Abstract
Associations between HLA class I antigen expression and efficacy of a melanoma vaccine (Melacine) were initially described in stage IV melanoma. Similar associations were observed in S9035, a phase III adjuvant trial evaluating Melacine for two years versus observation in patients with stage II melanoma. This report provides long-term results. The effects of treatment on relapse-free survival (RFS) and overall survival (OS) were evaluated, and pre-specified analyses investigated associations between treatment and HLA expression. Multivariable analyses were adjusted for tumor thickness, ulceration and site, method of nodal staging and sex. P=.01 was considered significant in subset analyses to account for multiple comparisons. For the entire study population of 689 patients, there were no significant differences in RFS or OS by arm. HLA serotyping was performed on 553 (80%) patients (vaccine 294, observation 259). Among the subpopulation with HLA-A2 and/or HLA-Cw3 serotype, vaccine arm patients (n=178) had marginally improved RFS (adjusted P=.02) and significantly improved OS compared with observation arm patients (n=145), with 10-year OS of 75% and 63%, respectively (hazard ratio 0.62, 99% CI 0.37-1.02, P=.01). There was no impact of HLA-A2 and/or HLA-Cw3 expression among observation arm patients. Analysis of mature data from S9035 indicates a significant OS benefit from adjuvant vaccine therapy for HLA-A2- and/or HLA-Cw3-expressing melanoma patients. The possibility of interactions between HLA type and outcome should be considered in future immunotherapy trials. Further investigations of melanoma-associated antigens present in Melacine and presented by HLA-A2 and HLA-Cw3 may be warranted.
Keywords: Vaccine, adjuvant immunotherapy, Melacine, melanoma, phase 3 clinical trial
INTRODUCTION
In 2013 there will be an estimated 76,690 new cases of invasive melanoma and 9,480 deaths from melanoma in the United States (1). The majority of patients with invasive melanoma present with clinically localized disease. Currently, high dose IFNα-2b and pegylated IFNα-2b are the only FDA-approved adjuvant therapy options for patients with high-risk melanoma, but there is a major unmet need for less toxic adjuvant therapies that could potentially improve outcomes for the large number of patients with intermediate-risk melanoma. Very few such patients receive adjuvant therapy today, and there is no therapy that has been shown to improve overall survival (OS) in patients with intermediate-thickness, node-negative melanoma (2).
In light of this, the SWOG initiated a phase III randomized clinical trial, S9035 in 1992, to evaluate the adjuvant use of an allogeneic melanoma vaccine. The vaccine chosen, Melacine (Corixa Corporation, Seattle, WA), is a polyvalent tumor-cell lysate derived from the melanoma metastasis of two patients (3). The vaccine contains numerous melanoma proteins that have shown the ability to mediate significant host immune reactivity in vivo (4). In a trial of stage IV melanoma patients, twelve of seventy patients exhibited a partial or complete response to Melacine (5). Additionally, Mitchell and colleagues (3, 6) indicated that Melacine had the ability to induce cytotoxic T lymphocyte-mediated immune responses and objective clinical responses in stage IV melanoma patients. Mitchell and colleagues utilized a hypothesis-generating Monte Carlo simulation to elicit all of the alleles most likely associated with clinical outcomes balanced against selecting criteria that apply broadly to the studied population (5). This resulted in the following criteria: two or more of five pre-specified HLA alleles (HLA-A2, HLA-A28, HLA-B44, HLA-B45, and HLA-Cw3) and at least one of the following alleles (HLA-A2 and –Cw3) (5). This association led to the incorporation of serotyping into the S9035 protocol, and the inclusion of pre-specified analyses of the correlation between these HLA serotypes and outcome.
S9035 accrued a total of 689 patients from April 1992 through November 1996, and results were reported after a median of follow-up among patients still living at 5.6 years from the start of the study (4). In that report, no statistically significant relapse-free survival (RFS) or OS benefit was found for the vaccine arm (4). However, in pre-specified subset analyses based on the work of Mitchell and colleagues, differences were found (5). Specifically, among patients with the HLA-A2 and/or HLA-Cw3 serotype the 5-year RFS was 77% for the vaccine arm, compared with 64% for the observation arm (P=.004) (7). Given these notable results, and the fact that the original analysis had insufficient events to adequately examine overall survival, this follow-up analysis evaluated long-term survival outcomes (RFS and OS) of S9035 by treatment arm and by HLA serotype.
