK and PTP components under pathological conditions.
In addition, mitochondrial JNK also appears to have a role in the regulation of mitochondrial bioenergetics. Mitochondrial JNK decreased respiration rates and ATP production during acetaminophen-induced liver injury (Hanawa et al., 2008). Oxidative stress induced by H2O2 in primary cortical neurons increased phosphorylation of JNK at OMM which was associated with the reduced activity of pyruvate dehydrogenase (PDH) due to its phosphorylation (Zhou et al., 2008). Similar observations were reported using anisomycin in aging brain mitochondria in which activation of JNK attenuated PDH activity (Zhou et al., 2009). PDH is a matrix-localized enzyme complex that is vital to energy metabolism, linking glycolysis in the cytoplasm to the Krebs cycle in mitochondria. Inhibition of PDH by JNK enhances a shift from aerobic toward anaerobic metabolism. As a result, JNK-induced mitochondrial dysfunction and reduced ATP production can initiate cell death. These studies confirm a key role of JNK in the regulation of mitochondrial energy metabolism.
Although most studies implicate JNK in stimulating cell death signaling, activation, rather than inhibition of JNK, has been shown to offer protective effects in cultured cardiomyocytes (Dougherty et al., 2002, Dougherty et al., 2004). Transfection/infection with JNK inhibitory mutants increased apoptosis in cardiomyocytes subjected to hypoxia/reoxygenation. The p38 inhibitor SB203580 provided only partial protection against apoptosis in neonatal rat cardiomyocytes, suggesting that JNK activation is protective and that the pathway is largely independent of p38 (Dougherty et al., 2002). However, our studies using the isolated Langendorff-perfused heart model revealed that inhibition of JNK leads to further compensatory activation of p38. SU3327, a JNK inhibitor, decreased IR-induced JNK phosphorylation and, interestingly, seemed to indirectly activate p38. IR greatly increased mitochondrial p38, while treatment with the JNK inhibitor increased the accumulation of both activated p38 and JNK in the mitochondrial fraction. This could, in part, account for the absence of the expected protective effect of JNK inhibition (Jang and Javadov, submitted for publication). MEKK1 knock-out mice demonstrated that activation of the MEKK1-JNK pathway prevented apoptosis and inflammation, thereby protecting the heart against HF and sudden death following cardiac pressure overload (Sadoshima et al., 2002). Oxidative stress induced by IR initiated JNK activation in mitochondria and required coupled electron transport, ROS generation, and calcium flux. These factors caused the selective and sequential activation of the calcium-dependent, proline-rich kinase Pyk2 and the small GTP binding factors Rac-1 and Cdc42. Interruption of these interactions prevented JNK activation and led to a pro-apoptotic phenotype during IR (Dougherty et al., 2004). In addition, JNK activation can play a role in preconditioning, which imparts resistance to sustained oxidative stress by various internal and external factors, such as IR, inflammation, xenobiotics, and UV radiation, among others.
Collectively, most in vivo and in vitro studies revealed the role of JNK activation in promoting mitochondria-mediated cell death in response to oxidative stress. Activity of JNK in subcellular compartments as well as type and severity of stimulus presumably play a decisive role in initiating pro-death and pro-survival.
7. Mitochondria modulate MAPK signaling
Mitochondria mediate pro-survival and pro-death pathways by modulating MAPK activity. A widely accepted model of mitochondria-mediated activation of MAPKs involves mild stress-induced opening of mitoKATP channels during IPC and pharmacological preconditioning. All three major MAPKs, ERK1/2, p38 and JNK, have been shown to be activated by IPC, although the precise mechanisms underlying MAPK activation during IPC still remain elusive (Fryer et al., 2001). mitoKATP channels are located in IMM and widely accepted as redox sensors and effectors of many survival signaling pathways implicated in IPC and pharmacological preconditioning (Hide et al., 1996, Gross, Fryer, 1999, Broadhead et al., 2004). Activation of protein kinases in the cytoplasm induced by short-term stress triggers the opening of mitoKATP channels, which in turn causes the loss of ΔΨmit and inhibition of ETC. Depolarization of IMM reduces Ca2+ overload, thereby protecting the heart from subsequent, sustained IR. One of the key regulators of mitoKATP channels is PKC, which is activated in response to various cellular stresses, including hypertrophic stimuli and oxidative stress (Sato et al., 1998). There are no data on the direct stimulation of mitoKATP channels by MAPKs. Importantly, the opening of mitoKATP channels in response to various cellular stresses induces a short mitochondrial ROS burst, which activates down-stream survival signaling molecules, including cytoplasmic MAPKs, to protect the heart. Oxidative phosphorylation accounts for ~90% of total cellular oxygen consumption, indicating that mitochondrial metabolism could significantly affect cellular ROS signaling (Marchi et al., 2012). Complexes I and III of the mitochondrial ETC generate superoxide anion (O2−) in the univalent reduction of O2 (Brand et al., 2004). Although ROS are frequently thought of as harmful byproducts of cellular metabolism that damage the cell, in fact, they are important second messengers that modulate cell signaling and can be involved in cell protection. Indeed, the latter partially explains rather unsuccessful clinical trials with antioxidants (Becker, 2004).
In addition, mitoKATP channels can also be activated by ROS produced in the cytoplasm and mitochondria. Non-mitochondrial ROS induced a significant increase in the activity of reconstituted myocardial mitoKATP channels in vitro, indicating that non-mitochondrial ROS can affect mitochondria through mitoKATP channels (Zhang et al., 2001). Interestingly, mitochondrial permeability transitions induced by exogenous ROS coincide with mitochondrial ROS generation, which leads to ROS accumulation in individual mitochondrion of isolated cardiomyocytes, a phenomenon known as “ROS-induced ROS release” (Zorov et al., 2000). This constitutes a ROS-amplification loop (Hänninen et al., 2010). If an increase in ROS reaches a threshold, it results in further ROS generation. ROS can be released into the cytosol and trigger ROS-induced ROS release in the cytoplasm and neighboring mitochondria. This signaling constitutes a positive feedback mechanism and can lead to mitochondrial dysfunction and cellular injury (Zorov et al., 2000, Zorov et al., 2006). In addition, mitochondrial Ca2+ uptake can stimulate ROS emission through the activation of the membrane permeability transition, implying that calcium could be a part of the mitochondrial ROS-amplification loop. Notably, ROS-induced ROS release can also occur through a PTP-independent mechanism in which increased ROS triggers the opening of the inner mitochondrial membrane anion channel (IMAC), thereby resulting in a brief increase in ETC-derived ROS (Brady et al., 2006).
The p38/JNK activator anisomycin reduced infarct size, an effect that was abolished by 5-hydroxydecanoate, an inhibitor of mitoKATP channels (Baines et al., 1999). AngII induced depolarization of ΔΨm within short-time periods following the increase in O2− generation in vascular smooth muscle cells (Kimura et al., 2005). Furthermore, cardioprotective effects of Ang II preconditioning against cardiac IR injury in vivo were eliminated by pretreatment with 5-hydroxydecanoate or apocynin, an NAD(P)H oxidase inhibitor. Both inhibitors suppressed AngII-induced activation of p38 and JNK, suggesting that preconditioning effects of Ang II against cardiac IR may be mediated through JNK and p38 activation by NAD(P)H oxidase-derived ROS-induced mitochondrial ROS (Kimura et al., 2005). Thus, ROS generation due to a short-time activation of NAD(P)H oxidase may serve as a trigger to induce the opening of mitoKATP channels and the subsequent mitochondrial ROS burst, which in turn can mediate the preconditioning effects of Ang II (Zhang et al., 2007). p38 and mitoKATP channels play important roles during adenosine-induced late preconditioning in mouse hearts. Adenosine A1 receptor-triggered delayed cardioprotection was mediated by p38 phosphorylation in Langendorff-mode perfused hearts (Zhao et al., 2001), and mitochondrial ROS initiated phosphorylation of p38 during hypoxia in embryonic chick cardiomyocytes (Kulisz et al., 2002). Activation of p38α and hypoxia-inducible factor 1 was dependent on the production of mitochondrial ROS. p38α−/− cells failed to activate hypoxia-inducible factor 1 under hypoxic conditions. Hypoxic activation of p38α and hypoxia-inducible factor 1 was abolished by the mitochondrial complex III inhibitor myxothiazol and the antioxidant protein glutathione peroxidase 1 (Emerling et al., 2005). Interestingly, p38 is part of a ROS-induced positive-feedback loop during heart development. Sustained activation of p38 soon after birth may contribute to the loss of cell division and binucleation in mammalian cardiomyocytes (Matsuyama and Kawahara, 2011).
Inhibition of the MEK-ERK1/2 pathway by PD98059 eliminated the cardioprotective effects of IPC (da Silva R et al., 2004). Likewise, the p38 antagonist SB203580 abolished IPC-induced cardioprotection and attenuated IPC when it was administered before the IPC stimulus (Fryer et al., 2001) or prior to and during the first 15 min of the lethal ischemia (Mocanu et al., 2000). Both short and prolonged activation of MAPKs in response to oxidative stress seem to be redox-sensitive. The extent of activation of the stress-regulated MAPKs p38 and JNK by hydrogen peroxide was similar to that induced by IR in isolated perfused hearts (Clerk et al., 1998). IPC stimulated p38 activation through mitochondrial ROS production (Zhao et al., 2001, Kulisz et al., 2002). ROS scavengers inhibited the activation of MAPKs, indicating that MAPKs are regulated by their redox states. Moreover, activation of MAPKs by mitochondrial ROS plays a central role in mediating cardioprotective signaling of IPC. Short-time increases of ROS generated by ETC act as a trigger to resist sustained IR injury through the posttranslational modification of redox-sensitive proteins, including MAPKs. Mitochondria-generated ROS activated p38α in cultured rat cardiomyocytes that had undergone prolonged hypoxia followed by reoxygenation (Kim et al., 2006).
In addition to IPC, ERK1/2 activation induced by temperature preconditioning was shown to be a downstream process of ROS generation. Both inhibition of ERK1/2 and scavenging ROS abolished the protective effect of temperature preconditioning in adult rat cardiomyocytes (Bhagatte et al., 2012). Opening of the mitoKATP channel stimulated the ERK1/2 pathway and exerted cell protection by regulating mitochondrial ROS. mitoKATP channel openers triggered ERK1/2 activation via mitochondria-derived ROS in THP-1 cells. Overexpression of manganese superoxide dismutase reduced ROS production and prevented ERK activation (Samavati et al., 2002). Ang II-induced activation of MAPK in the myocardium cardiovascular tissues (Zhang et al., 2004) and rat vascular smooth muscle cells (Kimura et al., 2005a) was sensitive to the ROS scavenger tempol, suggesting that activation of MAPK was redox-sensitive. In addition, AngII-induced activation of p38 and JNK was prevented by 5-hydroxydecanoate (Kimura et al., 2005b), indicating the important role of mitoKATP channels in the activation of p38 and JNK. Mitochondria-generated ROS stimulated hypoxia-induced activation of p38 in cardiomyocytes (Kulisz A et al., 2002) and ERK1/2 in endothelial cells (Schafer et al., 2003). Activation of p38 and ERK1/2 induced by PE in cultured neonatal cardiomyocytes was dependent on mitochondrial ROS (Javadov et al., 2006). Electron transport-coupled calcium flux and ROS produced in mitochondria stimulated JNK activation, which enhanced resistance of neonatal cardiomyocytes to hypoxia/reoxygenation-induced apoptosis (Dougherty et al., 2004).