PATIENTS AND METHODS
Clinical Trial Design
Eligible patients were 18 years or older with completely resected primary cutaneous melanoma that measured 1.5 to 4.0 mm in thickness or Clark's level IV (if thickness was unavailable), met all study eligibility criteria, were within 56 days of definitive surgery for the treatment of melanoma, and had no evidence of regional or metastatic disease on physical examination and chest x-ray (4). Patients were randomly assigned to either observation or 2 years of adjuvant therapy with a polyvalent, allogeneic melanoma cell-lysate vaccine (Melacine, Corixa Corporation, Seattle, WA) (4). The randomization was stratified to balance for sex, tumor thickness (1.5 to 3.0 mm, 3.1 to 4.0 mm, or Clark's level IV thickness unknown), and method of nodal staging (surgical, which could be elective lymphadenectomy or sentinel node biopsy, or clinical). Tumor ulceration, anatomic location of the primary tumor (extremity or non-extremity) and age were recorded as potential prognostic factors but not used in the stratification at the time of randomization. The Melacine vaccine is composed of an allogeneic melanoma cell lysate plus the immunologic adjuvant DETOX (detoxified Freund's adjuvant, containing mycobacterial cell wall skeleton plus monophosphoryl lipid A) (4). Each vaccine treatment consisted of two intramuscular injections of the vaccine/adjuvant combination (1.0 mL of Melacine cell lysate plus 0.25 mL DETOX split between two injection sites) given into extremities that were not involved with melanoma. The vaccines were delivered over the course of four 6-month cycles, each cycle consisting of 10 vaccinations (on weeks 1, 2, 3, 4, 6, 8, 12, 16, 20, and 24) followed by a 3 week rest (4).
Methods for HLA Class I Serologic Typing
All participating patients had their HLA class I alleles determined at a single, central laboratory as previously described using a panel of HLA class I antisera in an antigen/antibody test (7-9).
Statistical Analysis
The primary objective of this analysis was to evaluate long-term RFS and OS in patients from S9035 by arm and by HLA serotype. RFS is defined as the time from the date of randomization to the date of first clinical evidence of disease recurrence or death without evidence of recurrence, with patients last known to be alive and relapse-free censored at the date of last contact. OS is defined as the time from the date of randomization to the date of death due to any cause, with patients last known to be alive censored at the date of the last contact. Power and design specifications for the study were as previously presented (4). Survival plots were generated by the method of Kaplan and Meier (10). All multivariable RFS and OS comparisons were performed using Cox regression (11). Analyses by arm were adjusted for the design-specified stratification factors including tumor thickness, nodal staging method, and sex. Additional analyses by arm and by HLA phenotype status also were adjusted for ulceration and primary site location, which were recognized as prognostic factors in the overall trial results (4,8). Vaccine was coded as “1” and observation as “0”.
Previous trials of Melacine that enrolled stage IV patients indicated a relationship between five HLA class I serotypes and vaccine response (5). In pre-specified analyses, we examined if RFS and OS differed according to whether patients had ≥2 of the HLA alleles HLA-A2, -A28, -B44, -B45, and -Cw3, and by whether patients expressed HLA-A2 and/or -Cw3 vs. neither -A2 nor -Cw3. The analysis performed in 2002 examining the effect of HLA serotype on RFS used a critical level of P=.10 in tests for interactions, to minimize the possibility of missing a potentially significant interaction (12). Within HLA subsets, the analysis used a two-sided alpha level of P=.01 to account for multiple comparisons in evaluation of the series of pre-specified HLA hypotheses (7). For consistency, these statistical parameters are retained in this follow-up analysis of long term outcomes.