Acute ROS production can promote low-conductance (reversible) mitochondrial PTP opening. In contrast to high-conductance (pathological, irreversible) mode pores, the low-conductance PTP opening has a physiological role in the generation of mitochondrial depolarization spikes and the conveyance of calcium signals between individual mitochondria (Ichas et al., 1997). The low-conductance mode PTPs are permeable to small ions and molecules with a molecular mass less than 300 Da, and pore flickering does not induce detectable matrix swelling. Alternatively, acute oxidative stress may trigger irreversible PTP opening in stress-sensitive mitochondria of certain cell compartments, such as interfibrillar mitochondria, leading to ROS burst and activation of survival protein kinases in the cell. Cyclophilin D, a major regulator of the mitochondrial PTP was required by IPC to generate mitochondrial ROS and phosphorylate Akt and Erk1/2 in mouse cardiomyocytes (Hausenloy et al., 2010). In rat cardiomyocytes, inhibition of PTP by cyclosporine A, an inhibitor of cyclophilin D, prevented PE-induced activation of p38 and ERK1/2, as well as mitochondrial dysfunction (Javadov et al., 2006). These studies indicate the existence of a feedback mechanism in which mitochondrial stimuli initiate MAPK activation. Thus, mitochondrial ROS activate MAPK to mediate survival signaling pathways implicated in IPC and pharmacological preconditioning in the heart. However further studies are needed to elucidate the role of the mitoKATP-mitochondrial ROS-MAPK pathway in the pathogenesis of IR, hypertrophy and HF.
Altogether, current studies provide evidence of both direct and indirect interactions between MAPKs and mitochondria in response to different cellular stresses. However, the contributions of MAPKs to mitochondria-mediated cell survival and cell death mechanisms in healthy and diseased hearts remain to be elucidated. Along with extracellular stressors, mitochondrial dysfunction per se can initiate activation of MAPKs, although precise mechanisms of the activation loop are not clear. Importantly, spatiotemporal activation and regulation of MAPK signaling in subcellular compartments, especially in mitochondria, can stimulate pro-survival and pro-death pathways depending on the severity and type of stimuli. Considered together, these direct and indirect interactions provide evidence that a thorough understanding of mitochondria-MAPK interactions may provide novel insights into the pathogenesis of cardiac diseases. Also, interactions may be exploited to develop mitochondria-targeted therapy and improve the prognosis of heart disease.
8. Conclusions and perspectives
Many signaling networks, including the MAPKs family are involved in the pathogenesis of stress-related cardiac diseases such as MI, hypertrophy and HF. MAPKs respond transiently or permanently during myocardial stresses depending on timing, severity of stress, and types of stimuli, to mediate some of the most important signaling networks in the heart. Due to the complex interplay of regulatory mechanisms, current studies suggest multiple roles for MAPKs; in some cases, individual MAPKs foster cardioprotection, in other cases they mediate damage to the cell and cause cardiac dysfunction in response to oxidative stress. Mitochondria are powerhouses and important gate-keepers of cell life and death, and regulation of mitochondrial metabolism by MAPKs is critical for cardiac cells. Molecular mechanisms of activation of individual MAPKs and the cause-and-effect relationship between MAPK activation and cardiac diseases are not entirely clear. Understanding precise mechanisms of the role of MAPKs in cardiac diseases are obscured due to absence of highly specific inhibitors. Numerous studies revealed that the effects of MAPKs activation converge on mitochondria and modulate their metabolism. Though many questions remain unanswered, there exists enough evidence to consider the possibility of targeting MAPK-mitochondria interactions in the prevention and treatment of heart disease. A comprehensive understanding of relevant molecular mechanisms, as well as challenges in this area, will promote the development of new pharmacological agents and genetic manipulations for the treatment of cardiovascular diseases by targeting the MAPKs/mitochondria pathway.
Figure 1.
Main MAPK signaling pathways in the heart.
Acknowledgments
This study was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health through Research Grant SC1HL118669 to S. Javadov. The authors thank Ms. Jessica Soto Hernandez for her technical assistance in preparation of the manuscript.
Abbreviations
- AngII
angiotensin II
- CA
constitutively active
- CHIP
C terminus of Hsc70-interacting protein
- CT-1
cardiotrophin-1
- ΔΨmit
mitochondrial membrane potential
- DN
dominant negative
- ERK1/2
extracellular signal-regulated kinases 1 and 2
- ET-1
endothelin-1
- ETC
electron transport chain
- GPCR
G-protein-coupled receptor
- HF
heart failure
- Hsp27
heat shock protein 27
- ICER
inducible cAMP early repressor
- IMM
inner mitochondrial membrane
- IMS
intermembrane space
- IPC
ischemic preconditioning
- IR
ischemia-reperfusion
- JIP
JNK-interacting protein
- JNK
c-Jun NH2-terminal kinase
- LIF
leukemia inhibitory factor
- LTCC
L-type calcium channel
- MAPK
mitogen-activated protein kinase
- MAPKAP
MAPK activated protein kinase
- MEF
myocyte-specific enhancer factor
- MEK
MAPK/ERK kinase
- MEKK
MEK kinase
- MI
myocardial infarction
- mitoKATP channel
mitochondrial ATP-sensitive potassium channel
- MK2
MAPKAP kinase-2
- MKP
MAPK phosphatase
- NFAT
nuclear factor of activated T-cells
- NHE-1
sodium-hydrogen exchanger 1
- 6-OHDA
6-hydroxydopamine
- OMM
outer mitochondrial membrane
- PDE
phosphodiesterase
- PDH
pyruvate dehydrogenase
- PE
phenylephrine
- PKG
protein kinase G
- PTP
permeability transition pore
- ROS
reactive oxygen species
- RTK
receptor tyrosine kinase
- SAPK
stress-activated protein kinase
- Smac
second mitochondria-derived activator of caspase
- TG
transgenic
- TNFα
tumor necrosis factor alpha
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe J, Baines CP, Berk BC. Role of mitogen-activated protein kinases in ischemia and reperfusion injury : the good and the bad. Circ Res. 2000;86:607–609. doi: 10.1161/01.res.86.6.607. [DOI] [PubMed] [Google Scholar]
- Abe J, Kusuhara M, Ulevitch RJ, Berk BC, Lee JD. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J Biol Chem. 1996;271:16586–16590. doi: 10.1074/jbc.271.28.16586. [DOI] [PubMed] [Google Scholar]
- Akaike M, Che W, Marmarosh NL, Ohta S, Osawa M, Ding B, et al. The hinge-helix 1 region of peroxisome proliferator-activated receptor gamma1 (PPARgamma1) mediates interaction with extracellular signal-regulated kinase 5 and PPARgamma1 transcriptional activation: involvement in flow-induced PPARgamma activation in endothelial cells. Mol Cell Biol. 2004;24:8691–8704. doi: 10.1128/MCB.24.19.8691-8704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003;301:94–96. doi: 10.1126/science.1082015. [DOI] [PubMed] [Google Scholar]
- Alonso M, Melani M, Converso D, Jaitovich A, Paz C, Carreras MC, et al. Mitochondrial extracellular signal-regulated kinases 1/2 (ERK1/2) are modulated during brain development. J Neurochem. 2004;89:248–256. doi: 10.1111/j.1471-4159.2004.02323.x. [DOI] [PubMed] [Google Scholar]
- Andreka P, Zang J, Dougherty C, Slepak TI, Webster KA, Bishopric NH. Cytoprotection by Jun kinase during nitric oxide-induced cardiac myocyte apoptosis. Circ Res. 2001;88:305–312. doi: 10.1161/01.res.88.3.305. [DOI] [PubMed] [Google Scholar]
- Andrews C, Ho PD, Dillmann WH, Glembotski CC, McDonough PM. The MKK6-p38 MAPK pathway prolongs the cardiac contractile calcium transient, downregulates SERCA2, and activates NF-AT. Cardiovasc Res. 2003;59:46–56. doi: 10.1016/s0008-6363(03)00329-8. [DOI] [PubMed] [Google Scholar]
- Aoki H, Kang PM, Hampe J, Yoshimura K, Noma T, Matsuzaki M, et al. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem. 2002;277:10244–10250. doi: 10.1074/jbc.M112355200. [DOI] [PubMed] [Google Scholar]
- Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- Baines CP, Liu GS, Birincioglu M, Critz SD, Cohen MV, Downey JM. Ischemic preconditioning depends on interaction between mitochondrial KATP channels and actin cytoskeleton. American Journal of Physiology-Heart and Circulatory Physiology. 1999;276:H1361–H1368. doi: 10.1152/ajpheart.1999.276.4.H1361. [DOI] [PubMed] [Google Scholar]
- Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, et al. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- Ballard-Croft C, Kristo G, Yoshimura Y, Reid E, Keith BJ, Mentzer RM, et al. Acute adenosine preconditioning is mediated by p38 MAPK activation in discrete subcellular compartments. American Journal of Physiology-Heart and Circulatory Physiology. 2005;288:H1359–H1366. doi: 10.1152/ajpheart.01006.2004. [DOI] [PubMed] [Google Scholar]
- Barancik M, Htun P, Strohm C, Kilian S, Schaper W. Inhibition of the cardiac p38-MAPK pathway by SB203580 delays ischemic cell death. J Cardiovasc Pharmacol. 2000;35:474–483. doi: 10.1097/00005344-200003000-00019. [DOI] [PubMed] [Google Scholar]
- Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Bell JR, Eaton P, Shattock MJ. Role of p38-mitogen-activated protein kinase in ischaemic preconditioning in rat heart. Clin Exp Pharmacol Physiol. 2008;35:126–134. doi: 10.1111/j.1440-1681.2007.04794.x. [DOI] [PubMed] [Google Scholar]
- Bellahcene M, Jacquet S, Cao XB, Tanno M, Haworth RS, Layland J, et al. Activation of p38 mitogen-activated protein kinase contributes to the early cardiodepressant action of tumor necrosis factor. J Am Coll Cardiol. 2006;48:545–555. doi: 10.1016/j.jacc.2006.02.072. [DOI] [PubMed] [Google Scholar]
- Bernardi P. The mitochondrial permeability transition pore: a mystery solved? Front Physiol. 2013;4:95. doi: 10.3389/fphys.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagatte Y, Lodwick D, Storey N. Mitochondrial ROS production and subsequent ERK phosphorylation are necessary for temperature preconditioning of isolated ventricular myocytes. Cell death & disease. 2012;3:e345. doi: 10.1038/cddis.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch MA, Gillespie-Brown J, Ketterman AJ, Fuller SJ, Ben-Levy R, Ashworth A, et al. Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ Res. 1996;79:162–173. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Ketterman AJ, Sugden PH. Cellular stresses differentially activate c-Jun N-terminal protein kinases and extracellular signal-regulated protein kinases in cultured ventricular myocytes. J Biol Chem. 1995;270:29710–29717. doi: 10.1074/jbc.270.50.29710. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Ng DCH, Draper KA, Dhillon A, Abas L. Intact mitochondrial electron transport function is essential for signalling by hydrogen peroxide in cardiac myocytes. J Mol Cell Cardiol. 2000;32:1469–1480. doi: 10.1006/jmcc.2000.1187. [DOI] [PubMed] [Google Scholar]
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- Bonny C, Borsello T, Zine A. Targeting the JNK pathway as a therapeutic protective strategy for nervous system diseases. Rev Neurosci. 2005;16:57–67. doi: 10.1515/revneuro.2005.16.1.57. [DOI] [PubMed] [Google Scholar]
- Brady NR, Hamacher-Brady A, Westerhoff HV, Gottlieb RA. A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxid Redox Signal. 2006;8:1651–1665. doi: 10.1089/ars.2006.8.1651. [DOI] [PubMed] [Google Scholar]
- Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, et al. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichese L, Cazettes G, Valette A. JNK is associated with Bcl-2 and PP1 in mitochondria: paclitaxel induces its activation and its association with the phosphorylated form of Bcl-2. Cell Cycle. 2004;3:1312–1319. doi: 10.4161/cc.3.10.1166. [DOI] [PubMed] [Google Scholar]
- Broadhead MW, Kharbanda RK, Peters MJ, MacAllister RJ. KATP channel activation induces ischemic preconditioning of the endothelium in humans in vivo. Circulation. 2004;110:2077–2082. doi: 10.1161/01.CIR.0000144304.91010.F0. [DOI] [PubMed] [Google Scholar]
- Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschbeck M, Eickhoff J, Sommer MN, Ullrich A. Phosphotyrosine-specific phosphatase PTP-SL regulates the ERK5 signaling pathway. J Biol Chem. 2002;277:29503–29509. doi: 10.1074/jbc.M202149200. [DOI] [PubMed] [Google Scholar]
- Cameron SJ, Itoh S, Baines CP, Zhang C, Ohta S, Che W, et al. Activation of big MAP kinase 1 (BMK1/ERK5) inhibits cardiac injury after myocardial ischemia and reperfusion. FEBS Lett. 2004;566:255–260. doi: 10.1016/j.febslet.2004.03.120. [DOI] [PubMed] [Google Scholar]
- Capano M, Crompton M. Bax translocates to mitochondria of heart cells during simulated ischaemia: involvement of AMP-activated and p38 mitogen-activated protein kinases. Biochem J. 2006;395:57–64. doi: 10.1042/BJ20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JW, Cherry L, Laughlin JD, Figuera-Losada M, Lograsso PV. Selective inhibition of mitochondrial JNK signaling achieved using peptide mimicry of the Sab kinase interacting motif-1 (KIM1) ACS Chem Biol. 2011;6:808–818. doi: 10.1021/cb200062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JW, LoGrasso PV. Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. Journal of Biological Chemistry. 2011;286:16052–16062. doi: 10.1074/jbc.M111.223602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JW, Pachori A, Howard S, Iqbal S, LoGrasso PV. Inhibition of JNK mitochondrial localization and signaling is protective against ischemia/reperfusion injury in rats. Journal of Biological Chemistry. 2013;288:4000–4011. doi: 10.1074/jbc.M112.406777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, et al. JNK-dependent release of mitochondrial protein, Smac, during apoptosis in multiple myeloma (MM) cells. J Biol Chem. 2003;278:17593–17596. doi: 10.1074/jbc.C300076200. [DOI] [PubMed] [Google Scholar]
- Chen C, Sytkowski AJ. Apoptosis-linked gene-2 connects the Raf-1 and ASK1 signalings. Biochem Biophys Res Commun. 2005;333:51–57. doi: 10.1016/j.bbrc.2005.05.074. [DOI] [PubMed] [Google Scholar]
- Chen J, Fujii K, Zhang L, Roberts T, Fu H. Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism. Proc Natl Acad Sci U S A. 2001;98:7783–7788. doi: 10.1073/pnas.141224398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Kuo WW, Yang JJ, Wang SG, Yeh YL, Tsai FJ, et al. Eccentric cardiac hypertrophy was induced by long-term intermittent hypoxia in rats. Exp Physiol. 2007;92:409–416. doi: 10.1113/expphysiol.2006.036590. [DOI] [PubMed] [Google Scholar]
- Chen Y, Rajashree R, Liu Q, Hofmann P. Acute p38 MAPK activation decreases force development in ventricular myocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2578–2586. doi: 10.1152/ajpheart.00365.2003. [DOI] [PubMed] [Google Scholar]
- Cheng H, Kari G, Dicker AP, Rodeck U, Koch WJ, Force T. A novel preclinical strategy for identifying cardiotoxic kinase inhibitors and mechanisms of cardiotoxicity. Circ Res. 2011;109:1401–1409. doi: 10.1161/CIRCRESAHA.111.255695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien KR. Stress pathways and heart failure. Cell. 1999;98:555–558. doi: 10.1016/s0092-8674(00)80043-4. [DOI] [PubMed] [Google Scholar]
- Choukroun G, Hajjar R, Fry S, del Monte F, Haq S, Guerrero JL, et al. Regulation of cardiac hypertrophy in vivo by the stress-activated protein kinases/c-Jun NH(2)-terminal kinases. J Clin Invest. 1999;104:391–398. doi: 10.1172/JCI6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choukroun G, Hajjar R, Kyriakis JM, Bonventre JV, Rosenzweig A, Force T. Role of the stress-activated protein kinases in endothelin-induced cardiomyocyte hypertrophy. J Clin Invest. 1998;102:1311–1320. doi: 10.1172/JCI3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CW, Rincon M, Cavanagh J, Dickens M, Davis RJ. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science. 1997;278:1638–1641. doi: 10.1126/science.278.5343.1638. [DOI] [PubMed] [Google Scholar]
- Chu CT, Levinthal DJ, Kulich SM, Chalovich EM, DeFranco DB. Oxidative neuronal injury. The dark side of ERK1/2. Eur J Biochem. 2004;271:2060–2066. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicconi S, Ventura N, Pastore D, Bonini P, Di Nardo P, Lauro R, et al. Characterization of apoptosis signal transduction pathways in HL-5 cardiomyocytes exposed to ischemia/reperfusion oxidative stress model. J Cell Physiol. 2003;195:27–37. doi: 10.1002/jcp.10219. [DOI] [PubMed] [Google Scholar]
- Clark JE, Sarafraz N, Marber MS. Potential of p38-MAPK inhibitors in the treatment of ischaemic heart disease. Pharmacol Ther. 2007;116:192–206. doi: 10.1016/j.pharmthera.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Clerk A, Sugden PH. The p38-MAPK inhibitor, SB203580, inhibits cardiac stress-activated protein kinases/c-Jun N-terminal kinases (SAPKs/JNKs) FEBS Lett. 1998;426:93–96. doi: 10.1016/s0014-5793(98)00324-x. [DOI] [PubMed] [Google Scholar]
- Clerk A, Fuller SJ, Michael A, Sugden PH. Stimulation of “stress-regulated” mitogen-activated protein kinases (stress-activated protein kinases/c-Jun N-terminal kinases and p38-mitogen-activated protein kinases) in perfused rat hearts by oxidative and other stresses. J Biol Chem. 1998;273:7228–7234. doi: 10.1074/jbc.273.13.7228. [DOI] [PubMed] [Google Scholar]
- Cross HR, Li M, Petrich BG, Murphy E, Wang Y, Steenbergen C. Effect of p38 MAP kinases on contractility and ischemic injury in intact heart. Acta Physiol Hung. 2009;96:307–323. doi: 10.1556/APhysiol.96.2009.3.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva R, Grampp T, Pasch T, Schaub MC, Zaugg M. Differential activation of mitogen-activated protein kinases in ischemic and anesthetic preconditioning. Anesthesiology. 2004;100:59–69. doi: 10.1097/00000542-200401000-00013. [DOI] [PubMed] [Google Scholar]
- Dagda RK, Zhu J, Chu CT. Mitochondrial kinases in Parkinson’s disease: converging insights from neurotoxin and genetic models. Mitochondrion. 2009;9:289–298. doi: 10.1016/j.mito.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Zhu J, Kulich SM, Chu CT. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: implications for Parkinson’s disease. Autophagy. 2008;4:770–782. doi: 10.4161/auto.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem. 2008;283:29572–29585. doi: 10.1074/jbc.M801547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson SM, Morange M. Hsp25 and the p38 MAPK pathway are involved in differentiation of cardiomyocytes. Dev Biol. 2000;218:146–160. doi: 10.1006/dbio.1999.9596. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Degousee N, Martindale J, Stefanski E, Cieslak M, Lindsay TF, Fish JE, et al. MAP kinase kinase 6-p38 MAP kinase signaling cascade regulates cyclooxygenase-2 expression in cardiac myocytes in vitro and in vivo. Circ Res. 2003;92:757–764. doi: 10.1161/01.RES.0000067929.01404.03. [DOI] [PubMed] [Google Scholar]
- Deng Y, Yang J, McCarty M, Su B. MEKK3 is required for endothelium function but is not essential for tumor growth and angiogenesis. Am J Physiol Cell Physiol. 2007;293:C1404–1411. doi: 10.1152/ajpcell.00058.2007. [DOI] [PubMed] [Google Scholar]
- Deng X, Ruvolo P, Carr B, May WS., Jr Survival function of ERK1/2 as IL-3-activated, staurosporine-resistant Bcl2 kinases. Proc Natl Acad Sci U S A. 2000;97:1578–1583. doi: 10.1073/pnas.97.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denise Martin E, De Nicola GF, Marber MS. New therapeutic targets in cardiology: p38 alpha mitogen-activated protein kinase for ischemic heart disease. Circulation. 2012;126:357–368. doi: 10.1161/CIRCULATIONAHA.111.071886. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP. Scaffold proteins of MAP-kinase modules. Oncogene. 2007;26:3185–3202. doi: 10.1038/sj.onc.1210411. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra S, Sharma AK, Singla DK, Singal PK. p38 and ERK1/2 MAPKs mediate the interplay of TNF-alpha and IL-10 in regulating oxidative stress and cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2007;293:H3524–3531. doi: 10.1152/ajpheart.00919.2007. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res. 2006;70:191–199. doi: 10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Doczi R, Okresz L, Romero AE, Paccanaro A, Bogre L. Exploring the evolutionary path of plant MAPK networks. Trends Plant Sci. 2012;17:518–525. doi: 10.1016/j.tplants.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, et al. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW., 2nd Novel pharmacotherapies to abrogate postinfarction ventricular remodeling. Nat Rev Cardiol. 2009;6:283–291. doi: 10.1038/nrcardio.2009.12. [DOI] [PubMed] [Google Scholar]
- Dougherty CJ, Kubasiak LA, Frazier DP, Li H, Xiong WC, Bishopric NH, et al. Mitochondrial signals initiate the activation of c-Jun N-terminal kinase (JNK) by hypoxia-reoxygenation. The FASEB journal. 2004;18:1060–1070. doi: 10.1096/fj.04-1505com. [DOI] [PubMed] [Google Scholar]
- Dougherty CJ, Kubasiak LA, Prentice H, Andreka P, Bishopric NH, Webster KA. Activation of c-Jun N-terminal kinase promotes survival of cardiac myocytes after oxidative stress. Biochem J. 2002;362:561–571. doi: 10.1042/0264-6021:3620561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont E, Matsushita T, Kaba RA, Vozzi C, Coppen SR, Khan N, et al. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol. 2001;33:359–371. doi: 10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- Eisenberg LM, Eisenberg CA. Wnt signal transduction and the formation of the myocardium. Dev Biol. 2006;293:305–315. doi: 10.1016/j.ydbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Emerling BM, Platanias LC, Black E, Nebreda AR, Davis RJ, Chandel NS. Mitochondrial reactive oxygen species activation of p38 mitogen-activated protein kinase is required for hypoxia signaling. Mol Cell Biol. 2005;25:4853–4862. doi: 10.1128/MCB.25.12.4853-4862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eminel S, Klettner A, Roemer L, Herdegen T, Waetzig V. JNK2 translocates to the mitochondria and mediates cytochrome c release in PC12 cells in response to 6-hydroxydopamine. Journal of Biological Chemistry. 2004;279:55385–55392. doi: 10.1074/jbc.M405858200. [DOI] [PubMed] [Google Scholar]