RESULTS
Description of the Study Population
689 patients were enrolled into the study. Updated eligibility assessment showed 91 ineligible patients, mostly due to nonconforming pathology or inadequate surgical resection (95% of exclusions). Although the trial was activated in April 1992, sample collection for HLA serotyping began in September 1994 with the last patient accrued by 1996. A cohort of 553 (80%) of the entire 689 enrolled patients gave consent and underwent HLA class I serotyping. The total was composed of 383 (94%) of the 409 patients entered on or after September 1, 1994, who were all prospectively typed, and 170 (61%) of the 280 patients entered before September 1, 1994, who were retrospectively typed after earlier entry into the protocol (7). There were 294 patients in the vaccine arm and 259 in the observation arm included in this analysis. Patient characteristics within the HLA serotyped cohort were well balanced by treatment arm (7) Also, there was no difference in the proportion of patients by arm who were HLA-A2+ and/or -C3+ (p=.27) or who had ≥2 of the Mitchell 5 alleles (p=.47). This analysis inevitably excluded some patients relapsing within the initial 2 years of enrollment, before the institution of HLA phenotyping (7). However the results were confirmed in the subset of 383 prospectively serotyped patients, justifying inclusion of all 553 serotyped patients in the final analysis.
Analysis of RFS and OS by Treatment Arm
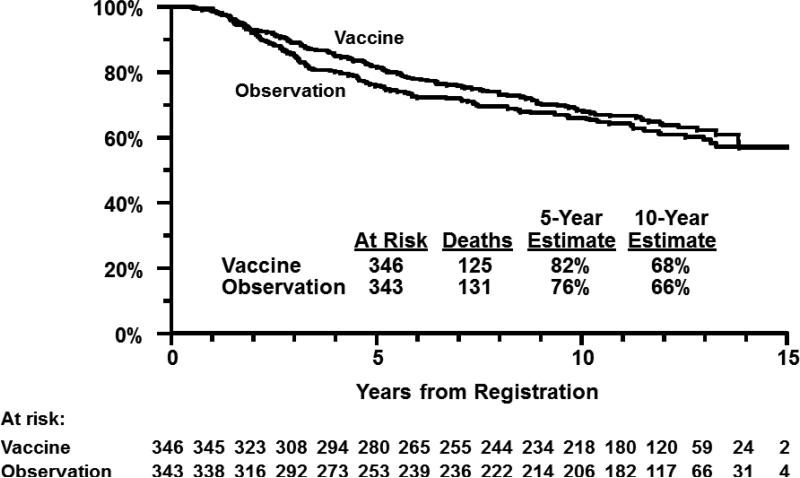
At the time of the present analysis, median follow-up among patients still alive was 12.1 years (maximum 15.2 years). In multivariable regression adjusting for stratification factors, RFS was not statistically different for patients assigned to the vaccine arm when either the eligible patients (P=.58) or all randomized patients (intent-to-treat analysis; P=.18) were evaluated. (Table 1) Similarly, OS did not differ significantly by treatment arm either in eligible patients (P=.61, Figure 1) or in all randomized patients (P=.31). Results were similar when primary site and tumor ulceration were included in the multivariable regression along with the stratification factors (data not shown).
Table 1.