- Engelbrecht AM, Niesler C, Page C, Lochner A. p38 and JNK have distinct regulatory functions on the development of apoptosis during simulated ischaemia and reperfusion in neonatal cardiomyocytes. Basic Res Cardiol. 2004;99:338–350. doi: 10.1007/s00395-004-0478-3. [DOI] [PubMed] [Google Scholar]
- English JM, Vanderbilt CA, Xu S, Marcus S, Cobb MH. Isolation of MEK5 and differential expression of alternatively spliced forms. J Biol Chem. 1995;270:28897–28902. doi: 10.1074/jbc.270.48.28897. [DOI] [PubMed] [Google Scholar]
- Erhardt P, Schremser EJ, Cooper GM. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol Cell Biol. 1999;19:5308–5315. doi: 10.1128/mcb.19.8.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M, Leppa S. Mitogen-activated protein kinases and activator protein 1 are required for proliferation and cardiomyocyte differentiation of P19 embryonal carcinoma cells. J Biol Chem. 2002;277:15992–16001. doi: 10.1074/jbc.M107340200. [DOI] [PubMed] [Google Scholar]
- Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res. 2005;66:22–32. doi: 10.1016/j.cardiores.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Feijoo C, Campbell DG, Jakes R, Goedert M, Cuenda A. Evidence that phosphorylation of the microtubule-associated protein Tau by SAPK4/p38delta at Thr50 promotes microtubule assembly. J Cell Sci. 2005;118:397–408. doi: 10.1242/jcs.01655. [DOI] [PubMed] [Google Scholar]
- Ferrandi C, Ballerio R, Gaillard P, Giachetti C, Carboni S, Vitte PA, et al. Inhibition of c-Jun N-terminal kinase decreases cardiomyocyte apoptosis and infarct size after myocardial ischemia and reperfusion in anaesthetized rats. Br J Pharmacol. 2004;142:953–960. doi: 10.1038/sj.bjp.0705873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R, Vitorino R, Padrao AI, Espadas G, Mancuso FM, Moreira-Goncalves D, et al. Lifelong exercise training modulates cardiac mitochondrial phosphoproteome in rats. J Proteome Res. 2014 doi: 10.1021/pr4011926. [DOI] [PubMed] [Google Scholar]
- Ferrell JE., Jr Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem Sci. 1996;21:460–466. doi: 10.1016/s0968-0004(96)20026-x. [DOI] [PubMed] [Google Scholar]
- Fischer TA, Ludwig S, Flory E, Gambaryan S, Singh K, Finn P, et al. Activation of cardiac c-Jun NH(2)-terminal kinases and p38-mitogen-activated protein kinases with abrupt changes in hemodynamic load. Hypertension. 2001;37:1222–1228. doi: 10.1161/01.hyp.37.5.1222. [DOI] [PubMed] [Google Scholar]
- Flesch M, Margulies KB, Mochmann HC, Engel D, Sivasubramanian N, Mann DL. Differential regulation of mitogen-activated protein kinases in the failing human heart in response to mechanical unloading. Circulation. 2001;104:2273–2276. doi: 10.1161/hc4401.099449. [DOI] [PubMed] [Google Scholar]
- Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- Fryer RM, Patel HH, Hsu AK, Gross GJ. Stress-activated protein kinase phosphorylation during cardioprotection in the ischemic myocardium. Am J Physiol Heart Circ Physiol. 2001;281:H1184–1192. doi: 10.1152/ajpheart.2001.281.3.H1184. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Meriin AB, Yaglom JA, Wei JY, Mosser DD, Sherman MY. Suppression of stress kinase JNK is involved in HSP72-mediated protection of myogenic cells from transient energy deprivation. HSP72 alleviates the stewss-induced inhibition of JNK dephosphorylation. J Biol Chem. 2000;275:38088–38094. doi: 10.1074/jbc.M006632200. [DOI] [PubMed] [Google Scholar]
- Galli S, Antico Arciuch VG, Poderoso C, Converso DP, Zhou Q, Bal de Kier Joffe E, et al. Tumor cell phenotype is sustained by selective MAPK oxidation in mitochondria. PLoS One. 2008;3:e2379. doi: 10.1371/journal.pone.0002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Hoz C, Sanchez-Fernandez G, Garcia-Escudero R, Fernandez-Velasco M, Palacios-Garcia J, Ruiz-Meana M, et al. Protein kinase C (PKC)zeta-mediated Galphaq stimulation of ERK5 protein pathway in cardiomyocytes and cardiac fibroblasts. J Biol Chem. 2012;287:7792–7802. doi: 10.1074/jbc.M111.282210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge B, Gram H, Di Padova F, Huang B, New L, Ulevitch RJ, et al. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- Gerits N, Kostenko S, Moens U. In vivo functions of mitogen-activated protein kinases: conclusions from knock-in and knock-out mice. Transgenic Res. 2007;16:281–314. doi: 10.1007/s11248-006-9052-0. [DOI] [PubMed] [Google Scholar]
- Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grethe S, Ares MP, Andersson T, Porn-Ares MI. p38 MAPK mediates TNF-induced apoptosis in endothelial cells via phosphorylation and downregulation of Bcl-x(L) Exp Cell Res. 2004;298:632–642. doi: 10.1016/j.yexcr.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Grethe S, Porn-Ares MI. p38 MAPK regulates phosphorylation of Bad via PP2A-dependent suppression of the MEK1/2-ERK1/2 survival pathway in TNF-alpha induced endothelial apoptosis. Cell Signal. 2006;18:531–540. doi: 10.1016/j.cellsig.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110(Pt 3):357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- Gustin MC, Albertyn J, Alexander M, Davenport K. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62:1264–1300. doi: 10.1128/mmbr.62.4.1264-1300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- Hall-Jackson CA, Goedert M, Hedge P, Cohen P. Effect of SB 203580 on the activity of c-Raf in vitro and in vivo. Oncogene. 1999;18:2047–2054. doi: 10.1038/sj.onc.1202603. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänninen SL, Ronkainen JJ, Leskinen H, Tavi P. Mitochondrial uncoupling downregulates calsequestrin expression and reduces SR Ca2 stores in cardiomyocytes. Cardiovasc Res. 2010;88:75–82. doi: 10.1093/cvr/cvq180. [DOI] [PubMed] [Google Scholar]
- Harris IS, Zhang S, Treskov I, Kovacs A, Weinheimer C, Muslin AJ. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110:718–723. doi: 10.1161/01.CIR.0000138190.50127.6A. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Lim SY, Ong SG, Davidson SM, Yellon DM. Mitochondrial cyclophilin-D as a critical mediator of ischaemic preconditioning. Cardiovasc Res. 2010;88:67–74. doi: 10.1093/cvr/cvq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Kim SW, Imanaka-Yoshida K, Yoshida T, Abel ED, Eliceiri B, et al. Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failure. J Clin Invest. 2004;113:1138–1148. doi: 10.1172/JCI19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Lee JD. Role of the BMK1/ERK5 signaling pathway: lessons from knockout mice. J Mol Med (Berl) 2004;82:800–808. doi: 10.1007/s00109-004-0602-8. [DOI] [PubMed] [Google Scholar]
- Hetman M, Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur J Biochem. 2004;271:2050–2055. doi: 10.1111/j.1432-1033.2004.04133.x. [DOI] [PubMed] [Google Scholar]
- Hide EJ, Thiemermann C. Limitation of myocardial infarct size in the rabbit by ischaemic preconditioning is abolished by sodium 5-hydroxydecanoate. Cardiovasc Res. 1996;31:941–946. [PubMed] [Google Scholar]
- Hilfiker-Kleiner D, Hilfiker A, Kaminski K, Schaefer A, Park JK, Michel K, et al. Lack of JunD promotes pressure overload-induced apoptosis, hypertrophic growth, and angiogenesis in the heart. Circulation. 2005;112:1470–1477. doi: 10.1161/CIRCULATIONAHA.104.518472. [DOI] [PubMed] [Google Scholar]
- Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J, Jr, et al. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97:189–198. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- Ho PD, Fan JS, Hayes NL, Saada N, Palade PT, Glembotski CC, et al. Ras reduces L-type calcium channel current in cardiac myocytes. Corrective effects of L-channels and SERCA2 on [Ca(2+)](i) regulation and cell morphology. Circ Res. 2001;88:63–69. doi: 10.1161/01.res.88.1.63. [DOI] [PubMed] [Google Scholar]
- Ho PD, Zechner DK, He H, Dillmann WH, Glembotski CC, McDonough PM. The Raf-MEK-ERK cascade represents a common pathway for alteration of intracellular calcium by Ras and protein kinase C in cardiac myocytes. J Biol Chem. 1998;273:21730–21735. doi: 10.1074/jbc.273.34.21730. [DOI] [PubMed] [Google Scholar]
- Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, et al. Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation. 2004;110:3544–3552. doi: 10.1161/01.CIR.0000148825.99184.50. [DOI] [PubMed] [Google Scholar]
- Hom J, Sheu SS. Morphological dynamics of mitochondria--a special emphasis on cardiac muscle cells. J Mol Cell Cardiol. 2009;46:811–820. doi: 10.1016/j.yjmcc.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover HE, Thuerauf DJ, Martindale JJ, Glembotski CC. alpha B-crystallin gene induction and phosphorylation by MKK6-activated p38. A potential role for alpha B-crystallin as a target of the p38 branch of the cardiac stress response. J Biol Chem. 2000;275:23825–23833. doi: 10.1074/jbc.M003864200. [DOI] [PubMed] [Google Scholar]
- Hoshijima M, Chien KR. Mixed signals in heart failure: cancer rules. J Clin Invest. 2002;109:849–855. doi: 10.1172/JCI15380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hreniuk D, Garay M, Gaarde W, Monia BP, McKay RA, Cioffi CL. Inhibition of c-Jun N-terminal kinase 1, but not c-Jun N-terminal kinase 2, suppresses apoptosis induced by ischemia/reoxygenation in rat cardiac myocytes. Mol Pharmacol. 2001;59:867–874. doi: 10.1124/mol.59.4.867. [DOI] [PubMed] [Google Scholar]
- Hunter JJ, Tanaka N, Rockman HA, Ross J, Jr, Chien KR. Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J Biol Chem. 1995;270:23173–23178. doi: 10.1074/jbc.270.39.23173. [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Aihara K, Sato T, Akaike M, Yoshizumi M, Suzaki Y, et al. Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis. J Biol Chem. 2005;280:29661–29666. doi: 10.1074/jbc.M411694200. [DOI] [PubMed] [Google Scholar]
- Isobe I, Maeno Y, Nagao M, Iwasa M, Koyama H, Seko-Nakamura Y, et al. Cytoplasmic vacuolation in cultured rat astrocytes induced by an organophosphorus agent requires extracellular signal-regulated kinase activation. Toxicol Appl Pharmacol. 2003;193:383–392. doi: 10.1016/j.taap.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Ito Y, Mishra NC, Yoshida K, Kharbanda S, Saxena S, Kufe D. Mitochondrial targeting of JNK/SAPK in the phorbol ester response of myeloid leukemia cells. Cell Death Differ. 2001;8:794–800. doi: 10.1038/sj.cdd.4400886. [DOI] [PubMed] [Google Scholar]
- Izumiya Y, Kim S, Izumi Y, Yoshida K, Yoshiyama M, Matsuzawa A, et al. Apoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II-induced cardiac hypertrophy and remodeling. Circ Res. 2003;93:874–883. doi: 10.1161/01.RES.0000100665.67510.F5. [DOI] [PubMed] [Google Scholar]
- Jaswal JS, Gandhi M, Finegan BA, Dyck JR, Clanachan AS. p38 mitogen-activated protein kinase mediates adenosine-induced alterations in myocardial glucose utilization via 5′-AMP-activated protein kinase. Am J Physiol Heart Circ Physiol. 2007;292:H1978–1985. doi: 10.1152/ajpheart.01121.2006. [DOI] [PubMed] [Google Scholar]
- Javadov S, Baetz D, Rajapurohitam V, Zeidan A, Kirshenbaum LA, Karmazyn M. Antihypertrophic effect of Na+/H+ exchanger isoform 1 inhibition is mediated by reduced mitogen-activated protein kinase activation secondary to improved mitochondrial integrity and decreased generation of mitochondrial-derived reactive oxygen species. J Pharmacol Exp Ther. 2006;317:1036–1043. doi: 10.1124/jpet.105.100107. [DOI] [PubMed] [Google Scholar]
- Javadov S, Karmazyn M, Escobales N. Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. Journal of Pharmacology and Experimental Therapeutics. 2009;330:670–678. doi: 10.1124/jpet.109.153213. [DOI] [PubMed] [Google Scholar]
- Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Mao XO, Zhu Y, Greenberg DA. MEK and ERK protect hypoxic cortical neurons via phosphorylation of Bad. J Neurochem. 2002;80:119–125. doi: 10.1046/j.0022-3042.2001.00678.x. [DOI] [PubMed] [Google Scholar]
- Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Kacimi R, Gerdes AM. Alterations in G protein and MAP kinase signaling pathways during cardiac remodeling in hypertension and heart failure. Hypertension. 2003;41:968–977. doi: 10.1161/01.HYP.0000062465.60601.CC. [DOI] [PubMed] [Google Scholar]
- Kai H, Muraishi A, Sugiu Y, Nishi H, Seki Y, Kuwahara F, et al. Expression of proto-oncogenes and gene mutation of sarcomeric proteins in patients with hypertrophic cardiomyopathy. Circ Res. 1998;83:594–601. doi: 10.1161/01.res.83.6.594. [DOI] [PubMed] [Google Scholar]
- Kaiser RA, Bueno OF, Lips DJ, Doevendans PA, Jones F, Kimball TF, et al. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. J Biol Chem. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
- Kaiser RA, Liang Q, Bueno O, Huang Y, Lackey T, Klevitsky R, et al. Genetic inhibition or activation of JNK1/2 protects the myocardium from ischemia-reperfusion-induced cell death in vivo. J Biol Chem. 2005;280:32602–32608. doi: 10.1074/jbc.M500684200. [DOI] [PubMed] [Google Scholar]
- Kamakura S, Moriguchi T, Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J Biol Chem. 1999;274:26563–26571. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- Kang BP, Urbonas A, Baddoo A, Baskin S, Malhotra A, Meggs LG. IGF-1 inhibits the mitochondrial apoptosis program in mesangial cells exposed to high glucose. Am J Physiol Renal Physiol. 2003;285:F1013–1024. doi: 10.1152/ajprenal.00209.2003. [DOI] [PubMed] [Google Scholar]
- Kang S, Chemaly ER, Hajjar RJ, Lebeche D. Resistin promotes cardiac hypertrophy via the AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) and c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathways. J Biol Chem. 2011;286:18465–18473. doi: 10.1074/jbc.M110.200022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasler HG, Victoria J, Duramad O, Winoto A. ERK5 is a novel type of mitogen-activated protein kinase containing a transcriptional activation domain. Mol Cell Biol. 2000;20:8382–8389. doi: 10.1128/mcb.20.22.8382-8389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Kravchenko VV, Tapping RI, Han J, Ulevitch RJ, Lee JD. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997;16:7054–7066. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, et al. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- Kim JK, Pedram A, Razandi M, Levin ER. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem. 2006;281:6760–6767. doi: 10.1074/jbc.M511024200. [DOI] [PubMed] [Google Scholar]
- Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, et al. Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci U S A. 2008;105:14555–14560. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, et al. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005;45:438–444. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, et al. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension. 2005;45:860–866. doi: 10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]
- Klein G, Schaefer A, Hilfiker-Kleiner D, Oppermann D, Shukla P, Quint A, et al. Increased collagen deposition and diastolic dysfunction but preserved myocardial hypertrophy after pressure overload in mice lacking PKCepsilon. Circ Res. 2005;96:748–755. doi: 10.1161/01.RES.0000161999.86198.1e. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Lackey T, Huang Y, Bisping E, Pu WT, Boxer LM, et al. Transcription factor gata4 regulates cardiac BCL2 gene expression in vitro and in vivo. FASEB J. 2006;20:800–802. doi: 10.1096/fj.05-5426fje. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Okumura N, Nakamoto T, Okada M, Hirai H, Nagai K. Activation of pp60c-src depending on cell density in PC12h cells. J Biol Chem. 1997;272:16262–16267. doi: 10.1074/jbc.272.26.16262. [DOI] [PubMed] [Google Scholar]
- Koivisto E, Kaikkonen L, Tokola H, Pikkarainen S, Aro J, Pennanen H, et al. Distinct regulation of B-type natriuretic peptide transcription by p38 MAPK isoforms. Mol Cell Endocrinol. 2011;338:18–27. doi: 10.1016/j.mce.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Kong JY, Klassen SS, Rabkin SW. Ceramide activates a mitochondrial p38 mitogen-activated protein kinase: a potential mechanism for loss of mitochondrial transmembrane potential and apoptosis. Molecular and cellular biochemistry. 2005;278:39–51. doi: 10.1007/s11010-005-1979-6. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Subramanian V, Singh M, Singh K. Beta1 integrins modulate beta-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Hypertension. 2007;49:865–872. doi: 10.1161/01.HYP.0000258703.36986.13. [DOI] [PubMed] [Google Scholar]
- Kudoh S, Komuro I, Mizuno T, Yamazaki T, Zou Y, Shiojima I, et al. Angiotensin II stimulates c-Jun NH2-terminal kinase in cultured cardiac myocytes of neonatal rats. Circ Res. 1997;80:139–146. doi: 10.1161/01.res.80.1.139. [DOI] [PubMed] [Google Scholar]
- Kulich SM, Horbinski C, Patel M, Chu CT. 6-Hydroxydopamine induces mitochondrial ERK activation. Free Radic Biol Med. 2007;43:372–383. doi: 10.1016/j.freeradbiomed.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulisz A, Chen N, Chandel NS, Shao Z, Schumacker PT. Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2002;282:L1324–L1329. doi: 10.1152/ajplung.00326.2001. [DOI] [PubMed] [Google Scholar]
- Kuma Y, Sabio G, Bain J, Shpiro N, Marquez R, Cuenda A. BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J Biol Chem. 2005;280:19472–19479. doi: 10.1074/jbc.M414221200. [DOI] [PubMed] [Google Scholar]
- Kumphune S, Bassi R, Jacquet S, Sicard P, Clark JE, Verma S, et al. A chemical genetic approach reveals that p38alpha MAPK activation by diphosphorylation aggravates myocardial infarction and is prevented by the direct binding of SB203580. J Biol Chem. 2010;285:2968–2975. doi: 10.1074/jbc.M109.079228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SH, Pimentel DR, Remondino A, Sawyer DB, Colucci WS. H(2)O(2) regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J Mol Cell Cardiol. 2003;35:615–621. doi: 10.1016/s0022-2828(03)00084-1. [DOI] [PubMed] [Google Scholar]
- Laderoute KR, Webster KA. Hypoxia/reoxygenation stimulates Jun kinase activity through redox signaling in cardiac myocytes. Circ Res. 1997;80:336–344. doi: 10.1161/01.res.80.3.336. [DOI] [PubMed] [Google Scholar]
- Lali FV, Hunt AE, Turner SJ, Foxwell BM. The pyridinyl imidazole inhibitor SB203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J Biol Chem. 2000;275:7395–7402. doi: 10.1074/jbc.275.10.7395. [DOI] [PubMed] [Google Scholar]
- Le NT, Takei Y, Shishido T, Woo CH, Chang E, Heo KS, et al. p90RSK targets the ERK5-CHIP ubiquitin E3 ligase activity in diabetic hearts and promotes cardiac apoptosis and dysfunction. Circ Res. 2012;110:536–550. doi: 10.1161/CIRCRESAHA.111.254730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Bach JH, Chae HS, Lee SH, Joo WS, Choi SH, et al. Mitogen-activated protein kinase/extracellular signal-regulated kinase attenuates 3-hydroxykynurenine-induced neuronal cell death. J Neurochem. 2004;88:647–656. doi: 10.1111/j.1471-4159.2004.02191.x. [DOI] [PubMed] [Google Scholar]
- Lee KS, Park JH, Lim HJ, Park HY. HB-EGF induces cardiomyocyte hypertrophy via an ERK5-MEF2A-COX2 signaling pathway. Cell Signal. 2011;23:1100–1109. doi: 10.1016/j.cellsig.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, et al. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol Cell Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnefsky EJ, Hoppel CL. Ischemia-reperfusion injury in the aged heart: role of mitochondria. Arch Biochem Biophys. 2003;420:287–297. doi: 10.1016/j.abb.2003.09.046. [DOI] [PubMed] [Google Scholar]
- Li G, Ali IS, Currie RW. Insulin-induced myocardial protection in isolated ischemic rat hearts requires p38 MAPK phosphorylation of Hsp27. Am J Physiol Heart Circ Physiol. 2008;294:H74–87. doi: 10.1152/ajpheart.00675.2007. [DOI] [PubMed] [Google Scholar]
- Li Q, Guo HC, Maslov LN, Qiao XW, Zhou JJ, Zhang Y. Mitochondrial permeability transition pore plays a role in the cardioprotection of CB2 receptor against ischemia-reperfusion injury. Can J Physiol Pharmacol. 2014;92:205–214. doi: 10.1139/cjpp-2013-0293. [DOI] [PubMed] [Google Scholar]
- Li Z, Ma JY, Kerr I, Chakravarty S, Dugar S, Schreiner G, et al. Selective inhibition of p38alpha MAPK improves cardiac function and reduces myocardial apoptosis in rat model of myocardial injury. Am J Physiol Heart Circ Physiol. 2006;291:H1972–1977. doi: 10.1152/ajpheart.00043.2006. [DOI] [PubMed] [Google Scholar]
- Liang Q, Bueno OF, Wilkins BJ, Kuan CY, Xia Y, Molkentin JD. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J. 2003;22:5079–5089. doi: 10.1093/emboj/cdg474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Molkentin JD. Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy between cultured myocytes and animal models. J Mol Cell Cardiol. 2003;35:1385–1394. doi: 10.1016/j.yjmcc.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Liang Q, Wiese RJ, Bueno OF, Dai YS, Markham BE, Molkentin JD. The transcription factor GATA4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol Cell Biol. 2001;21:7460–7469. doi: 10.1128/MCB.21.21.7460-7469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, et al. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci U S A. 2001;98:12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao P, Wang SQ, Wang S, Zheng M, Zhang SJ, Cheng H, et al. p38 Mitogen-activated protein kinase mediates a negative inotropic effect in cardiac myocytes. Circ Res. 2002;90:190–196. doi: 10.1161/hh0202.104220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HW, New L, Han J, Molkentin JD. Calcineurin enhances MAPK phosphatase-1 expression and p38 MAPK inactivation in cardiac myocytes. J Biol Chem. 2001;276:15913–15919. doi: 10.1074/jbc.M100452200. [DOI] [PubMed] [Google Scholar]
- Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–1941. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- Liu J, Mao W, Ding B, Liang CS. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295:H1956–1965. doi: 10.1152/ajpheart.00407.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Dillon AR, Tillson M, Makarewich C, Nguyen V, Dell’Italia L, et al. Volume overload induces differential spatiotemporal regulation of myocardial soluble guanylyl cyclase in eccentric hypertrophy and heart failure. J Mol Cell Cardiol. 2013;60:72–83. doi: 10.1016/j.yjmcc.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YH, Wang D, Rhaleb NE, Yang XP, Xu J, Sankey SS, et al. Inhibition of p38 mitogen-activated protein kinase protects the heart against cardiac remodeling in mice with heart failure resulting from myocardial infarction. J Card Fail. 2005;11:74–81. doi: 10.1016/j.cardfail.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Lorenz K, Schmitt JP, Schmitteckert EM, Lohse MJ. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med. 2009;15:75–83. doi: 10.1038/nm.1893. [DOI] [PubMed] [Google Scholar]
- Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, et al. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99:1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- Mackay K, Mochly-Rosen D. An inhibitor of p38 mitogen-activated protein kinase protects neonatal cardiac myocytes from ischemia. J Biol Chem. 1999;274:6272–6279. doi: 10.1074/jbc.274.10.6272. [DOI] [PubMed] [Google Scholar]
- MacLellan WR, Schneider MD. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol. 2000;62:289–319. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- Majewski M, Nieborowska-Skorska M, Salomoni P, Slupianek A, Reiss K, Trotta R, et al. Activation of mitochondrial Raf-1 is involved in the antiapoptotic effects of Akt. Cancer Res. 1999;59:2815–2819. [PubMed] [Google Scholar]
- Marber MS, Rose B, Wang Y. The p38 mitogen-activated protein kinase pathway--a potential target for intervention in infarction, hypertrophy, and heart failure. J Mol Cell Cardiol. 2011;51:485–490. doi: 10.1016/j.yjmcc.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, et al. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct. 2012;2012:329635. doi: 10.1155/2012/329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Avkiran M, Quinlan RA, Cohen P, Marber MS. Antiischemic effects of SB203580 are mediated through the inhibition of p38alpha mitogen-activated protein kinase: Evidence from ectopic expression of an inhibition-resistant kinase. Circ Res. 2001;89:750–752. doi: 10.1161/hh2101.099504. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Csiszar A, Dutta D, Balagopal G, Calvani R, Leeuwenburgh C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am J Physiol Heart Circ Physiol. 2013;305:H459–476. doi: 10.1152/ajpheart.00936.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale JJ, Wall JA, Martinez-Longoria DM, Aryal P, Rockman HA, Guo Y, et al. Overexpression of mitogen-activated protein kinase kinase 6 in the heart improves functional recovery from ischemia in vitro and protects against myocardial infarction in vivo. J Biol Chem. 2005;280:669–676. doi: 10.1074/jbc.M406690200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama D, Kawahara K. Oxidative stress-induced formation of a positive-feedback loop for the sustained activation of p38 MAPK leading to the loss of cell division in cardiomyocytes soon after birth. Basic Res Cardiol. 2011;106:815–828. doi: 10.1007/s00395-011-0178-8. [DOI] [PubMed] [Google Scholar]
- Maulik N, Yoshida T, Zu YL, Sato M, Banerjee A, Das DK. Ischemic preconditioning triggers tyrosine kinase signaling: a potential role for MAPKAP kinase 2. Am J Physiol. 1998;275:H1857–1864. doi: 10.1152/ajpheart.1998.275.5.H1857. [DOI] [PubMed] [Google Scholar]
- McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26:3113–3121. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- Mihl C, Dassen WR, Kuipers H. Cardiac remodelling: concentric versus eccentric hypertrophy in strength and endurance athletes. Neth Heart J. 2008;16:129–133. doi: 10.1007/BF03086131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano G, Morel S, Bonny C, Samaja M, von Segesser LK, Nicod P, et al. A peptide inhibitor of c-Jun NH2-terminal kinase reduces myocardial ischemia-reperfusion injury and infarct size in vivo. Am J Physiol Heart Circ Physiol. 2007;292:H1828–1835. doi: 10.1152/ajpheart.01117.2006. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Ota A, Foster W, Zhang B, Fang Z, Patel S, et al. Distinct gene expression profiles in adult mouse heart following targeted MAP kinase activation. Physiol Genomics. 2006;25:50–59. doi: 10.1152/physiolgenomics.00224.2005. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Takeishi Y, Takahashi H, Shishido T, Arimoto T, Tomoike H, et al. Activation of distinct signal transduction pathways in hypertrophied hearts by pressure and volume overload. Basic Res Cardiol. 2004;99:328–337. doi: 10.1007/s00395-004-0482-7. [DOI] [PubMed] [Google Scholar]
- Mocanu MM, Baxter GF, Yue Y, Critz SD, Yellon DM. The p38 MAPK inhibitor, SB203580, abrogates ischaemic preconditioning in rat heart but timing of administration is critical. Basic Res Cardiol. 2000;95:472–478. doi: 10.1007/s003950070023. [DOI] [PubMed] [Google Scholar]
- Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Dorn GW., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monick MM, Powers LS, Barrett CW, Hinde S, Ashare A, Groskreutz DJ, et al. Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. The Journal of Immunology. 2008;180:7485–7496. doi: 10.4049/jimmunol.180.11.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor AN, Fliegel L. Protein kinase-mediated regulation of the Na(+)/H(+) exchanger in the rat myocardium by mitogen-activated protein kinase-dependent pathways. J Biol Chem. 1999;274:22985–22992. doi: 10.1074/jbc.274.33.22985. [DOI] [PubMed] [Google Scholar]
- Muro C, Grigoriev SM, Pietkiewicz D, Kinnally KW, Campo ML. Comparison of the TIM and TOM channel activities of the mitochondrial protein import complexes. Biophys J. 2003;84:2981–2989. doi: 10.1016/S0006-3495(03)70024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadruz W, Jr, Corat MA, Marin TM, Guimaraes Pereira GA, Franchini KG. Focal adhesion kinase mediates MEF2 and c-Jun activation by stretch: role in the activation of the cardiac hypertrophic genetic program. Cardiovasc Res. 2005;68:87–97. doi: 10.1016/j.cardiores.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Nadruz W, Jr, Kobarg CB, Kobarg J, Franchini KG. c-Jun is regulated by combination of enhanced expression and phosphorylation in acute-overloaded rat heart. Am J Physiol Heart Circ Physiol. 2004;286:H760–767. doi: 10.1152/ajpheart.00430.2003. [DOI] [PubMed] [Google Scholar]
- Nagarkatti DS, Sha’afi RI. Role of p38 MAP kinase in myocardial stress. J Mol Cell Cardiol. 1998;30:1651–1664. doi: 10.1006/jmcc.1998.0733. [DOI] [PubMed] [Google Scholar]
- Naito AT, Tominaga A, Oyamada M, Oyamada Y, Shiraishi I, Monzen K, et al. Early stage-specific inhibitions of cardiomyocyte differentiation and expression of Csx/Nkx-2.5 and GATA-4 by phosphatidylinositol 3-kinase inhibitor LY294002. Exp Cell Res. 2003;291:56–69. doi: 10.1016/s0014-4827(03)00378-1. [DOI] [PubMed] [Google Scholar]
- Nakaoka Y, Nishida K, Fujio Y, Izumi M, Terai K, Oshima Y, et al. Activation of gp130 transduces hypertrophic signal through interaction of scaffolding/docking protein Gab1 with tyrosine phosphatase SHP2 in cardiomyocytes. Circ Res. 2003;93:221–229. doi: 10.1161/01.RES.0000085562.48906.4A. [DOI] [PubMed] [Google Scholar]
- Nantel A, Huber M, Thomas DY. Localization of endogenous Grb10 to the mitochondria and its interaction with the mitochondrial-associated Raf-1 pool. J Biol Chem. 1999;274:35719–35724. doi: 10.1074/jbc.274.50.35719. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Sheng Z, Lin A. Opposing effects of Jun kinase and p38 mitogen-activated protein kinases on cardiomyocyte hypertrophy. Mol Cell Biol. 1998;18:3518–3526. doi: 10.1128/mcb.18.6.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DC, Court NW, dos Remedios CG, Bogoyevitch MA. Activation of signal transducer and activator of transcription (STAT) pathways in failing human hearts. Cardiovasc Res. 2003;57:333–346. doi: 10.1016/s0008-6363(02)00664-8. [DOI] [PubMed] [Google Scholar]
- Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, Olson EN. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001;20:2757–2767. doi: 10.1093/emboj/20.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, et al. p38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak G. Protein kinase C-alpha and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na+ transport, and cisplatin-induced apoptosis in renal cells. J Biol Chem. 2002;277:43377–43388. doi: 10.1074/jbc.M206373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak G, Clifton GL, Godwin ML, Bakajsova D. Activation of ERK1/2 pathway mediates oxidant-induced decreases in mitochondrial function in renal cells. Am J Physiol Renal Physiol. 2006;291:F840–855. doi: 10.1152/ajprenal.00219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Otani H, Wu Y, Kyoi S, Enoki C, Fujiwara H, et al. Role of F-actin organization in p38 MAP kinase-mediated apoptosis and necrosis in neonatal rat cardiomyocytes subjected to simulated ischemia and reoxygenation. Am J Physiol Heart Circ Physiol. 2005;289:H2310–2318. doi: 10.1152/ajpheart.00462.2005. [DOI] [PubMed] [Google Scholar]
- Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- Pargellis C, Tong L, Churchill L, Cirillo PF, Gilmore T, Graham AG, et al. Inhibition of p38 MAP kinase by utilizing a novel allosteric binding site. Nat Struct Biol. 2002;9:268–272. doi: 10.1038/nsb770. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Petrich BG, Eloff BC, Lerner DL, Kovacs A, Saffitz JE, Rosenbaum DS, et al. Targeted activation of c-Jun N-terminal kinase in vivo induces restrictive cardiomyopathy and conduction defects. J Biol Chem. 2004;279:15330–15338. doi: 10.1074/jbc.M314142200. [DOI] [PubMed] [Google Scholar]
- Petrich BG, Gong X, Lerner DL, Wang X, Brown JH, Saffitz JE, et al. c-Jun N-terminal kinase activation mediates downregulation of connexin43 in cardiomyocytes. Circ Res. 2002;91:640–647. doi: 10.1161/01.res.0000035854.11082.01. [DOI] [PubMed] [Google Scholar]
- Pi X, Yan C, Berk BC. Big mitogen-activated protein kinase (BMK1)/ERK5 protects endothelial cells from apoptosis. Circ Res. 2004;94:362–369. doi: 10.1161/01.RES.0000112406.27800.6F. [DOI] [PubMed] [Google Scholar]
- Ping P, Murphy E. Role of p38 mitogen-activated protein kinases in preconditioning: a detrimental factor or a protective kinase? Circ Res. 2000;86:921–922. doi: 10.1161/01.res.86.9.921. [DOI] [PubMed] [Google Scholar]
- Ping P, Zhang J, Cao X, Li RC, Kong D, Tang XL, et al. PKC-dependent activation of p44/p42 MAPKs during myocardial ischemia-reperfusion in conscious rabbits. Am J Physiol. 1999;276:H1468–1481. doi: 10.1152/ajpheart.1999.276.5.H1468. [DOI] [PubMed] [Google Scholar]
- Porter CM, Havens MA, Clipstone NA. Identification of amino acid residues and protein kinases involved in the regulation of NFATc subcellular localization. J Biol Chem. 2000;275:3543–3551. doi: 10.1074/jbc.275.5.3543. [DOI] [PubMed] [Google Scholar]
- Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, et al. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A. 2007;104:14074–14079. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Ramirez MT, Sah VP, Zhao XL, Hunter JJ, Chien KR, Brown JH. The MEKK- JNK pathway is stimulated by alpha1-adrenergic receptor and ras activation and is associated with in vitro and in vivo cardiac hypertrophy. J Biol Chem. 1997;272:14057–14061. doi: 10.1074/jbc.272.22.14057. [DOI] [PubMed] [Google Scholar]
- Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol. 2008;40:2707–2719. doi: 10.1016/j.biocel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Ravingerova T, Barancik M, Strniskova M. Mitogen-activated protein kinases: a new therapeutic target in cardiac pathology. Mol Cell Biochem. 2003;247:127–138. doi: 10.1023/a:1024119224033. [DOI] [PubMed] [Google Scholar]
- Regan CP, Li W, Boucher DM, Spatz S, Su MS, Kuida K. Erk5 null mice display multiple extraembryonic vascular and embryonic cardiovascular defects. Proc Natl Acad Sci U S A. 2002;99:9248–9253. doi: 10.1073/pnas.142293999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid EA, Kristo G, Yoshimura Y, Ballard-Croft C, Keith BJ, Mentzer RM, et al. In vivo adenosine receptor preconditioning reduces myocardial infarct size via subcellular ERK signaling. American Journal of Physiology-Heart and Circulatory Physiology. 2005;288:H2253–H2259. doi: 10.1152/ajpheart.01009.2004. [DOI] [PubMed] [Google Scholar]
- Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, et al. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- Ren J, Zhang S, Kovacs A, Wang Y, Muslin AJ. Role of p38alpha MAPK in cardiac apoptosis and remodeling after myocardial infarction. J Mol Cell Cardiol. 2005;38:617–623. doi: 10.1016/j.yjmcc.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Ricci R, Eriksson U, Oudit GY, Eferl R, Akhmedov A, Sumara I, et al. Distinct functions of junD in cardiac hypertrophy and heart failure. Genes Dev. 2005;19:208–213. doi: 10.1101/gad.327005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol Rev. 2009;228:212–224. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- Roberts OL, Holmes K, Muller J, Cross DA, Cross MJ. ERK5 is required for VEGF-mediated survival and tubular morphogenesis of primary human microvascular endothelial cells. J Cell Sci. 2010;123:3189–3200. doi: 10.1242/jcs.072801. [DOI] [PubMed] [Google Scholar]
- Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiological Reviews. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumora L, Lovric J, Sairam MR, Maysinger D. Impairments of heat shock protein expression and MAPK translocation in the central nervous system of follitropin receptor knockout mice. Exp Gerontol. 2007;42:619–628. doi: 10.1016/j.exger.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Ruppert C, Deiss K, Herrmann S, Vidal M, Oezkur M, Gorski A, et al. Interference with ERK(Thr188) phosphorylation impairs pathological but not physiological cardiac hypertrophy. Proc Natl Acad Sci U S A. 2013;110:7440–7445. doi: 10.1073/pnas.1221999110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J, Montagne O, Wang Q, Yang G, Warden J, Liu J, et al. The MEKK1-JNK pathway plays a protective role in pressure overload but does not mediate cardiac hypertrophy. J Clin Invest. 2002;110:271–279. doi: 10.1172/JCI14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavati L, Monick MM, Sanlioglu S, Buettner GR, Oberley LW, Hunninghake GW. Mitochondrial KATP channel openers activate the ERK kinase by an oxidant-dependent mechanism. American Journal of Physiology-Cell Physiology. 2002;283:C273–C281. doi: 10.1152/ajpcell.00514.2001. [DOI] [PubMed] [Google Scholar]
- Sano Y, Harada J, Tashiro S, Gotoh-Mandeville R, Maekawa T, Ishii S. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-beta signaling. J Biol Chem. 1999;274:8949–8957. doi: 10.1074/jbc.274.13.8949. [DOI] [PubMed] [Google Scholar]
- Sato T, O’Rourke B, Marban E. Modulation of mitochondrial ATP-dependent K+ channels by protein kinase C. Circ Res. 1998;83:110–114. doi: 10.1161/01.res.83.1.110. [DOI] [PubMed] [Google Scholar]
- Saurin AT, Martin JL, Heads RJ, Foley C, Mockridge JW, Wright MJ, et al. The role of differential activation of p38-mitogen-activated protein kinase in preconditioned ventricular myocytes. FASEB J. 2000;14:2237–2246. doi: 10.1096/fj.99-0671com. [DOI] [PubMed] [Google Scholar]
- Schafer M, Schafer C, Ewald N, Piper HM, Noll T. Role of redox signaling in the autonomous proliferative response of endothelial cells to hypoxia. Circ Res. 2003;92:1010–1015. doi: 10.1161/01.RES.0000070882.81508.FC. [DOI] [PubMed] [Google Scholar]
- Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- Schwertz H, Carter JM, Abdudureheman M, Russ M, Buerke U, Schlitt A, et al. Myocardial ischemia/reperfusion causes VDAC phosphorylation which is reduced by cardioprotection with a p38 MAP kinase inhibitor. Proteomics. 2007;7:4579–4588. doi: 10.1002/pmic.200700734. [DOI] [PubMed] [Google Scholar]
- See F, Thomas W, Way K, Tzanidis A, Kompa A, Lewis D, et al. p38 mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J Am Coll Cardiol. 2004;44:1679–1689. doi: 10.1016/j.jacc.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Sharma V, Abraham T, So A, Allard MF, McNeill JH. Functional effects of protein kinases and peroxynitrite on cardiac carnitine palmitoyltransferase-1 in isolated mitochondria. Mol Cell Biochem. 2010;337:223–237. doi: 10.1007/s11010-009-0303-2. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Yoshiyama M, Omura T, Hanatani A, Kim S, Takeuchi K, et al. Activation of mitogen-activated protein kinases and activator protein-1 in myocardial infarction in rats. Cardiovasc Res. 1998;38:116–124. doi: 10.1016/s0008-6363(97)00327-1. [DOI] [PubMed] [Google Scholar]
- Shiroto K, Otani H, Yamamoto F, Huang CK, Maulik N, Das DK. MK2−/− gene knockout mouse hearts carry anti-apoptotic signal and are resistant to ischemia reperfusion injury. J Mol Cell Cardiol. 2005;38:93–97. doi: 10.1016/j.yjmcc.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Shishido T, Woo CH, Ding B, McClain C, Molina CA, Yan C, et al. Effects of MEK5/ERK5 association on small ubiquitin-related modification of ERK5: implications for diabetic ventricular dysfunction after myocardial infarction. Circ Res. 2008;102:1416–1425. doi: 10.1161/CIRCRESAHA.107.168138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu E, Matsuno H, Akamastu S, Kanno Y, Suga H, Nakajima K, et al. alphaB-crystallin is phosphorylated during myocardial infarction: involvement of platelet-derived growth factor-BB. Arch Biochem Biophys. 2005;438:111–118. doi: 10.1016/j.abb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Song ZF, Ji XP, Li XX, Wang SJ, Wang SH, Zhang Y. Inhibition of the activity of poly (ADP-ribose) polymerase reduces heart ischaemia/reperfusion injury via suppressing JNK-mediated AIF translocation. J Cell Mol Med. 2008;12:1220–1228. doi: 10.1111/j.1582-4934.2008.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio S, Poksay K, de Belle I, Lafuente MJ, Liu B, Nasir J, et al. Paraptosis: mediation by MAP kinases and inhibition by AIP-1/Alix. Cell Death Differ. 2004;11:1066–1075. doi: 10.1038/sj.cdd.4401465. [DOI] [PubMed] [Google Scholar]
- Steenbergen C. The role of p38 mitogen-activated protein kinase in myocardial ischemia/reperfusion injury; relationship to ischemic preconditioning. Basic Res Cardiol. 2002;97:276–285. doi: 10.1007/s00395-002-0364-9. [DOI] [PubMed] [Google Scholar]
- Sucher R, Gehwolf P, Kaier T, Hermann M, Maglione M, Oberhuber R, et al. Intracellular signaling pathways control mitochondrial events associated with the development of ischemia/reperfusion-associated damage. Transplant International. 2009;22:922–930. doi: 10.1111/j.1432-2277.2009.00883.x. [DOI] [PubMed] [Google Scholar]
- Sucher R, Gehwolf P, Kaier T, Hermann M, Maglione M, Oberhuber R, et al. Intracellular signaling pathways control mitochondrial events associated with the development of ischemia/reperfusion-associated damage. Transpl Int. 2009;22:922–930. doi: 10.1111/j.1432-2277.2009.00883.x. [DOI] [PubMed] [Google Scholar]
- Sugden PH, Clerk A. Cellular mechanisms of cardiac hypertrophy. J Mol Med (Berl) 1998;76:725–746. doi: 10.1007/s001090050275. [DOI] [PubMed] [Google Scholar]
- Sumida T, Otani H, Kyoi S, Okada T, Fujiwara H, Nakao Y, et al. Temporary blockade of contractility during reperfusion elicits a cardioprotective effect of the p38 MAP kinase inhibitor SB-203580. Am J Physiol Heart Circ Physiol. 2005;288:H2726–2734. doi: 10.1152/ajpheart.01183.2004. [DOI] [PubMed] [Google Scholar]
- Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- Tachibana H, Perrino C, Takaoka H, Davis RJ, Naga Prasad SV, Rockman HA. JNK1 is required to preserve cardiac function in the early response to pressure overload. Biochem Biophys Res Commun. 2006;343:1060–1066. doi: 10.1016/j.bbrc.2006.03.065. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Saito Y, Kuwahara K, Harada M, Tanimoto K, Nakagawa Y, et al. Hypertrophic responses to cardiotrophin-1 are not mediated by STAT3, but via a MEK5-ERK5 pathway in cultured cardiomyocytes. J Mol Cell Cardiol. 2005;38:185–192. doi: 10.1016/j.yjmcc.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Takeishi Y, Abe J, Lee JD, Kawakatsu H, Walsh RA, Berk BC. Differential regulation of p90 ribosomal S6 kinase and big mitogen-activated protein kinase 1 by ischemia/reperfusion and oxidative stress in perfused guinea pig hearts. Circ Res. 1999;85:1164–1172. doi: 10.1161/01.res.85.12.1164. [DOI] [PubMed] [Google Scholar]
- Takeishi Y, Huang Q, Abe J, Che W, Lee JD, Kawakatsu H, et al. Activation of mitogen-activated protein kinases and p90 ribosomal S6 kinase in failing human hearts with dilated cardiomyopathy. Cardiovasc Res. 2002;53:131–137. doi: 10.1016/s0008-6363(01)00438-2. [DOI] [PubMed] [Google Scholar]
- Takeishi Y, Huang Q, Abe J, Glassman M, Che W, Lee JD, et al. Src and multiple MAP kinase activation in cardiac hypertrophy and congestive heart failure under chronic pressure-overload: comparison with acute mechanical stretch. J Mol Cell Cardiol. 2001;33:1637–1648. doi: 10.1006/jmcc.2001.1427. [DOI] [PubMed] [Google Scholar]
- Tamura K, Sudo T, Senftleben U, Dadak AM, Johnson R, Karin M. Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell. 2000;102:221–231. doi: 10.1016/s0092-8674(00)00027-1. [DOI] [PubMed] [Google Scholar]
- Taniike M, Yamaguchi O, Tsujimoto I, Hikoso S, Takeda T, Nakai A, et al. Apoptosis signal-regulating kinase 1/p38 signaling pathway negatively regulates physiological hypertrophy. Circulation. 2008;117:545–552. doi: 10.1161/CIRCULATIONAHA.107.710434. [DOI] [PubMed] [Google Scholar]
- Teos LY, Zhao A, Alvin Z, Laurence GG, Li C, Haddad GE. Basal and IGF-I-dependent regulation of potassium channels by MAP kinases and PI3-kinase during eccentric cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2008;295:H1834–1845. doi: 10.1152/ajpheart.321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn J, McMahon M, Thorburn A. Raf-1 kinase activity is necessary and sufficient for gene expression changes but not sufficient for cellular morphology changes associated with cardiac myocyte hypertrophy. J Biol Chem. 1994;269:30580–30586. [PubMed] [Google Scholar]