Relapse-Free Survival and Overall Survival Analysis Results
| 5 Year Estimate | 10 Year Estimate | ||||||
|---|---|---|---|---|---|---|---|
| Analysis | Vaccine | Obs. | Vaccine | Obs. | Hazard Ratio | Confidence Interval* | p-value |
| Relapse Free Survival | |||||||
| Eligible | 66% | 64% | 56% | 54% | 0.94 | 0.74-1.18 | .58 |
| All Randomized | 67% | 63% | 57% | 53% | 0.86 | 0.70-1.07 | .18 |
| A2+ and/or Cw3+ | 78% | 65% | 66% | 54% | 0.67 | 0.43-1.04 | .02 |
| A2− and Cw3− | 64% | 66% | 54% | 57% | 1.14 | 0.70-1.88 | .49 |
| ≥2 of Mitchell 5 | 84% | 61% | 72% | 49% | 0.46 | 0.24-0.86 | .002 |
| 0 or 1 of Mitchell 5 | 67% | 67% | 56% | 58% | 1.07 | 0.72-1.59 | .65 |
| Overall Survival | |||||||
| Eligible | 81% | 77% | 67% | 67% | 0.93 | 0.72-1.21 | .61 |
| All Randomized | 82% | 76% | 68% | 66% | 0.88 | 0.69-1.13 | .31 |
| A2+ and/or Cw3+ | 90% | 76% | 75% | 63% | 0.62 | 0.37-1.02 | .01 |
| A2− and Cw3− | 80% | 84% | 67% | 75% | 1.33 | 0.73-2.41 | .22 |
| ≥2 of Mitchell 5 | 93% | 74% | 78% | 61% | 0.50 | 0.24-1.03 | .01 |
| 0 or 1 of Mitchell 5 | 83% | 82% | 69% | 72% | 1.07 | 0.59-1.47 | .69 |
95% confidence intervals are provided for the analyses of RFS and OS by intervention arm. Consistent with the design, which specifies a two-sided alpha level of p=.01 to account for multiple comparisons within HLA subsets, 99% confidence intervals are provided for the HLA subset analyses (i.e., A2+ and/or Cw3+; A2− and Cw3−; ≥2 of Mitchell 5; 0 or 1 of Mitchell 5).
Figure 1.
Overall survival by treatment arm in eligible patients.
Analysis of Relapse-Free and Overall Survival by HLA Serotype
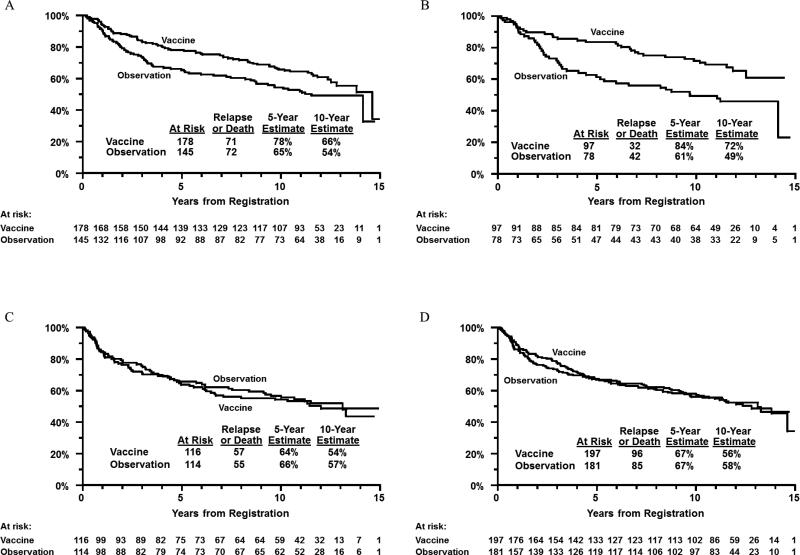
There was a significant test for interaction between treatment arm and HLA-A2/Cw3 status (A2+ and/or Cw3+ versus both A2- and Cw3-; P=.06). This analysis of the effect of HLA serotype used a critical level of P=.10 in tests for interactions, to minimize the possibility of missing a potentially significant interaction (12). In the A2+ and/or Cw3+ groups, the 10-year RFS for vaccine arm patients was 66% versus 54% for observation arm patients (P=.02, Figure 2, panel A). In the A2- and Cw3- groups, the 10-year RFS for vaccine arm patients was 54% versus 57% for observation arm patients (P=.49, Figure 2, panel C).
Figure 2.
Panel A: relapse-free survival by treatment arm in HLA-A2+ and/or -Cw3+ patients. Panel B: relapse-free survival by treatment arm in patients expressing ≥2 of the “Mitchell 5” alleles. Panel C: relapse-free survival by treatment arm in HLA-A2- and -Cw3- patients. Panel D: relapse-free survival by treatment arm in patients expressing none or one of the “Mitchell 5” alleles.
There was a highly significant test for interaction between treatment arm and the expression of at least two of the five HLA serotypes initially described by Mitchell and colleagues (P=.005). Among patients expressing at least two of the five alleles, the 10-year RFS for vaccine arm patients was 72% versus 49% for observation arm patients (P=.002, Figure 2, panel B). Among patients expressing one or none of the five alleles, 10-year RFS for vaccine arm patients was 56% versus 58% for observation arm patients (P=.65, Figure 2, panel D). Thus, there was improved RFS primarily among vaccine arm patients expressing at least two of the five noted alleles at 10 years following registration.
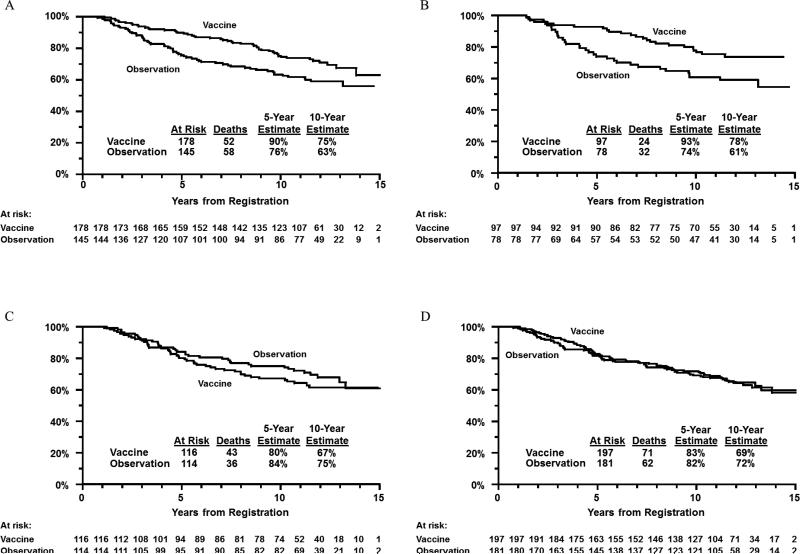
There was a statistically significant test for interaction between treatment arm and HLA A2/Cw3 status (P=.01). In the A2+ and/or Cw3+ group, the 10-year OS for vaccine arm patients was 75% versus 63% for observation arm patients (P=.01, Figure 3, panel A). In the A2- and Cw3- group, the 10-year OS for vaccine arm patients was 67% versus 75% for observation arm patients (P=.22, Figure 3, panel C).
Figure 3.
Panel A: overall survival by treatment arm in HLA-A2+ and/or -Cw3+ patients only. Panel B: overall survival by treatment arm in patients expressing ≥2 of the “Mitchell 5” alleles. Panel C: overall survival by treatment arm in HLA-A2- and -Cw3- patients only. Panel D: overall survival by treatment arm in patients expressing none or one of the “Mitchell 5” alleles.
There was also a significant interaction between treatment arm and the expression of at least two of the five HLA serotypes (P=.02). Among patients expressing at least two of the five alleles, the 10-year OS for vaccine arm patients was 78% versus 61% for observation arm patients (P=.01, Figure 3, panel B). Among patients expressing one or none of the five alleles, the 10-year OS for vaccine arm patients was 69% versus 72% for observation arm patients (P=.69, Figure 3, panel D). Thus, there was improved OS primarily among vaccine arm patients expressing at least two of the five noted alleles at 10 years following registration.
Since all analyses of the interaction between intervention and HLA serotype were derived in multivariable regressions, the effects noted above are independent of the adjustment variables including tumor thickness, nodal staging method, sex, ulceration, and primary site location.