- Ueyama T, Kawashima S, Sakoda T, Rikitake Y, Ishida T, Kawai M, et al. Requirement of activation of the extracellular signal-regulated kinase cascade in myocardial cell hypertrophy. J Mol Cell Cardiol. 2000;32:947–960. doi: 10.1006/jmcc.2000.1135. [DOI] [PubMed] [Google Scholar]
- Vahebi S, Ota A, Li M, Warren CM, de Tombe PP, Wang Y, et al. p38-MAPK induced dephosphorylation of alpha-tropomyosin is associated with depression of myocardial sarcomeric tension and ATPase activity. Circ Res. 2007;100:408–415. doi: 10.1161/01.RES.0000258116.60404.ad. [DOI] [PubMed] [Google Scholar]
- Wall JA, Wei J, Ly M, Belmont P, Martindale JJ, Tran D, et al. Alterations in oxidative phosphorylation complex proteins in the hearts of transgenic mice that overexpress the p38 MAP kinase activator, MAP kinase kinase 6. American Journal of Physiology-Heart and Circulatory Physiology. 2006;291:H2462–H2472. doi: 10.1152/ajpheart.01311.2005. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial DNA mutations in disease and aging. Environ Mol Mutagen. 2010;51:440–450. doi: 10.1002/em.20586. [DOI] [PubMed] [Google Scholar]
- Wang HG, Rapp UR, Reed JC. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- Wang X, Merritt AJ, Seyfried J, Guo C, Papadakis ES, Finegan KG, et al. Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol Cell Biol. 2005;25:336–345. doi: 10.1128/MCB.25.1.336-345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tournier C. Regulation of cellular functions by the ERK5 signalling pathway. Cell Signal. 2006;18:753–760. doi: 10.1016/j.cellsig.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang S, Sah VP, Ross J, Jr, Brown JH, Han J, et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- Wang Y, Su B, Sah VP, Brown JH, Han J, Chien KR. Cardiac hypertrophy induced by mitogen-activated protein kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells. J Biol Chem. 1998;273:5423–5426. doi: 10.1074/jbc.273.10.5423. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ma M, Hirabayashi K, Gurusamy N, Veeraveedu PT, Prakash P, et al. Swimming stress in DN 14-3-3 mice triggers maladaptive cardiac remodeling: role of p38 MAPK. Am J Physiol Heart Circ Physiol. 2007;292:H1269–1277. doi: 10.1152/ajpheart.00550.2006. [DOI] [PubMed] [Google Scholar]
- Weinbrenner C, Liu GS, Cohen MV, Downey JM. Phosphorylation of tyrosine 182 of p38 mitogen-activated protein kinase correlates with the protection of preconditioning in the rabbit heart. J Mol Cell Cardiol. 1997;29:2383–2391. doi: 10.1006/jmcc.1997.0473. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Whiteside ST, Goodbourn S. Signal transduction and nuclear targeting: regulation of transcription factor activity by subcellular localisation. J Cell Sci. 1993;104(Pt 4):949–955. doi: 10.1242/jcs.104.4.949. [DOI] [PubMed] [Google Scholar]
- Wiltshire C, Gillespie DA, May GH. Sab (SH3BP5), a novel mitochondria-localized JNK-interacting protein. Biochem Soc Trans. 2004;32:1075–1077. doi: 10.1042/BST0321075. [DOI] [PubMed] [Google Scholar]
- Wiltshire C, Matsushita M, Tsukada S, Gillespie DA, May GH. A new c-Jun N-terminal kinase (JNK)-interacting protein, Sab (SH3BP5), associates with mitochondria. Biochem J. 2002;367:577–585. doi: 10.1042/BJ20020553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. Journal of Biological Chemistry. 2011;286:35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert KC, Taga T, Saito M, Narazaki M, Kishimoto T, Glembotski CC, et al. Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy. Assembly of sarcomeric units in series VIA gp130/leukemia inhibitory factor receptor-dependent pathways. J Biol Chem. 1996;271:9535–9545. doi: 10.1074/jbc.271.16.9535. [DOI] [PubMed] [Google Scholar]
- Woo CH, Le NT, Shishido T, Chang E, Lee H, Heo KS, et al. Novel role of C terminus of Hsc70-interacting protein (CHIP) ubiquitin ligase on inhibiting cardiac apoptosis and dysfunction via regulating ERK5-mediated degradation of inducible cAMP early repressor. FASEB J. 2010;24:4917–4928. doi: 10.1096/fj.10-162636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CH, Shishido T, McClain C, Lim JH, Li JD, Yang J, et al. Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ Res. 2008;102:538–545. doi: 10.1161/CIRCRESAHA.107.156877. [DOI] [PubMed] [Google Scholar]
- Yamaguchi O, Watanabe T, Nishida K, Kashiwase K, Higuchi Y, Takeda T, et al. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J Clin Invest. 2004;114:937–943. doi: 10.1172/JCI20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Ding B, Shishido T, Woo CH, Itoh S, Jeon KI, et al. Activation of extracellular signal-regulated kinase 5 reduces cardiac apoptosis and dysfunction via inhibition of a phosphodiesterase 3A/inducible cAMP early repressor feedback loop. Circ Res. 2007;100:510–519. doi: 10.1161/01.RES.0000259045.49371.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Luo H, Lee JD, Abe J, Berk BC. Molecular cloning of mouse ERK5/BMK1 splice variants and characterization of ERK5 functional domains. J Biol Chem. 2001;276:10870–10878. doi: 10.1074/jbc.M009286200. [DOI] [PubMed] [Google Scholar]
- Yang SH, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- Yang TT, Xiong Q, Enslen H, Davis RJ, Chow CW. Phosphorylation of NFATc4 by p38 mitogen-activated protein kinases. Mol Cell Biol. 2002;22:3892–3904. doi: 10.1128/MCB.22.11.3892-3904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Zhang J, Lin H, Wang R, Qiao Y, Wang B, et al. p38 mitogen-activated protein kinase inhibition decreases TNFalpha secretion and protects against left ventricular remodeling in rats with myocardial ischemia. Inflammation. 2008;31:65–73. doi: 10.1007/s10753-007-9050-2. [DOI] [PubMed] [Google Scholar]
- Yin T, Sandhu G, Wolfgang CD, Burrier A, Webb RL, Rigel DF, et al. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. J Biol Chem. 1997;272:19943–19950. doi: 10.1074/jbc.272.32.19943. [DOI] [PubMed] [Google Scholar]
- Yu L, Li M, She T, Shi C, Meng W, Wang B, et al. Endothelin-1 stimulates the expression of L-type Ca2+ channels in neonatal rat cardiomyocytes via the extracellular signal-regulated kinase 1/2 pathway. J Membr Biol. 2013;246:343–353. doi: 10.1007/s00232-013-9538-7. [DOI] [PubMed] [Google Scholar]
- Yue Y, Qin Q, Cohen MV, Downey JM, Critz SD. The relative order of mKATP channels, free radicals and p38 MAPK in preconditioning’s protective pathway in rat heart. Cardiovasc Res. 2002;55:681–689. doi: 10.1016/s0008-6363(02)00452-2. [DOI] [PubMed] [Google Scholar]
- Yung HW, Wyttenbach A, Tolkovsky AM. Aggravation of necrotic death of glucose-deprived cells by the MEK1 inhibitors U0126 and PD184161 through depletion of ATP. Biochem Pharmacol. 2004;68:351–360. doi: 10.1016/j.bcp.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Xu R, Chen J, Cong X, Hu S, Chen X. Lovastatin protects mesenchymal stem cells against hypoxia- and serum deprivation-induced apoptosis by activation of PI3K/Akt and ERK1/2. J Cell Biochem. 2008;103:256–269. doi: 10.1002/jcb.21402. [DOI] [PubMed] [Google Scholar]
- Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- Zechner D, Thuerauf DJ, Hanford DS, McDonough PM, Glembotski CC. A role for the p38 mitogen-activated protein kinase pathway in myocardial cell growth, sarcomeric organization, and cardiac-specific gene expression. J Cell Biol. 1997;139:115–127. doi: 10.1083/jcb.139.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Chen YF, Campbell WB, Zou AP, Gross GJ, Li PL. Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circ Res. 2001;89:1177–1183. doi: 10.1161/hh2401.101752. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Kimura S, Nishiyama A, Shokoji T, Rahman M, Abe Y. ROS during the acute phase of Ang II hypertension participates in cardiovascular MAPK activation but not vasoconstriction. Hypertension. 2004;43:117–124. doi: 10.1161/01.HYP.0000105110.12667.F8. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Lu XM, Kimura S, Nishiyama A. Role of mitochondria in angiotensin II-induced reactive oxygen species and mitogen-activated protein kinase activation. Cardiovasc Res. 2007;76:204–212. doi: 10.1016/j.cardiores.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li XX, Bian HJ, Liu XB, Ji XP, Zhang Y. Inhibition of the activity of Rho-kinase reduces cardiomyocyte apoptosis in heart ischemia/reperfusion via suppressing JNK-mediated AIF translocation. Clin Chim Acta. 2009;401:76–80. doi: 10.1016/j.cca.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Zhang S, Weinheimer C, Courtois M, Kovacs A, Zhang CE, Cheng AM, et al. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111:833–841. doi: 10.1172/JCI16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao TC, Hines DS, Kukreja RC. Adenosine-induced late preconditioning in mouse hearts: role of p38 MAP kinase and mitochondrial KATP channels. American Journal of Physiology-Heart and Circulatory Physiology. 2001;280:H1278–H1285. doi: 10.1152/ajpheart.2001.280.3.H1278. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Herdegen T. Cerebral ischemia provokes a profound exchange of activated JNK isoforms in brain mitochondria. Mol Cell Neurosci. 2009;41:186–195. doi: 10.1016/j.mcn.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Zheng M, Dilly K, Dos Santos Cruz J, Li M, Gu Y, Ursitti JA, et al. Sarcoplasmic reticulum calcium defect in Ras-induced hypertrophic cardiomyopathy heart. Am J Physiol Heart Circ Physiol. 2004;286:H424–433. doi: 10.1152/ajpheart.00110.2003. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Lam PY, Han D, Cadenas E. c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J Neurochem. 2008;104:325–335. doi: 10.1111/j.1471-4159.2007.04957.x. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Lam PY, Han D, Cadenas E. Activation of c-Jun-N-terminal kinase and decline of mitochondrial pyruvate dehydrogenase activity during brain aging. FEBS Lett. 2009;583:1132–1140. doi: 10.1016/j.febslet.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JH, Guo F, Shelburne J, Watkins S, Chu CT. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 2003;13:473–481. doi: 10.1111/j.1750-3639.2003.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JH, Kulich SM, Oury TD, Chu CT. Cytoplasmic aggregates of phosphorylated extracellular signal-regulated protein kinases in Lewy body diseases. Am J Pathol. 2002;161:2087–2098. doi: 10.1016/S0002-9440(10)64487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Kinsey GR, Yan Y, Han J, Schnellmann RG. Extracellular signal-regulated kinase activation mediates mitochondrial dysfunction and necrosis induced by hydrogen peroxide in renal proximal tubular cells. J Pharmacol Exp Ther. 2008;325:732–740. doi: 10.1124/jpet.108.136358. [DOI] [PubMed] [Google Scholar]
- Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive Oxygen Species (Ros-Induced) Ros Release A New Phenomenon Accompanying Induction of the Mitochondrial Permeability Transition in Cardiac Myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]