DISCUSSION
The observation that the polyvalent melanoma cell lysate vaccine, Melacine was consistent with improved OS in a subset of intermediate-thickness node-negative melanoma patients defined by HLA serotype extends the findings of the previous report on S9035 by Sosman and colleagues, which indicated a highly significant benefit of adjuvant therapy with Melacine with respect to RFS among patients expressing two or more of the so-called “Mitchell 5” class I antigens (7). The consistent effect of pre-specified HLA serotypes on vaccination outcomes across studies in different disease stages and maintained over many years of follow-up suggests an effect that is unlikely to be due to chance alone. Moreover, the lack of impact of HLA serotype on outcome in the observation arm directly implicates the host (vaccine recipient in this case) immune response to Melacine and not selection of a subset of patients with an inherently favorable prognosis. The mechanism of action of this allogeneic cell lysate and the critical antigens involved has not been elucidated to date. While the most likely explanation would seem to be the existence of HLAA2- and HLA-Cw3-restricted antigenic peptides in the cell lysate that were important to the vaccine's antitumor immune effect; however, a possible effect of the vaccine adjuvant (detoxified Freund's adjuvant) in stimulating host immunity must also be considered. Why any such adjuvant effect should be HLA-restricted in the precise pattern observed is unexplained at this time (13). Mitchell and colleagues originally hypothesized that the HLA-restricted effect was due to a “match” between the HLA haplotype of the two patients whose tumors comprised the cell lysate (which formed the basis for the selection of the Mitchell 5 HLA antigens) and the vaccine recipient (5). However, our current understanding of antigen presentation would indicate that allogeneic peptide fragments are recognized in the context of the recipient HLA haplotype, regardless of the haplotype of the antigen “donor” through the process of antigen cross-presentation. Irrespective of the precise mechanism, the existence of a distinct and identifiable subset of patients with clinical benefit from allogeneic vaccination suggests continued exploration of immune-based adjuvant strategies in melanoma, with particular reference to melanoma-associated antigens present in Melacine and presented by HLA-A2 and HLA-Cw3. Moreover, the possibility of interactions between HLA haplotype and treatment with other types of immunotherapy, such as IFNα, ipilimumab, IL-2, anti-PD-1 or anti-PD-L1 antibody therapies, may deserve further study, although previous efforts to find such interactions have been inconclusive (14,15).
Although the results of this analysis closely mirror those of the prior analysis conducted a decade earlier, some caveats must be considered. Collection of samples for HLA class I serotyping was only initiated after trial recruitment had already begun. Therefore 136 patients (20%) were not serotyped. The patients not serotyped likely had more aggressive disease leading to progression and removal from the trial before serotyping was initiated (7). However, the main findings were confirmed in analyses restricted to only the 383 prospectively serotyped patients, so it is unlikely that the inclusion of some patients retrospectively serotyped or the loss of some patients with progressive disease prior to the initiation of serotyping materially affected the conclusions. Additionally, this analysis was limited to well-defined hypotheses about pre-specified antigens and groups of antigens that were established when serotyping was added to the protocol. The Cox regression model included adjustments for all the stratification and important prognostic factors that were available at the time.7
Since the trial began, major changes have occurred in the specificity and sensitivity of HLA haplotyping. The analyses initially performed by Mitchell and colleagues and those done as part of S9035 involved serotyping methodologies that are now considered out of date. Current molecularly-based HLA typing techniques have refined and further segmented some of the HLA serotypes used in the present analysis (16). This development hinders the translation of the results from S9035 to the care of present day patients, but presents an opportunity for future trials to more completely characterize the patient populations being studied.
Additionally, since the S9035 trial began in 1992, the standard of care for surgical treatment of intermediate-thickness clinically node-negative melanoma has changed, with the abandonment of elective lymphadenectomy and the widespread use of sentinel node biopsy now the norm in this patient population. In S9035, only a quarter of all patients enrolled underwent surgical staging of the regional nodes, and sentinel node biopsy was rarely used, so micrometastases would have been present in some patients who were classified as being node-negative in this trial. The inability to detect microscopic lymph node involvement in S9035 means this patient population likely contained both stage II and III melanoma patients. The prognosis of such patients would be worse than that of present day melanoma patients with intermediate thickness tumors, and the effect of the adjuvant therapy could have been different between those patients with and without microscopic nodal involvement, making it more difficult to compare these results to more recent studies. Other groups have examined several different antigens as vaccines in early phase clinical trials. These include the antigens MAGE-A3, MART-1, gp100 and tyrosinase, but it remains unclear whether any of these have antitumor efficacy when used in the adjuvant setting (17-21). Melacine contains all the above antigens, along with others that have not been characterized. The only positive phase III vaccine trial in melanoma reported to date utilized gp100:209-217 peptide fragments in patients with unresectable stage III and IV melanoma along with high-dose IL-2 (20). In this trial, patients had to express the HLA*A201 genotype to be eligible (the molecular counterpart of the HLA-A2 serotype in our study). Patients receiving IL-2 combined with the gp100 peptide vaccine demonstrated a significantly higher response rate (16% vs. 6%, P=.03) and a trend toward longer median OS (17.2 months vs. 11.2 months; P=.06) (20). The addition of interleukin-2 was hypothesized to work via its ability to overcome a weak immune response to gp100. Although not directly analogous to our own findings, the results of this phase III trial lend support to the notion that melanocyte antigen peptide vaccines in properly HLA-restricted patients can have clinical significance. Further evidence is provided by a trial involving ESO, a cancer antigen group, which when administered as a vaccine led to CD4+ T cell responses only in patients with a specific MHC class II allele, HLA-DR52b, that is present in about half of the Caucasian population (22). The identification of specific MHC class II epitopes that correlate with response supports the role of immunologic evaluation of host HLA alleles in tumor vaccine trials.
Patients with surgically resected melanoma at risk for relapse need an active and durable therapy to prevent melanoma recurrence. This final report of S9035 indicates a potentially clinically significant OS benefit from adjuvant vaccine therapy for patients with HLA-A2 and/or HLA-Cw3 serotypes that were not seen in the entire group of treated patients. These results suggest that our understanding of the optimum ways to stimulate an antitumor immune response in melanoma patients is still incomplete, and the possibility of interactions between HLA haplotype and outcome should be considered in future immunotherapy trials.
Acknowledgments
Funding: This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA20319, CA27057, CA22433, CA58723, CA58861, CA46441, CA46113, CA37981, CA76448, CA35281, CA04919, CA58686, CA46136, CA35090, CA35176, CA12644, CA35262, CA35119, CA14028, CA16385, CA45450, CA58658, CA45377, CA46282, CA42777, CA35178, CA35192, CA58416, CA76429, CA35431, CA45807, CA28862, CA13612, CA58415, CA58348, CA45560, CA63844, CA12213, CA58882, CA35117, CA76447
Footnotes
AUTHOR DISCLOSURE DECLARATION Employment or leadership position:
Consultant or advisory role: J. Sosman, self/compensated (GSK, Amgen, Genentech)
Stock Ownership: none
Honoraria: none
Research funding: J. Sosman, self (BMS, Novartis)
Expert testimony: none
Patents, licenses or royalties: none
Other remuneration: none
None: W. Carson, L. Flaherty, R. Kempf, M. Othus, M. Porter, A. Ribas, V. Sondak, J. Thompson, R.
Tuthill, J Unger
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA-Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Aamdal S. Current approaches to adjuvant therapy of melanoma. Eur J Cancer. 2011;47:336–7. doi: 10.1016/S0959-8049(11)70193-9. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell MS, Kan-Mitchell J, Kempf RA, Harel W, Shau HY, Lind S. Active specific immunotherapy for melanoma: phase I trial of allogeneic lysates and a novel adjuvant. Cancer Res. 1988;48:5883–93. [PubMed] [Google Scholar]
- 4.Sondak VK, Liu PY, Tuthill RJ, Kempf RA, Unger JM, Sosman JA, et al. Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: overall results of a randomized trial of the Southwest Oncology Group. J Clin Oncol. 2002;20:2058–66. doi: 10.1200/JCO.2002.08.071. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell MS, Harel W, Groshen S. Association of HLA phenotype with response to active specific immunotherapy of melanoma. J Clin Oncol. 1992;10:1158–64. doi: 10.1200/JCO.1992.10.7.1158. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell MS, Harel W, Kempf RA, Hu E, Kan-Mitchell J, Boswell WD, et al. Active-specific immunotherapy for melanoma. J Clin Oncol. 1990;8:856–69. doi: 10.1200/JCO.1990.8.5.856. [DOI] [PubMed] [Google Scholar]
- 7.Sosman JA, Unger JM, Liu PY, Flaherty LE, Park MS, Kempf RA, et al. Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: impact of HLA class I antigen expression on outcome. J Clin Oncol. 2002;20:2067–75. doi: 10.1200/JCO.2002.08.072. [DOI] [PubMed] [Google Scholar]
- 8.Terasaki PI. Histocompatibility testing in transplantation. Arch Pathol Lab Med. 1991;115:250–4. [PubMed] [Google Scholar]
- 9.Hopkins KA. The basic lymphocyte microcytotoxicity tests. In: Phelan D, Mickelson E, Noreen H, editors. ASHI Laboratory Manual. ed 3. American Society for Histocompatibility and Immunogenetics; Lenexer, KS: Section I.B.1.1-I.B.1.13. [Google Scholar]
- 10.Kaplan EL, Meier Paul. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–81. [Google Scholar]
- 11.Cox DR. Regression Models and Life-Tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 12.Green S, Liu PY, O'Sullivan J. Factorial design considerations. J Clin Oncol. 2002;20:3424–30. doi: 10.1200/JCO.2002.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Gnjatic S, Sawhney NB, Bhardwaj N. Toll-like receptor agonists: are they good adjuvants? Cancer J. 2010;16:382–91. doi: 10.1097/PPO.0b013e3181eaca65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogas H, Kirkwood JM, Falk CS, Dafni U, Sondak VK, Tsoutsos D, et al. Correlation of molecular human leukocyte antigen typing and outcome in high-risk melanoma patients receiving adjuvant interferon. Cancer. 2010;116:4326–33. doi: 10.1002/cncr.25211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoon DS, Okamoto T, Wang HJ, Elashoff R, Nizze AJ, Foshag LJ, et al. Is the survival of melanoma patients receiving polyvalent melanoma cell vaccine linked to the human leukocyte antigen phenotype of patients? J Clin Oncol. 1998;16:1430–7. doi: 10.1200/JCO.1998.16.4.1430. [DOI] [PubMed] [Google Scholar]
- 16.Barouch D, Friede T, Stevanović S, Tussey L, Smith K, Rowland-Jones S, et al. HLA-A2 subtypes are functionally distinct in peptide binding and presentation. J Exp Med. 1995;182:1847–56. doi: 10.1084/jem.182.6.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995;154:3961–68. [PubMed] [Google Scholar]
- 18.Roeder C, Schuler-Thurner B, Berchtold S, Vieth G, Driesch P, Schuler G, et al. MAGE-A3 is a frequent tumor antigen of metastasized melanoma. Arch Dermatol Res. 2005;296:314–9. doi: 10.1007/s00403-004-0527-7. [DOI] [PubMed] [Google Scholar]
- 19.Kruit WH, Suciu S, Dreno B, Mortier L, Robert C, Chiarion-Sileni V, et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in metastatic melanoma. J Clin Oncol. 2013;31:2413–20. doi: 10.1200/JCO.2012.43.7111. [DOI] [PubMed] [Google Scholar]
- 20.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–27. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drexler I, Antunes E, Schmitz M, Woelfel T, Huber C, Erfle V, et al. Modified vaccinia virus ankara for delivery of human tyrosinase as melonoma-associated antigen: Induction of tyrosinase- and melonoma-specific human leukocyte antigen A*^0201-restricted cytotoxic T cells in vitro and in vivo. Cancer Res. 1999;59:4955–63. [PubMed] [Google Scholar]
- 22.Bioley G, Dousset C, Yeh A, Dupont B, Bhardwaj N, Mears G, et al. Vaccination with recombinant NYESO-1 protein elicits immunodominant HLA-DR52b-restricted CD4+ T cell responses with a conserved T cell receptor repertoire. Clin Cancer Res. 2009;15:4467–74. doi: 10.1158/1078-0432.CCR-09-0582. [DOI] [PubMed] [Google Scholar]